Abstract
Lipid droplets (LDs) are subcellular organelles secreted from the endoplasmic reticulum (ER) that play a major role in lipid homeostasis. Recent research elucidates additional roles of LDs in cellular bioenergetics and innate immunity. LDs activate signaling cascades for interferon response and secretion of pro-inflammatory cytokines. Since balanced lipid homeostasis is critical for neuronal health, LDs play a crucial role in neurodegenerative diseases. RNA viruses enhance the secretion of LDs to support various phases of their life cycle in neurons which further leads to neurodegeneration. Targeting the excess LD formation in the brain could give us a new arsenal of antiviral therapeutics against neuroviruses. Liposomes are a suitable drug delivery system that could be used for drug delivery in the brain by crossing the Blood–Brain Barrier. Utilizing this, various pharmacological inhibitors and non-coding RNAs can be delivered that could inhibit the biogenesis of LDs or reduce their sizes, reversing the excess lipid-related imbalance in neurons.
Graphical Abstract
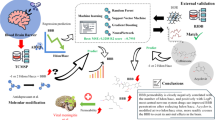
Liposome-Mediated Antiviral Drug Delivery Across Blood-Brain Barrier.
Developing effective antiviral drug is challenging and it doubles against neuroviruses that needs delivery across the Blood–Brain Barrier (BBB). Lipid Droplets (LDs) are interesting targets for developing antivirals, hence targeting LD formation by drugs delivered using Liposomes can be game changers.






Similar content being viewed by others
Data Availability
Not Applicable.
Abbreviations
- LDs:
-
Lipid droplets
- ER:
-
Endoplasmic reticulum
- TAGs:
-
Triacylglycerols
- DAGs:
-
Diacylglycerols
- SEs:
-
Sterol esters
- BBB:
-
Blood–Brain Barrier
- DGAT:
-
Diacylglycerol acyltransferase
- GPAT:
-
Glycerol 3 phosphate acyltransferase
- AGPAT:
-
Acylglycerol 3 phosphate
- PAP:
-
Phosphatidate phosphate
- FATP:
-
Fatty acid transport protein
- MGAT2:
-
Monoacylglycerol acyltransferase
- SCD1:
-
Stearoyl-CoA Desaturase 1
- nLDs:
-
Nuclear lipid droplets
- PLIN5:
-
Perilipin 5
- PLIN1:
-
Perilipn 1
- PLIN2:
-
Perilipn 2
- PLIN3:
-
Perilipn 3
- PKA:
-
Protein kinase A
- PKB:
-
Protein kinase B
- SIRT1:
-
Sirtuin1
- CRABP1:
-
Cellular retinoic acid-binding protein 1
- ATRA:
-
All-transretinoic Acid
- TLR:
-
Toll-like receptor
- EGF:
-
Epidermal growth factor
- RIG-I:
-
Retinoic acid-inducible gene-I
- PRR:
-
Patter recognition receptor
- IFNs:
-
Interferons
- NDRG1:
-
N-myc downstream-regulated gene 1
- USP15:
-
Ubiquitin Specific Peptidase 15
- PGC-1:
-
Peroxisome proliferator-activated receptor gamma 1
- AMPK:
-
AMP-activated protein kinase
- LDMC:
-
Lipid droplets mitochondrial coupling
- MFN2:
-
Mitofusin2
- CMA:
-
Chaperon-mediated autophagy
- LDAF1:
-
Lipid droplet assembly factor 1
- MGAT2:
-
Monoacylglycerol acetyltransferase 2
- FATP:
-
Fatty acid transporter protein
- SCD1:
-
Stearoyl COA desaturase 1
- IGTP:
-
Interferon-gamma inducible guanine
- IFI47:
-
Interferon-gamma inducible protein
- IIGP1:
-
Interferon-inducible guanine triphosphatase 1
- TGTP1:
-
T cell-specific GTPase1
- cPLA2:
-
Phospholipase A2
- SREBP:
-
Sterol regulatory binding element protein
- SKI-1/S1P:
-
Subtilisin kexin isozyme-1/site 1 protease
- PI3K:
-
Phosphoinositide 3-kinase
- AUP1:
-
Ancient ubiquitous protein 1
- NS4A:
-
Nonstructural protein 4A
- NS4B:
-
Nonstructural protein 4B
- NS5A:
-
Nonstructural protein 5A
- NS3:
-
Nonstructural protein 3
- DENV:
-
Dengue virus
- JEV:
-
Japanese encephalitis virus
- ADRP:
-
Adipose differentiation-related protein
- PARγ:
-
Peroxisome proliferator-activated receptor gamma
- HSL:
-
Hormone-sensitive lipase
- ATGL:
-
Adipose triglyceride lipase
- ZIKAV:
-
Zika virus
- HCV:
-
Hepatitis C virus
- MAP1LC3:
-
Microtube-associated protein 1 light chain 3
- AD:
-
Alzheimer's disease
- PD:
-
Parkinson's disease
- ALS:
-
Amyotrophic lateral sclerosis
- MCT:
-
Monocarboxylate transporter
- JNK:
-
C jun N terminal kinase
- αSyn:
-
Alpha-synuclein
- CNS:
-
Central nervous system
- ROS:
-
Reactive oxygen species
- MRP:
-
Multidrug resistance-associated protein
- ABC:
-
ATP binding cassette
- P-gp:
-
P glycoprotein
- DMPC:
-
Dimyristoyl-sn-glycerol-3-phosphocholine
- DMPG:
-
1,2-Dimyristoyl-sn-glycerol-3-phosphoglycerol
- DSPE:
-
1,2-Distearoyl-sn-glycerol-3-phosphoethanolamine
- PEG:
-
Polyethylene glycol
- FIT:
-
Fat storage-inducing transmembrane protein
References
Acevedo N, Waggoner J, Rodriguez M et al (2017) Zika virus, chikungunya virus, and dengue virus in cerebrospinal fluid from adults with neurological manifestations, Guayaquil, Ecuador. Front Microbiol. https://doi.org/10.3389/fmicb.2017.00042
Achar A, Myers R, Ghosh C (2021) Drug delivery challenges in brain disorders across the blood-brain barrier: novel methods and future considerations for improved therapy. Biomedicines 9:1834. https://doi.org/10.3390/biomedicines9121834
Akbarzadeh A, Rezaei-Sadabady R, Davaran S et al (2013) Liposome: classification, preparation, and applications. Nanoscale Res Lett 8:102. https://doi.org/10.1186/1556-276X-8-102
