Abstract
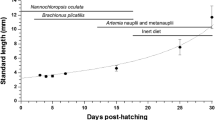
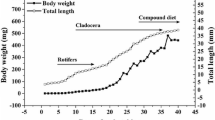
Knowledge of the developmental ontogeny of the digestive system and nutritional requirements of marine fish larvae is a primary requisite for their successful rearing under an optimal feeding regime. In this context, we assessed the activity profile of key digestive enzymes viz., trypsin, chymotrypsin, leucine aminopeptidase, lipase, amylase, and alkaline phosphatase during the early ontogeny of milkfish, Chanos chanos (0 day, 3 days, 6 days, 9 days, 12 days, 15 days, 18 days, 21 days, 25 days, and 30 days post-hatch). Larvae for this study were obtained from the successful breeding of milkfish at ICAR-Central Institute of Brackishwater Aquaculture, India. Growth curves (length and weight) of the larvae indicated a positive morphological development under a standardized feeding regime that comprised Chlorella salina, Brachionus plicatilis, Artemia salina nauplii, and commercial weaning feed for different larval stages. With respect to protein digestion, the specific activity of pancreatic enzymes trypsin and chymotrypsin and intestinal brush border leucine aminopeptidase showed two peaks at 3 dph and 15 dph, following the introduction of rotifer and Artemia nauplii. Similar bimodal peaks were observed for alkaline phosphatase and amylase activities, with the first peak at 3 dph and the second peak at 18 dph and 21 dph, respectively. Whereas in the case of lipase, high activity levels were observed at 0 dph, 3 dph, and 18 dph, with subsequent decreases and fluctuations. Overall, as most of the enzymes were found to have peak activities at 15 to 21 dph, this period can be potentially considered as the developmental window for weaning larvae from live to formulated feeds in milkfish hatcheries.








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the lead author.
References
Alvarez-Gonzalez CA, Moyano-Lopez FJ, Civera-Cerecedo R, Carrasco-Chávez V, Ortiz-Galindo JL, Dumas S (2008) Development of digestive enzyme activity in larvae of spotted sand bass Paralabrax maculatofasciatus. Fish Physiol and Biochem 34(4):373–384
Alvarez-Gonzalez CA, Cervantes-Trujano M, Tovar-Ramírez D, Conklin DE, Nolasco H, Gisbert E, Piedrahita R (2006) Development in digestive enzymes in California halibut Paralichthys californicus larvae. Fish Physiol Biochem 31:83–93
Asgari R, Rafiee G, Eagderi S, Noori F, Agh N, Poorbagher H, Gisbert E (2013) Ontogeny of the digestive enzyme activities in hatchery produced Beluga (Huso huso). Aquaculture 416:33–40. https://doi.org/10.1016/j.aquaculture.2013.08.014
Baglole CJ, Goff GP, Wright GM (1998) Distribution and ontogeny of digestive enzymes in larval yellowtail and winter flounder. J Fish Biol 53:767–784
Beccaria C, Diaz JP, Connes R, Chatain B (1991) Organogenesis of the exocrine pancreas in the sea bass, Dicentrarchus labrax L., reared extensively and intensively. Aquaculture 99:339–354
Bera A, Kailasam M, Mandal B, Sukumaran K, Makesh M, Hussain T, Vijayan K (2019) Effect of tank colour on foraging capacity, growth, and survival of milkfish (Chanos chanos) larvae. Aquaculture 512:734347. https://doi.org/10.1016/j.aquaculture.2019.734347
Bera A, Kailasam M, Mandal B, Padiyar A, Ambasankar K, Sukumaran K, Makesh M, Kumararaja P, Subburaj R, Thiagarajan G, Vijayan KK (2021) Maturity induction and extended spawning kinetics of milkfish (Chanos chanos) administered with combined GnRHa and 17α-methyl testosterone pellet at varied frequencies. Aquaculture:736993
Bessey OA, Love RH (1952) Preparation and measurement of the purity of the phosphatase reagent, disodium p-nitrophenyl phosphate. J Biol Chem 196:175–178
Buchet V, Infante JZ, Cahu C (2000) Effect of lipid level in a compound diet on the development of red drum (Sciaenops ocellatus) larvae. Aquaculture 184:339–347
Buddington RK, Diamond JM (1989) Ontogenetic development of intestinal nutrient transport. Ann Rev Physiol 51:601–619
Cahu CL, Zambonino-Infante JL (2001) Substitution of live food by formulated diets in marine fish larvae. Aquaculture 200:161–180
Cahu CL, Ronnestad I, Grangier V, Zambonino-Infante JL (2004) Expression and activities of pancreatic enzymes in developing sea bass larvae (Dicentrarchus labrax) in relation to intact and hydrolysed dietary protein; involvement of cholecystokinin. Aquaculture 238:295–308
Campoverde C, Rodríguez C, Perez JA, Gisbert E, Estevez A (2017) Early weaning in meagre Argyrosomus regius: effects on growth, survival, digestion and skeletal deformities. Aquac Res 48. https://doi.org/10.1111/are.13342
Cara JB, Moyano FJ, Cardenas S, Fernandez- Diaz C, Yufera M. (2003) Assessment of digestive enzyme activities during larval development of white bream. J Fish Biol 63:48–58
Chakrabarti R, Rathore MR, Mittal P, Kumar S (2006) Functional changes in digestive enzymes and characterization of proteases of silver carp (♂) and bighead carp (♀) hybrid, during early ontogeny. Aquaculture 253(1–4):694–702. https://doi.org/10.1016/jaquaculture.2005.08.018
Chen BN, Qin JG, Kumar MS, Hutchinson W, Clarke S (2006) Ontogenetic development of digestive enzymes in yellowtail kingfish Seriola lalandi larvae. Aquaculture 260:264–271. https://doi.org/10.1016/j.aquaculture.2006.06.021
Comabella Y, Mendoza R, Aguilera C, Carrillo O, Hurtado A, García GT (2006) Digestive enzyme activity during early larval development of the Cuban gar (Atractosteus tristoechus). Fish Physiol Biochem 32:147–157. https://doi.org/10.1007/s10695-006-0007-4
Cordova-Montejo M, Alvarez-Gonzalez C, Lopez L, Frias-Quintana C, Galaviz M (2019) Changes of digestive enzymes in totoaba (Totoaba macdonaldi Gilbert, 1890) during early ontogeny. Latin American J Aquac Res 47:102–113. https://doi.org/10.3856/vol47-issue1-fulltext-11
Cousin JB, Baudin-Laurencin F, Gabaudan J (1987) Ontogeny of enzymatic activities in fed and fasting turbot, Scophtalmus maximus L. J Fish Biol 30:15–33
Cuvier-Peres A, Kestemont P (2002) Development of some digestive enzymes in Eurasian perch larvae Perca fluviatilis. Fish Physiol Biochem 24:279–285
Dayal JS, Ali SA, Thirunavukkarasu A, Kailasam M, Subburaj R (2003) Nutrient and amino acid profiles of egg and larvae of Asian seabass, Lates calcarifer (Bloch). Fish Physiol Biochem 29(2):141–147
Erlanger B, Kokowsky N, Cohen W (1961) The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys 95:271–278
Erlanger BF, Edel F, Cooper AG (1966) The action of chymotrypsin on two new chromogenic substrates. Arch Biochem Biophys 115(1):206–210
Frias-Quintana CA, Márquez-Couturier G, Alvarez-González CA, Tovar-Ramírez D, Nolasco-Soria H, Galaviz-Espinosa MA, Martínez-García R, Camarillo-Coop S, Martínez-Yañez R, Gisbert E (2016) Development of digestive tract and enzyme activities during the early ontogeny of the tropical gar Atractosteus tropicus. Fish Physiol Biochem 41(5):1075–1091. https://doi.org/10.1007/s10695-015-0070-9
Galaviz MA, Garcia-Gasca A, Drawbridge M, Alvarez-Gonzalez CA, Lopez LM (2011) Ontogeny of the digestive tract and enzymatic activity in white seabass, Atractoscion nobilis, larvae. Aquaculture 318:162–168
Gawlicka A, Teh SJ, Hung SSO, Hinton DE, Noue J (1995) Histological and histochemical changes in the digestive tract of white sturgeon larvae during ontogeny. Fish Physiol Biochem 14:357–371. https://doi.org/10.1007/BF00003374
Gawlicka A, Parent B, Horn MH, Ross N, Opstad I, Torrissen OJ (2000) Activity of digestive enzymes in yolk-sac larvae of Atlantic halibut (Hippoglossus hippoglossus): indication of readiness for first feeding. Aquaculture 184:303–314
German DP, Bittong RA (2009) Digestive enzyme activities and gastrointestinal fermentation in wood-eating catfishes. J Comp Physiol B 179(8):1025–1042