Al Asmari AK, Ullah Z, Tariq M, Fatani A (2016) Preparation, characterization, and in vivo evaluation of intranasally administered liposomal formulation of donepezil. Drug Des Dev Ther 10:205–215. https://doi.org/10.2147/DDDT.S93937
Apaydın ÇB, Çınar G, Cihan-Üstündağ G (2021) Small-molecule antiviral agents in ongoing clinical trials for COVID-19. Curr Drug Targets 22:1986–2005. https://doi.org/10.2174/1389450122666210215112150
Araujo AQC, Silva MTT, Araujo APQC (2016) Zika virus-associated neurological disorders: a review. Brain 139:2122–2130. https://doi.org/10.1093/brain/aww158
Awadh AA (2023) The role of cytosolic lipid droplets in hepatitis C virus replication, assembly, and release. Biomed Res Int 2023:1–15. https://doi.org/10.1155/2023/5156601
Baek Y-B, Kwon H-J, Sharif M et al (2022) Therapeutic strategy targeting host lipolysis limits infection by SARS-CoV-2 and influenza A virus. Signal Transduct Target Ther 7:367. https://doi.org/10.1038/s41392-022-01223-4
Bang B-R, Li M, Tsai K-N et al (2019) Regulation of hepatitis C virus infection by cellular retinoic acid binding proteins through the modulation of lipid droplet abundance. J Virol. https://doi.org/10.1128/JVI.02302-18
Bechmann I, Woodroofe N (2014) Immune privilege of the brain. Neuroinflammation and CNS disorders. Wiley, Chichester, pp 1–8
Becuwe M, Bond LM, Pinto AFM et al (2020) FIT2 is an acyl–coenzyme A diphosphatase crucial for endoplasmic reticulum homeostasis. J Cell Biol. https://doi.org/10.1083/jcb.202006111
Begley D (2004) ABC transporters and the blood-brain barrier. Curr Pharm Des 10:1295–1312. https://doi.org/10.2174/1381612043384844
Belhadj Z, Ying M, Cao X et al (2017) Design of Y-shaped targeting material for liposome-based multifunctional glioblastoma-targeted drug delivery. J Control Release 255:132–141. https://doi.org/10.1016/j.jconrel.2017.04.006
Bhatt-Wessel B, Jordan TW, Miller JH, Peng L (2018) Role of DGAT enzymes in triacylglycerol metabolism. Arch Biochem Biophys 655:1–11. https://doi.org/10.1016/j.abb.2018.08.001
Binns D, Januszewski T, Chen Y et al (2006) An intimate collaboration between peroxisomes and lipid bodies. J Cell Biol 173:719–731. https://doi.org/10.1083/jcb.200511125
Bosch M, Sánchez-Álvarez M, Fajardo A et al (2020) Mammalian lipid droplets are innate immune hubs integrating cell metabolism and host defense. Science 370:8085. https://doi.org/10.1126/science.aay8085
Bosch M, Sweet MJ, Parton RG, Pol A (2021) Lipid droplets and the host–pathogen dynamic: FATal attraction? J Cell Biol. https://doi.org/10.1083/jcb.202104005
Boutant M, Kulkarni SS, Joffraud M et al (2017) Mfn2 is critical for brown adipose tissue thermogenic function. EMBO J 36:1543–1558. https://doi.org/10.15252/embj.201694914
Bulbake U, Doppalapudi S, Kommineni N, Khan W (2017) Liposomal formulations in clinical use: an updated review. Pharmaceutics. https://doi.org/10.3390/pharmaceutics9020012
Casares D, Escribá PV, Rosselló CA (2019) Membrane lipid composition: effect on membrane and organelle structure, function and compartmentalization and therapeutic avenues. Int J Mol Sci 20:2167. https://doi.org/10.3390/ijms20092167
Cerletti A, Drewe J, Fricker G et al (2000) Endocytosis and transcytosis of an immunoliposome-based brain drug delivery system. J Drug Target 8:435–446. https://doi.org/10.3109/10611860008997919
Chang LK, , Chan TG, Raguseo F, Mishra A, Chattenton D, de Rosales RTM, Long NJ, Morse SV (2023) Rapid short-pulses of focused ultrasound and microbubbles deliver a range of agent sizes to the brain. Sci Rep 13(1):6963
Chastagner P, Devictor B, Geoerger B, Aerts I, Leblond P, Frappaz D, Gentet J-C, Bracard S, André N (2015) Phase I study of non-pegylated liposomal doxorubicin in children with recurrent/refractory high-grade glioma. Cancer Chemother Pharmacol 76(2):425–432. https://doi.org/10.1007/s00280-015-2781-0
Checkoway H, Lundin JI, Kelada SN (2011) Neurodegenerative diseases. IARC Sci Publ 407–19
Chen C, Duan Z, Yuan Y et al (2017) Peptide-22 and cyclic RGD functionalized liposomes for glioma targeting drug delivery overcoming BBB and BBTB. ACS Appl Mater Interfaces 9:5864–5873. https://doi.org/10.1021/acsami.6b15831
Cheng X, Gao J, Ding Y et al (2021) Multi-functional liposome: a powerful theranostic nano-platform enhancing photodynamic therapy. Adv Sci 8:2100876. https://doi.org/10.1002/advs.202100876
Choudhary V, Ojha N, Golden A, Prinz WA (2015) A conserved family of proteins facilitates nascent lipid droplet budding from the ER. J Cell Biol 211:261–271. https://doi.org/10.1083/jcb.201505067
Chung J, Wu X, Lambert TJ et al (2019) LDAF1 and seipin form a lipid droplet assembly complex. Dev Cell 51:551-563.e7. https://doi.org/10.1016/j.devcel.2019.10.006
Chung GY, Shim KH, Kim HJ, Min SK, Shin HS (2018) Chitosan-coated C-phycocyanin liposome for extending the neuroprotective time window against ischemic brain stroke. Curr Pharm Des 24(17):1859–1864. https://doi.org/10.2174/1381612824666180515123543