Gisbert E, Gimenez G, Fernandez I, Kotzamanis Y, Estevez A (2009) Development of digestive enzymes in common dentex, Dentex dentex, during early ontogeny. Aquaculture 287:381–387
Hamza N, Mhetli M, Kestemont P (2007) Effects of weaning age and diets on ontogeny of digestive activities and structures of pikeperch (Sander lucioperca) larvae. Fish Physiol Biochem 33:121–133. https://doi.org/10.1007/s10695-006-9123-4
Horn MH, Neighbors M, Murray S (1986) Herbivore responses to a seasonally fluctuating food supply: growth potential of two temperate intertidal fishes based on the protein and energy assimilated from their macroalgal diets. J Exp Mar Biol Ecol 103:217–234
Jimenez-Martınez LD, Alvarez-Gonzalez CA, Tovar-Ramırez D, Gaxiola G, Sanchez-Zamora A, Moyano FJ, Alarcon FJ, Marquez-Couturier G, Gisbert E, Contreras-Sanchez WM, Perales-Garcıa N, Arias-Rodrıguez L, Indy JR, Paramo-Delgadillo S, Palomino-Albarran IG (2012) Digestive enzyme activities during early ontogeny in Common snook (Centropomus undecimalis). Fish Physiol Biochem 38:441–454
Khoa TND, Waqalevu V, Honda A, Shiozaki K, Kotani T (2019) Early ontogenetic development, digestive enzymatic activity and gene expression in red sea bream (Pagrus major). Aquaculture 512:734283. https://doi.org/10.1016/j.aquaculture.2019.734283
Kim BG, Divakaran S, Brown CL, Ostrowski AC (2001) Comparative digestive enzyme ontogeny in two marine larval fishes: Pacific threadfin (Polydactylus sexfilis) and bluefin trevally (Caranx melampygus). Fish Physiol Biochem 24:225–241
Kurokawa T, Suzuki T (1996) Formation of the diffuse pancreas and the development of digestive enzyme synthesis in larvae of the Japanese flounder Paralichthys oliÍaceus. Aquaculture 141:267–276
Kurokawa T, Suzuki T (1998) Development of intestinal brush border aminopeptidase in the larval Japanese flounder Paralichthys olivaceus. Aquaculture 162:113–124
Liao IC, Juario JV, Kumagai S, Nakajim H, Natividad M, Buri P (1979) On the induced spawning and larval rearing of milkfish, Chanos chanos (Forskal). Aquaculture 18(2):75–93
Lim C, Borlongan IG, Pascual FP (2002) Milkfish, Chanos chanos, in nutrient requirements and feeding of finfish for aquaculture, webster. C. D. and C. E. Lim. Cabi Publishing, New York, USA, pp 172–183
Ma H, Cahu C, Zambonino J, Yu H, Duan Q, Le Gall MM, Mai K (2005) Activities of selected digestive enzymes during larval development of large yellow croaker (Pseudosciaena crocea). Aquaculture 245:239–248
Ma Z, Guo H, Zheng P, Wang L, Jiang S, Qin JG, Zhang D (2014) Ontogenetic development of digestive functionality in golden pompano Trachinotus ovatus (Linnaeus 1758). Fish Physiol Biochem 40(4):1157–1167. https://doi.org/10.1007/s10695-014-9912-0
Mendoza R, Aguilera C, Rodríguez G, González M, Castro R (2002) Morphophysiological studies on alligator gar (Atractosteus spatula) larval development as a basis for their culture and repopulation of their natural habitats. Fish Biol 12:133–142
Pedersen BH, Nilssen EM, Hjeldman K (1987) Variations in the content of trypsin and trypsinogen in larval herring (Clupea harengus) digesting copepod nauplii. Mar Biol 94:171–181
Peres A, Cahu CL, Zambonino Infante JL, Legall MM, Quazuguel P (1996) Amylase and trypsin responses to intake of dietary carbohydrate and protein depend on the developmental stage in sea bass (Dicentrarchus labrax) larvae. Fish Physiol Biochem 15:237–242
Rainuzzo JR, Reitan KI, Jørgensen L (1992) Comparative study on the fatty acid and lipid composition of four marine fish larvae. Comp. Biochem. Physiol. 103B:21–26
Ribeiro L, Zambonino-Infante JL, Cahu C, Dinis MT (1999) Development of digestive enzymes in larvae of Solea senegalensis, Kaup 1858. Aquaculture 179:465–473
Rajesh M, Kamalam BS, Sharma P, Verma VC, Pandey A, Dubey MK, Ciji A, Akhtar MS, Pandey N, Sarma D, Kaushik SJ (2022) Evaluation of a novel methanotroph bacteria meal grown on natural gas as fish meal substitute in rainbow trout, Oncorhynchus mykiss. Aquac Res 53(6):2159–2174
Rønnestad I, Finn RN, Lein I, Lie Ø (1995) Compartmental changes in the contents of total lipid, lipid classes and their associated fatty acids in developing yolk-sac larvae of Atlantic halibut Hippoglossus hippoglossus _L. Aquac Res 1:119–130
Rønnestad I, Yúfera M, Ueberschär B, Ribeiro L, Sæle Ø, Boglione C (2013) Feeding behaviour and digestive physiology in larval fish: current knowledge and gaps and bottlenecks in research. Rev Aquac 5:559–598. https://doi.org/10.1111/raq.12010
Santos A, Pedreira M, Santos T, Moura G, Santos J, Silva R (2016) Development of the digestive system in larvae of the Neotropical fish Prochilodus argenteus (Characiformes, Prochilodontidae). Acta Scientiarum Ani Sci 38:9. https://doi.org/10.4025/actascianimsci.v38i1.28824
Segner H, Storch V, Reinecke M (1994) The development of functional digestive and metabolic organs in turbot, Scophthalmus maximus. Mar Biol 119(3):471–486
Shan X-J, Huang W, Cao L, Xiao Z-Z, Dou S-Z (2009) Ontogentic development of digestive enzymes and effect of starvation in miiuy croaker Miichthys miiuy larvae. Fish Physiol Biochemi 35:385–98
Silva JFX, Ribeiro K, Silva JF, Cahú TB, Bezerra RS (2014) Utilization of tilapia processing waste for the production of fish protein hydrolysate. Animal Feed Sci Technol 196:96–106
Sivaramakrishnan T, Ambasankar K, Kumaraguru Vasagam KP, Syama Dayal J, Sandeep KP, Bera A, Makesh M, Kailasam M, Vijayan KK (2021) Effect of dietary soy lecithin inclusion levels on growth, feed utilization, fatty acid profile, deformity and survival of milkfish (Chanos chanos) larvae. Aquac Res 52(11):5366–5374
Sivaramakrishnan T, Syama Dayal J, Ambasankar K, Felix N, Sandeep KP, Aritra Bera, Kumaraguru Vasagam KP, Thiyagarajan G, Kailasam M (2023) Amino acid and fatty acid compositions of various stages of Chanos chanos larvae: implications for larval feed formulation. Ind J Fish 70(1). https://doi.org/10.21077/ijf.2023.70.1.130207-12
Srichanun M, Tantikitti C, Vatanakul V, Musikarune P (2013) Digestive enzyme activity during ontogenetic development and effect of live feed in green catfish larvae (Mystus nemurus Cuv. and Val). Songklanakarin J Sci Technol 34(3):247–254
Suzer C, Firat K, Saka S (2006) Ontogenic development of the digestive enzymes in common pandora, Pagellus erythrinus, L. larvae. Aquac Res 37:1565–1571
Suzer C, Kamaci HO, Coban D, Saka S, Firat K, Ozkara B, Ozkara A (2007) Digestive enzyme activity of the red porgy (Pagrus pagrus, L.) during larval development under culture conditions. Aquac Res 38:1778–1785
Suzer C, Coban D, Yildirim Ş, Hekimoğlu M, Kamacı HO, Firat K, Saka Ş (2014) Stage-specific ontogeny of digestive enzymes in the cultured Common Dentex (Dentex dentex) larvae. Turkish J Fish Aquat Sci 14:759–768. https://doi.org/10.4194/1303-2712-v14_3_18
Teles A, Salas-Leiva J, Alvarez-Gonzalez CA, Tovar-Ramirez D (2018) Changes in digestive enzyme activities during early ontogeny of Seriola rivoliana. Fish Physiol Biochem 45:733–742. https://doi.org/10.1007/s10695-018-0598-6
Toledo-Solis FJ, Hilerio-Ruiz AG, Delgadin T (2021) Changes in digestive enzyme activities during the early ontogeny of the South American cichlid (Cichlasoma dimerus). Fish Physiol Biochem 47:1211–1227. https://doi.org/10.1007/s10695-021-00976-z
Toledo-Solis FJ, Uscanga-Martínez A, Guerrero-Zárate R, Márquez-Couturier G, Martínez-García R, Camarillo-Coop S, Perales-García N, Rodríguez-Valencia W, Gómez-Gómez MA, Álvarez-González CA (2014) Changes on digestive enzymes during initial ontogeny in the three-spot cichlid Cichlasoma trimaculatum. Fish Physiol Biochem 41:267–279