Cloherty APM, Olmstead AD, Ribeiro CMS, Jean F (2020) Hijacking of lipid droplets by hepatitis C, dengue and Zika viruses—from viral protein moonlighting to extracellular release. Int J Mol Sci 21:7901. https://doi.org/10.3390/ijms21217901
Cole NB, Murphy DD, Grider T et al (2002) Lipid droplet binding and oligomerization properties of the Parkinson’s disease protein α-synuclein. J Biol Chem 277:6344–6352. https://doi.org/10.1074/jbc.M108414200
Correale J, Villa A (2009) Cellular elements of the blood-brain barrier. Neurochem Res 34:2067–2077. https://doi.org/10.1007/s11064-009-0081-y
Coyaud E, Ranadheera C, Cheng D et al (2018) Global interactomics uncovers extensive organellar targeting by Zika virus. Mol Cell Proteomics 17:2242–2255. https://doi.org/10.1074/mcp.TIR118.000800
den Brok MH, Raaijmakers TK, Collado-Camps E, Adema GJ (2018) Lipid droplets as immune modulators in myeloid cells. Trends Immunol 39:380–392. https://doi.org/10.1016/j.it.2018.01.012
Deodhar S, Dash AK (2018) Long circulating liposomes: challenges and opportunities. Ther Deliv 9:857–872. https://doi.org/10.4155/tde-2018-0035
Dias SSG, Cunha-Fernandes T, Souza-Moreira L, Soares VC, Lima GB, Azevedo-Quintanilha IG, Santos J, Pereira-Dutra F, Freitas C, Reis PA, Rehen SK, Bozza FA, Souza TML, de Almeida CJG, Bozza PT (2023) Metabolic reprogramming and lipid droplets are involved in Zika virus replication in neural cells. J Neuroinflamm 20(1):61. https://doi.org/10.1186/s12974-023-02736-7
Dias SSG, Soares VC, Ferreira AC et al (2020) Lipid droplets fuel SARS-CoV-2 replication and production of inflammatory mediators. PLoS Pathog 16:e1009127. https://doi.org/10.1371/journal.ppat.1009127
Dinesh DC, Tamilarasan S, Rajaram K, Bouřa E (2020) Antiviral drug targets of single-stranded RNA viruses causing chronic human diseases. Curr Drug Targets 21:105–124. https://doi.org/10.2174/1389450119666190920153247
dos Santos Rodrigues B, Arora S, Kanekiyo T, Singh J (2020) Efficient neuronal targeting and transfection using RVG and transferrin-conjugated liposomes. Brain Res 1734:146738. https://doi.org/10.1016/j.brainres.2020.146738
Egorova N, Dhollander T, Khlif MS et al (2020) Pervasive white matter fiber degeneration in ischemic stroke. Stroke 51:1507–1513. https://doi.org/10.1161/STROKEAHA.119.028143
Estes RE, Lin B, Khera A, Davis MY (2021) Lipid metabolism influence on neurodegenerative disease progression: is the vehicle as important as the cargo? Front Mol Neurosci. https://doi.org/10.3389/fnmol.2021.788695
Finck BN, Gropler MC, Chen Z et al (2006) Lipin 1 is an inducible amplifier of the hepatic PGC-1α/PPARα regulatory pathway. Cell Metab 4:199–210. https://doi.org/10.1016/j.cmet.2006.08.005
Fu Q, Zhao Y, Yang Z et al (2019a) Liposomes actively recognizing the glucose transporter GLUT 1 and integrin αvβ3 for dual-targeting of glioma. Arch Pharm (weinheim) 352:1800219. https://doi.org/10.1002/ardp.201800219
Fu Y, Chen J, Huang Z (2019b) Recent progress in microRNA-based delivery systems for the treatment of human disease. ExRNA 1:24. https://doi.org/10.1186/s41544-019-0024-y
Ghaferi M, Raza A, Koohi M, Zahra W, Akbarzadeh A, Ebrahimi Shahmabadi H, Alavi SE (2022) Impact of PEGylated liposomal doxorubicin and carboplatin combination on glioblastoma. Pharmaceutics 14(10):2183. https://doi.org/10.3390/pharmaceutics14102183
Gallardo-Montejano VI, Saxena G, Kusminski CM et al (2016) Nuclear Perilipin 5 integrates lipid droplet lipolysis with PGC-1α/SIRT1-dependent transcriptional regulation of mitochondrial function. Nat Commun 7:12723. https://doi.org/10.1038/ncomms12723
Galli A, Ramirez S, Bukh J (2021) Lipid droplets accumulation during hepatitis C virus infection in cell-culture varies among genotype 1–3 strains and does not correlate with virus replication. Viruses 13:389. https://doi.org/10.3390/v13030389
García CC, Vázquez CA, Giovannoni F et al (2020) Cellular organelles reorganization during Zika virus infection of human cells. Front Microbiol. https://doi.org/10.3389/fmicb.2020.01558
Gaillard PJ, Appeldoorn CCM, Dorland R, van Kregten J, Manca F, Vugts DJ, Windhorst B, van Dongen GAMS, de Vries HE, Maussang D, van Tellingen O (2014) Pharmacokinetics, brain delivery, and efficacy in brain tumor-bearing mice of glutathione pegylated liposomal doxorubicin (2B3-101). PLoS ONE 9(1):e82331. https://doi.org/10.1371/journal.pone.0082331
Girard V, Jollivet F, Knittelfelder O et al (2021) Abnormal accumulation of lipid droplets in neurons induces the conversion of alpha-Synuclein to proteolytic resistant forms in a Drosophila model of Parkinson’s disease. PLoS Genet 17:e1009921. https://doi.org/10.1371/journal.pgen.1009921
Gosk S, Vermehren C, Storm G, Moos T (2004) Targeting anti-transferrin receptor antibody (OX26) and OX26-conjugated liposomes to brain capillary endothelial cells using in situ perfusion. J Cereb Blood Flow Metab 24:1193–1204. https://doi.org/10.1097/01.WCB.0000135592.28823.47
Grafals-Ruiz N, Rios-Vicil CI, Lozada-Delgado EL, Quiñones-Díaz BI, Noriega-Rivera RA, Martínez-Zayas G, Santana-Rivera Y, Santiago-Sánchez GS, Valiyeva F, Vivas-Mejía PE (2020) Brain targeted gold liposomes improve RNAi delivery for glioblastoma. Int J Nanomed 15:2809–2828. https://doi.org/10.2147/IJN.S241055
Guan J, Jiang Z, Wang M et al (2019) Short peptide-mediated brain-targeted drug delivery with enhanced immunocompatibility. Mol Pharm 16:907–913. https://doi.org/10.1021/acs.molpharmaceut.8b01216
Guan J, Shen Q, Zhang Z et al (2018) Enhanced immunocompatibility of ligand-targeted liposomes by attenuating natural IgM absorption. Nat Commun 9:2982. https://doi.org/10.1038/s41467-018-05384-1
Heaton NS, Randall G (2010) Dengue virus-induced autophagy regulates lipid metabolism. Cell Host Microbe 8:422–432. https://doi.org/10.1016/j.chom.2010.10.006
Herms A, Bosch M, Reddy BJN et al (2015) AMPK activation promotes lipid droplet dispersion on detyrosinated microtubules to increase mitochondrial fatty acid oxidation. Nat Commun 6:7176. https://doi.org/10.1038/ncomms8176
Hernandez C, Shukla S (2022) Liposome based drug delivery as a potential treatment option for Alzheimer’s disease. Neural Regen Res 17:1190. https://doi.org/10.4103/1673-5374.327328
Huang Z, Niu L (2019) Developing RNA aptamers for potential treatment of neurological diseases. Future Med Chem 11(6):551–565. https://doi.org/10.4155/fmc-2018-0364
Idrees D, Kumar V (2021) SARS-CoV-2 spike protein interactions with amyloidogenic proteins: potential clues to neurodegeneration. Biochem Biophys Res Commun 554:94–98. https://doi.org/10.1016/j.bbrc.2021.03.100
Ishida K, Goto S, Ishimura M, Amanuma M, Hara Y, Suzuki R, Katoh K, Morita E (2019) Functional correlation between subcellular localizations of Japanese encephalitis virus capsid protein and virus production. J Virol. https://doi.org/10.1128/JVI.00612-19
Jackson CL (2019) Lipid droplet biogenesis. Curr Opin Cell Biol 59:88–96. https://doi.org/10.1016/j.ceb.2019.03.018
Jarvis JN, Lawrence DS, Meya DB, Kagimu E, Kasibante J, Mpoza E, Rutakingirwa MK, Ssebambulidde K, Tugume L, Rhein J, Boulware DR, Mwandumba HC, Moyo M, Mzinganjira H, Kanyama C, Hosseinipour MC, Chawinga C, Meintjes G, Schutz C, Harrison TS (2022) Single-dose liposomal amphotericin B treatment for cryptococcal meningitis. New Engl J Med 386(12):1109–1120. https://doi.org/10.1056/NEJMoa2111904
Jin Y, McFie PJ, Banman SL et al (2014) Diacylglycerol Acyltransferase-2 (DGAT2) and Monoacylglycerol Acyltransferase-2 (MGAT2) Interact to Promote Triacylglycerol Synthesis. J Biol Chem 289:28237–28248. https://doi.org/10.1074/jbc.M114.571190
Johnsen KB, Burkhart A, Melander F et al (2017) Targeting transferrin receptors at the blood-brain barrier improves the uptake of immunoliposomes and subsequent cargo transport into the brain parenchyma. Sci Rep 7:10396. https://doi.org/10.1038/s41598-017-11220-1
Joshi S, Singh-Moon R, Wang M et al (2014) Cationic surface charge enhances early regional deposition of liposomes after intracarotid injection. J Neurooncol 120:489–497. https://doi.org/10.1007/s11060-014-1584-1
Juhairiyah F, de Lange ECM (2021) Understanding drug delivery to the brain using liposome-based strategies: studies that provide mechanistic insights are essential. AAPS J 23:114. https://doi.org/10.1208/s12248-021-00648-z
Kadereit B, Kumar P, Wang W-J et al (2008) Evolutionarily conserved gene family important for fat storage. Proc Natl Acad Sci 105:94–99. https://doi.org/10.1073/pnas.0708579105
Kahana M, Weizman A, Gabay M, Loboda Y, Segal-Gavish H, Gavish A, Barhum Y, Offen D, Finberg J, Allon N, Gavish M (2021) Liposome-based targeting of dopamine to the brain: a novel approach for the treatment of Parkinson’s disease. Mol Psychiatry 26(6):2626–2632. https://doi.org/10.1038/s41380-020-0742-4
Kapoor M, Burgess DJ (2012) Efficient and safe delivery of siRNA using anionic lipids: formulation optimization studies. Int J Pharm 432:80–90. https://doi.org/10.1016/j.ijpharm.2012.04.058
Kasenda B, König D, Manni M et al (2022) Targeting immunoliposomes to EGFR-positive glioblastoma. ESMO Open 7:100365. https://doi.org/10.1016/j.esmoop.2021.100365
Kato T, Natsume A, Toda H, Iwamizu H, Sugita T, Hachisu R, Watanabe R, Yuki K, Motomura K, Bankiewicz K, Wakabayashi T (2010) Efficient delivery of liposome-mediated MGMT-siRNA reinforces the cytotoxity of temozolomide in GBM-initiating cells. Gene Ther 17(11):1363–1371. https://doi.org/10.1038/gt.2010.88
Käufer C, Schreiber CS, Hartke A-S et al (2022) Microgliosis and neuronal proteinopathy in brain persist beyond viral clearance in SARS-CoV-2 hamster model. EBioMedicine 79:103999. https://doi.org/10.1016/j.ebiom.2022.103999
Kaushik S, Cuervo AM (2015) Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat Cell Biol 17:759–770. https://doi.org/10.1038/ncb3166
Khabazian E, Vakhshiteh F, Norouzi P et al (2022) Cationic liposome decorated with cyclic RGD peptide for targeted delivery of anti-STAT3 siRNA to melanoma cancer cells. J Drug Target 30:522–533. https://doi.org/10.1080/1061186X.2021.1973481
Kien B, Kolleritsch S, Kunowska N et al (2022) Lipid droplet-mitochondria coupling via perilipin 5 augments respiratory capacity but is dispensable for FA oxidation. J Lipid Res 63:100172. https://doi.org/10.1016/j.jlr.2022.100172