Ueberschar B (1993) Measurement of proteolytic enzyme activity: significance and application in larval fish research. In: Walter BT, Fyhn HJ (eds) Physiological and biochemical aspects of fish development. University of Bergen, Bergen, pp 233–239
Uscanga-Martinez A, Perales-Garcia N, Alvarez-Gonzalez CA, Moyano FJ, Tovar-Ramirez D, Gisbert E, Marquez-Couturier G, Contreras-Sanchez WM, Arias-Rodriguez L, Indy JR (2011) Changes in digestive enzyme activity during initial ontogeny of bay snook Petenia splendida. Fish Physiol Biochem 37:667–680
Woolley LD, Partridge GJ, Qin JG (2012) Mortality reduction in yellowtail kingfish (Seriola lalandi) larval rearing by optimising Artemia feeding regimes. Aquaculture 344:161–167
Yufera M, Moyano FJ, Martinez-Rodriguez G (2018) The digestive function in developing fish larvae and fry. From molecular gene expression to enzymatic activity. Emer Issues Fish Larvae Res:51–86. https://doi.org/10.1007/978-3-319-73244-2_3
Zambonino Infante JL, Cahu C (1994) Development and response to a diet change of some digestive enzymes in sea bass Dicentrarchus labrax larvae. Fish Physiol Biochem 12:399–408. https://doi.org/10.1007/BF00004304
Zambonino-Infante JL, Cahu CL (2001) Ontogeny of the gastrointestinal tract in marine fish larvae. Comp Biochem Physiol 130C:477–487. https://doi.org/10.1016/S1532-0456(01)00274-5
Zambonino-Infante J, Gisber E, Sarasquete C (2008) Ontogeny and physiology of the digestive system of marine fish larvae. In: Cyrino JEO, Bureau D, Kapoor BG (eds) Feeding and digestive functions of fish. Science Publishers. Inc, Enfield, USA, pp 277–344
Zueva NN, Dalev PG, Lazarova DL (1993) Properties, preparation, and practical use of alkaline phosphatase. Biokimiya 58:1009–1023
Acknowledgements
The authors thank the Indian Council of Agricultural Research for providing financial support to carry out this research program (FISHCIBASIL 201800800147). The authors are grateful to the director of ICAR-CIBA for his facilitation, support, and guidance. We also sincerely appreciate the fish hatchery and feed mill staff at Muttukadu Experimental Station of ICAR-CIBA for their help in conducting the experiments and laboratory analysis.
Funding
Indian Council of Agricultural Research, FISHCIBASIL 201800800136.
Author information
Authors and Affiliations
Contributions
Thirugnanmurthy Sivaramakrishnan conceived, concept developed, and designed the study. Thirugnanmurthy Sivaramakrishnan and Aritra Bera conducted the experiments and collected the data. Thirugnanmurthy Sivaramakrishnan and Biju Sam Kamalam enzyme analyzed and revised the manuscript, and Kondusamy Ambasankar supervised the study. Thirugnanmurthy Sivaramakrishnan wrote the manuscript. K.P. Kumaraguru Vasagam and Muniyandi Kailasam, Nathan Felix, and Ambasankar Kondusamy revised the manuscript. All authors edited and approved the final manuscript for submission to the journal.
Corresponding author
Ethics declarations
Ethical approval
The institute animal ethical committee clearance was obtained for this study at the Central Institute of Brackishwater Aquaculture, Chennai, India. Milkfish, Chanos chanos larvae used in the experiment, is obtained from the hatchery, and it is not an endangered fish as per the provisions of the Govt. of India’s Wildlife Protection Act of 1972.
Consent to participate
Participation in this article has been consented to by all authors.
Consent for publication
All authors approved the manuscript for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sivaramakrishnan, T., Ambasankar, K., Felix, N. et al. Changes in digestive enzyme activities during the early ontogeny of milkfish, Chanos chanos larvae. Fish Physiol Biochem 49, 867–882 (2023). https://doi.org/10.1007/s10695-023-01225-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-023-01225-1