Kong L, Li X, Ni Y et al (2020) Transferrin-modified Osthole PEGylated liposomes travel the blood-brain barrier and mitigate Alzheimer’s disease-related pathology in APP/PS-1 mice. Int J Nanomed 15:2841–2858. https://doi.org/10.2147/IJN.S239608
Kusakabe S, Suzuki T, Sugiyama Y et al (2019) USP15 participates in hepatitis C virus propagation through regulation of viral RNA translation and lipid droplet formation. J Virol. https://doi.org/10.1128/JVI.01708-18
Lagrutta LC, Layerenza JP, Bronsoms S et al (2021) Nuclear-lipid-droplet proteome: carboxylesterase as a nuclear lipase involved in lipid-droplet homeostasis. Heliyon 7:e06539. https://doi.org/10.1016/j.heliyon.2021.e06539
Langen UH, Ayloo S, Gu C (2019) Development and cell biology of the blood-brain barrier. Annu Rev Cell Dev Biol 35:591–613. https://doi.org/10.1146/annurev-cellbio-100617-062608
Langner M, Kral TE (1999) Liposome-based drug delivery systems. Pol J Pharmacol 51:211–222
Lardizabal KD, Mai JT, Wagner NW et al (2001) DGAT2 is a new diacylglycerol acyltransferase gene family. J Biol Chem 276:38862–38869. https://doi.org/10.1074/jbc.M106168200
Li J, Zeng H, You Y, Wang R, Tan T, Wang W, Yin L, Zeng Z, Zeng Y, Xie T (2021) Active targeting of orthotopic glioma using biomimetic liposomes co-loaded elemene and cabazitaxel modified by transferritin. J Nanobiotechnol 19(1):289. https://doi.org/10.1186/s12951-021-01048-3
Li G-H, Ning Z-J, Liu Y-M, Li X-H (2017) Neurological manifestations of dengue infection. Front Cell Infect Microbiol. https://doi.org/10.3389/fcimb.2017.00449
Lippens RJJ (1999) Liposomal daunorubicin (daunoxome) in children with recurrent or progressive brain tumors. Pediatr Hematol Oncol 16(2):131–139. https://doi.org/10.1080/088800199277452
Liu L, Zhang K, Sandoval H et al (2015) Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell 160:177–190. https://doi.org/10.1016/j.cell.2014.12.019
Liu L, Zhang Y-Q, Tschapalda K, et al (2010) ML360, a potent inhibitor of lipid Droplet formation
Loyse A, Thangaraj H, Easterbrook P, Ford N, Roy M, Chiller T, Govender N, Harrison TS, Bicanic T (2013) Cryptococcal meningitis: improving access to essential antifungal medicines in resource-poor countries. Lancet Infect Dis 13(7):629–637. https://doi.org/10.1016/S1473-3099(13)70078-1
Lu B, Lu Y, Moser AH et al (2008) LPS and proinflammatory cytokines decrease lipin-1 in mouse adipose tissue and 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab 295:E1502–E1509. https://doi.org/10.1152/ajpendo.90323.2008
Lundin C, Nordström R, Wagner K et al (2006) Membrane topology of the human seipin protein. FEBS Lett 580:2281–2284. https://doi.org/10.1016/j.febslet.2006.03.040
Lu Y-J, Hsu H-L, Lan Y-H, Chen J-P (2023) Thermosensitive cationic magnetic liposomes for thermoresponsive delivery of CPT-11 and SLP2 shRNA in glioblastoma treatment. Pharmaceutics 15(4):1169. https://doi.org/10.3390/pharmaceutics15041169
Man WC, Miyazaki M, Chu K, Ntambi J (2006) Colocalization of SCD1 and DGAT2: implying preference for endogenous monounsaturated fatty acids in triglyceride synthesis. J Lipid Res 47:1928–1939. https://doi.org/10.1194/jlr.M600172-JLR200
Marina NM, Cochrane D, Harney E, Zomorodi K, Blaney S, Winick N, Bernstein M, Link MP (2002) Dose escalation and pharmacokinetics of pegylated liposomal doxorubicin (Doxil) in children with solid tumors: a pediatric oncology group study. Clin Cancer Res 8(2):413–418
Martina BEE, Koraka P, Osterhaus ADME (2009) Dengue Virus Pathogenesis: an Integrated View. Clin Microbiol Rev 22:564–581. https://doi.org/10.1128/CMR.00035-09
Marschallinger J, Iram T, Zardeneta M et al (2020) Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat Neurosci 23:194–208. https://doi.org/10.1038/s41593-019-0566-1
Mathew S, Faheem M, Ibrahim SM et al (2016) Hepatitis C virus and neurological damage. World J Hepatol 8:545. https://doi.org/10.4254/wjh.v8.i12.545
Mehrabian A, Mashreghi M, Dadpour S et al (2022) Nanocarriers call the last shot in the treatment of brain cancers. Technol Cancer Res Treat 21:153303382210809. https://doi.org/10.1177/15330338221080974
Melo RCN, Dvorak AM (2012) Lipid body-phagosome interaction in macrophages during infectious diseases: host defense or pathogen survival strategy? PLoS Pathog 8:e1002729. https://doi.org/10.1371/journal.ppat.1002729
Meyers A, Weiskittel TM, Dalhaimer P (2017) Lipid droplets: formation to breakdown. Lipids 52:465–475. https://doi.org/10.1007/s11745-017-4263-0
Milla P, Dosio F, Cattel L (2012) PEGylation of proteins and liposomes: a powerful and flexible strategy to improve the drug delivery. Curr Drug Metab 13:105–119. https://doi.org/10.2174/138920012798356934
Minn A, Ghersi-Egea J-F, Perrin R et al (1991) Drug metabolizing enzymes in the brain and cerebral microvessels. Brain Res Rev 16:65–82. https://doi.org/10.1016/0165-0173(91)90020-9
Mohamed M, Abu Lila AS, Shimizu T et al (2019) PEGylated liposomes: immunological responses. Sci Technol Adv Mater 20:710–724. https://doi.org/10.1080/14686996.2019.1627174
Monson EA, Crosse KM, Das M, Helbig KJ (2018) Lipid droplet density alters the early innate immune response to viral infection. PLoS ONE 13:e0190597. https://doi.org/10.1371/journal.pone.0190597
Monson EA, Crosse KM, Duan M et al (2021a) Intracellular lipid droplet accumulation occurs early following viral infection and is required for an efficient interferon response. Nat Commun 12:4303. https://doi.org/10.1038/s41467-021-24632-5
Monson EA, Trenerry AM, Laws JL et al (2021b) Lipid droplets and lipid mediators in viral infection and immunity. FEMS Microbiol Rev. https://doi.org/10.1093/femsre/fuaa066
Morse SV, Mishra A, Chan TG, de Rosales TM (2022) Liposome delivery to the brain with rapid short-pulses of focused ultrasound and microbubbles. J Control Release 341:605–615. https://doi.org/10.1016/j.jconrel.2021.12.005
Mullauer FB, van Bloois L, Daalhuisen JB et al (2011) Betulinic acid delivered in liposomes reduces growth of human lung and colon cancers in mice without causing systemic toxicity. Anticancer Drugs 22:223–233. https://doi.org/10.1097/CAD.0b013e3283421035
Mustafa Khidir A, Saeed AA (2020) Ligand-targeted liposomes. Health Primary Care. https://doi.org/10.15761/HPC.1000188
Nageeb El-Helaly S, Abd Elbary A, Kassem MA, El-Nabarawi MA (2017) Electrosteric stealth Rivastigmine loaded liposomes for brain targeting: preparation, characterization, ex vivo, bio-distribution and in vivo pharmacokinetic studies. Drug Delivery 24(1):692–700. https://doi.org/10.1080/10717544.2017.1309476
Nardacci R, Colavita F, Castilletti C, Lapa D, Matusali G, Meschi S, Del Nonno F, Colombo D, Capobianchi MR, Zumla A, Ippolito G, Piacentini M, Falasca L (2021) Evidences for lipid involvement in SARS-CoV-2 cytopathogenesis. Cell Death Dis 12(3):263. https://doi.org/10.1038/s41419-021-03527-9
Nakhaei P, Margiana R, Bokov DO et al (2021) Liposomes: structure, biomedical applications, and stability parameters with emphasis on cholesterol. Front Bioeng Biotechnol 9. https://doi.org/10.3389/fbioe.2021.705886
Niranjan R, Muthukumaravel S, Jambulingam P (2019) The involvement of neuroinflammation in dengue viral disease: importance of innate and adaptive immunity. NeuroImmunoModulation 26:111–118. https://doi.org/10.1159/000501209
Noble GT, Stefanick JF, Ashley JD et al (2014) Ligand-targeted liposome design: challenges and fundamental considerations. Trends Biotechnol 32:32–45. https://doi.org/10.1016/j.tibtech.2013.09.007
Noe CR, Noe-Letschnig M, Handschuh P et al (2020) Dysfunction of the blood-brain barrier—a key step in neurodegeneration and dementia. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2020.00185
Nsairat H, Khater D, Sayed U et al (2022) Liposomes: structure, composition, types, and clinical applications. Heliyon 8:e09394. https://doi.org/10.1016/j.heliyon.2022.e09394
Obeid MA, Tate RJ, Mullen AB, Ferro VA (2018) Lipid-based nanoparticles for cancer treatment. Lipid nanocarriers for drug targeting. Elsevier, Amsterdam, pp 313–359
Ohsaki Y, Sołtysik K, Fujimoto T (2017) The lipid droplet and the endoplasmic reticulum. pp 111–120
Olzmann JA, Carvalho P (2019) Dynamics and functions of lipid droplets. Nat Rev Mol Cell Biol 20:137–155. https://doi.org/10.1038/s41580-018-0085-z
Partoazar A, Nasoohi S, Rezayat SM, Gilani K, Mehr SE, Amani A, Rahimi N, Dehpour AR (2017) Nanoliposome containing cyclosporine A reduced neuroinflammation responses and improved neurological activities in cerebral ischemia/reperfusion in rat. Fundam Clin Pharmacol 31(2):185–193. https://doi.org/10.1111/fcp.12244
Peng Y, Zhao Y, Chen Y et al (2018) Dual-targeting for brain-specific liposomes drug delivery system: synthesis and preliminary evaluation. Bioorg Med Chem 26:4677–4686. https://doi.org/10.1016/j.bmc.2018.08.006
Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD (2006) Blood–brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol 1:223–236. https://doi.org/10.1007/s11481-006-9025-3
Phuphanich S, Maria B, Braeckman R, Chamberlain M (2006) A pharmacokinetic study of intra-CSF administered encapsulated cytarabine (DepoCyt®) for the treatment of neoplastic meningitis in patients with leukemia, lymphoma, or solid tumors as part of a phase III study. J Neurooncol 81(2):201–208. https://doi.org/10.1007/s11060-006-9218-x
Pillai AN, Shukla S, Rahaman A (2017) An evolutionarily conserved phosphatidate phosphatase maintains lipid droplet number and ER morphology but not nuclear morphology. Biol Open. https://doi.org/10.1242/bio.028233
Qin Z-L, Yao Q-F, Ren H, Zhao P, Qi Z-T (2022) Lipid droplets and their participation in Zika virus infection. Int J Mol Sci 23(20):12584. https://doi.org/10.3390/ijms232012584
Qu B, Li X, Guan M et al (2014) Design, synthesis and biological evaluation of multivalent glucosides with high affinity as ligands for brain targeting liposomes. Eur J Med Chem 72:110–118. https://doi.org/10.1016/j.ejmech.2013.10.007
Randall G (2018) Lipid droplet metabolism during dengue virus infection. Trends Microbiol 26(8):640–642. https://doi.org/10.1016/j.tim.2018.05.010
Renne MF, Klug YA, Carvalho P (2020) Lipid droplet biogenesis: a mystery “unmixing”? Semin Cell Dev Biol 108:14–23. https://doi.org/10.1016/j.semcdb.2020.03.001
Rip J, Chen L, Hartman R et al (2014) Glutathione PEGylated liposomes: pharmacokinetics and delivery of cargo across the blood–brain barrier in rats. J Drug Target 22:460–467. https://doi.org/10.3109/1061186X.2014.888070
Robinson RF, Nahata MC (1999) A comparative review of conventional and lipid formulations of amphotericin B. J Clin Pharm Ther 24(4):249–257. https://doi.org/10.1046/j.1365-2710.1999.00220.x
Rodhain F (1996) Recent data on the epidemiology of Japanese encephalitis]. Bull Acad Natl Med 180:1325–37; discussion 1338–1340
Roger S, Ducancelle A, Le Guillou-Guillemette H et al (2021) HCV virology and diagnosis. Clin Res Hepatol Gastroenterol 45:101626. https://doi.org/10.1016/j.clinre.2021.101626
Rotman M, Welling MM, Bunschoten A, de Backer ME, Rip J, Nabuurs RJA, Gaillard PJ, van Buchem MA, van der Maarel SM, van der Weerd L (2015) Enhanced glutathione PEGylated liposomal brain delivery of an anti-amyloid single domain antibody fragment in a mouse model for Alzheimer’s disease. J Control Release 203:40–50. https://doi.org/10.1016/j.jconrel.2015.02.012
Roy SK, Bhattacharjee S (2021) Dengue virus: epidemiology, biology, and disease aetiology. Can J Microbiol 67:687–702. https://doi.org/10.1139/cjm-2020-0572
Sarkar R, Sharma KB, Kumari A et al (2021) Japanese encephalitis virus capsid protein interacts with non-lipidated MAP1LC3 on replication membranes and lipid droplets. J Gen Virol. https://doi.org/10.1099/jgv.0.001508
Sato Y, Murase K, Kato J, Kobune M, Sato T, Kawano Y, Takimoto R, Takada K, Miyanishi K, Matsunaga T, Takayama T, Niitsu Y (2008) Resolution of liver cirrhosis using vitamin A–coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat Biotechnol 26(4):431–442. https://doi.org/10.1038/nbt1396
Sawant RR, Torchilin VP (2012) Challenges in development of targeted liposomal therapeutics. AAPS J 14:303–315. https://doi.org/10.1208/s12248-012-9330-0
Schnyder A, Huwyler J (2005) Drug transport to brain with targeted liposomes. NeuroRx 2:99–107. https://doi.org/10.1602/neurorx.2.1.99
Schuldiner M, Bohnert M (2017) A different kind of love – lipid droplet contact sites. Biochim Biophys Acta 1862:1188–1196. https://doi.org/10.1016/j.bbalip.2017.06.005
Schweitzer CJ, Zhang F, Boyer A et al (2018) N-Myc Downstream-regulated gene 1 restricts hepatitis C virus propagation by regulating lipid droplet biogenesis and viral assembly. J Virol. https://doi.org/10.1128/JVI.01166-17
Sembongi H, Miranda M, Han G-S et al (2013) Distinct roles of the phosphatidate phosphatases lipin 1 and 2 during adipogenesis and lipid droplet biogenesis in 3T3-L1 cells. J Biol Chem 288:34502–34513. https://doi.org/10.1074/jbc.M113.488445
Sercombe L, Veerati T, Moheimani F et al (2015) Advances and challenges of liposome assisted drug delivery. Front Pharmacol. https://doi.org/10.3389/fphar.2015.00286
Shannahan H (2015) Implications of scavenger receptors in the safe development of nanotherapeutics. Recept Clin Investig. https://doi.org/10.14800/rci.811
Sharma KB, Vrati S, Kalia M (2021) Pathobiology of Japanese encephalitis virus infection. Mol Aspects Med 81:100994. https://doi.org/10.1016/j.mam.2021.100994
Shin J, Kong C, Cho JS, Lee J, Koh CS, Yoon M-S, Na YC, Chang WS, Chang JW (2018) Focused ultrasound–mediated noninvasive blood-brain barrier modulation: preclinical examination of efficacy and safety in various sonication parameters. Neurosurg Focus 44(2):E15. https://doi.org/10.3171/2017.11.FOCUS17627
Sun C, Wang J, Liu J, Qiu L, Zhang W, Zhang L (2013) Liquid proliposomes of nimodipine drug delivery system: preparation, characterization, and pharmacokinetics. AAPS PharmSciTech 14(1):332–338. https://doi.org/10.1208/s12249-013-9924-6
Suzuki M, Shinohara Y, Ohsaki Y, Fujimoto T (2011) Lipid droplets: size matters. Microscopy 60:S101–S116. https://doi.org/10.1093/jmicro/dfr016
Tang W-C, Lin R-J, Liao C-L, Lin Y-L (2014) Rab18 facilitates dengue virus infection by targeting fatty acid synthase to sites of viral replication. J Virol 88:6793–6804. https://doi.org/10.1128/JVI.00045-14
Tracey TJ, Kirk SE, Steyn FJ, Ngo ST (2021) The role of lipids in the central nervous system and their pathological implications in amyotrophic lateral sclerosis. Semin Cell Dev Biol 112:69–81. https://doi.org/10.1016/j.semcdb.2020.08.012
Vieira D, Gamarra L (2016) Getting into the brain: liposome-based strategies for effective drug delivery across the blood–brain barrier. Int J Nanomed 11:5381–5414. https://doi.org/10.2147/IJN.S117210
Vieyres G, Pietschmann T (2019) HCV pit stop at the lipid droplet: refuel lipids and put on a lipoprotein coat before exit. Cells 8:233. https://doi.org/10.3390/cells8030233
Walther TC, Chung J, Farese RV (2017) Lipid droplet biogenesis. Annu Rev Cell Dev Biol 33:491–510. https://doi.org/10.1146/annurev-cellbio-100616-060608
Wang G, Yin W, Shin H et al (2021) Neuronal accumulation of peroxidated lipids promotes demyelination and neurodegeneration through the activation of the microglial NLRP3 inflammasome. Nat Aging 1:1024–1037. https://doi.org/10.1038/s43587-021-00130-7
Wang H, Becuwe M, Housden BE et al (2016) Seipin is required for converting nascent to mature lipid droplets. Elife. https://doi.org/10.7554/eLife.16582
Wang T, Jiang Y, Chu H et al (2019) Doxorubicin and Lovastatin co-delivery liposomes for synergistic therapy of liver cancer. J Drug Deliv Sci Technol 52:452–459. https://doi.org/10.1016/j.jddst.2019.04.045
Wei X, Gao J, Zhan C et al (2015) Liposome-based glioma targeted drug delivery enabled by stable peptide ligands. J Control Release 218:13–21. https://doi.org/10.1016/j.jconrel.2015.09.059
Whittaker A, Anson M, Harky A (2020) Neurological manifestations of COVID-19: a systematic review and current update. Acta Neurol Scand 142:14–22. https://doi.org/10.1111/ane.13266
Whittaker R, Loy PA, Sisman E et al (2010) Identification of MicroRNAs that control lipid droplet formation and growth in hepatocytes via high-content screening. SLAS Discovery 15:798–805. https://doi.org/10.1177/1087057110374991
Wilfling F, Haas JT, Walther TC Jr, RVF, (2014) Lipid droplet biogenesis. Curr Opin Cell Biol 29:39–45. https://doi.org/10.1016/j.ceb.2014.03.008
Wilfling F, Wang H, Haas JT et al (2013) Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets. Dev Cell 24:384–399. https://doi.org/10.1016/j.devcel.2013.01.013
Winkle M, El-Daly SM, Fabbri M, Calin GA (2021) Noncoding RNA therapeutics — challenges and potential solutions. Nat Rev Drug Discov 20:629–651. https://doi.org/10.1038/s41573-021-00219-z
Xing H, Hwang K, Lu Y (2016) Recent developments of liposomes as nanocarriers for theranostic applications. Theranostics 6:1336–1352. https://doi.org/10.7150/thno.15464
Xu N, Zhang SO, Cole RA et al (2012) The FATP1–DGAT2 complex facilitates lipid droplet expansion at the ER–lipid droplet interface. J Cell Biol 198:895–911. https://doi.org/10.1083/jcb.201201139
Yadav RS, Tiwari NK (2014) Lipid integration in neurodegeneration: an overview of Alzheimer’s disease. Mol Neurobiol 50:168–176. https://doi.org/10.1007/s12035-014-8661-5
Yan J, Horng T (2020) Lipid metabolism in regulation of macrophage functions. Trends Cell Biol 30:979–989. https://doi.org/10.1016/j.tcb.2020.09.006
Yang D, Wang X, Zhang L et al (2022) Lipid metabolism and storage in neuroglia: role in brain development and neurodegenerative diseases. Cell Biosci 12:106. https://doi.org/10.1186/s13578-022-00828-0
Ye Z, Gastfriend BD, Umlauf BJ et al (2022) Antibody-targeted liposomes for enhanced targeting of the blood-brain barrier. Pharm Res 39:1523–1534. https://doi.org/10.1007/s11095-022-03186-1
Ying M, Zhan C, Wang S et al (2016) Liposome-based systemic glioma-targeted drug delivery enabled by all- <scp>d</scp> peptides. ACS Appl Mater Interfaces 8:29977–29985. https://doi.org/10.1021/acsami.6b10146
Yingchoncharoen P, Kalinowski DS, Richardson DR (2016) Lipid-based drug delivery systems in cancer therapy: what is available and what is yet to come. Pharmacol Rev 68:701–787. https://doi.org/10.1124/pr.115.012070
Yuan S, Yan B, Cao J et al (2021) SARS-CoV-2 exploits host DGAT and ADRP for efficient replication. Cell Discov 7:100. https://doi.org/10.1038/s41421-021-00338-2
Zabel MD, Mollnow L, Bender H (2019) Lipopeptide Delivery of siRNA to the central nervous system. pp 389–403
Zhang I, Cui Y, Amiri A et al (2016) Pharmacological inhibition of lipid droplet formation enhances the effectiveness of curcumin in glioblastoma. Eur J Pharm Biopharm 100:66–76. https://doi.org/10.1016/j.ejpb.2015.12.008
Zhang J, Lan Y, Li MY et al (2018) Flaviviruses exploit the lipid droplet protein AUP1 to trigger lipophagy and drive virus production. Cell Host Microbe 23:819-831.e5. https://doi.org/10.1016/j.chom.2018.05.005
Zhang J, Lan Y, Sanyal S (2017) Modulation of lipid droplet metabolism—a potential target for therapeutic intervention in flaviviridae infections. Front Microbiol. https://doi.org/10.3389/fmicb.2017.02286
Zhang Y, He J, Shen L et al (2021) Brain-targeted delivery of obidoxime, using aptamer-modified liposomes, for detoxification of organophosphorus compounds. J Control Release 329:1117–1128. https://doi.org/10.1016/j.jconrel.2020.10.039
Zhao N, Francis NL, Calvelli HR, Moghe PV (2020) Microglia-targeting nanotherapeutics for neurodegenerative diseases. APL Bioeng. https://doi.org/10.1063/5.0013178
Zhu JJ, Luo J, Sun YT et al (2015) Short communication: effect of inhibition of fatty acid synthase on triglyceride accumulation and effect on lipid metabolism genes in goat mammary epithelial cells. J Dairy Sci 98:3485–3491. https://doi.org/10.3168/jds.2014-8202
Zoni V, Khaddaj R, Campomanes P et al (2021) Pre-existing bilayer stresses modulate triglyceride accumulation in the ER versus lipid droplets. Elife. https://doi.org/10.7554/eLife.62886
Acknowledgements
SG, SM acknowledge the support from CSIR-IICB; SM acknowledges Academy of Scientific and Innovative Research (AcSIR), Ghaziabad- 201002, India.
Funding
This work was supported by the Ramalingaswami Re-entry Fellowship, Department of Biotechnology, India, (BT/RLF/Re-entry/46/2021) awarded to SG. Fellowship of SM is supported by UGC, India.
Author information
Authors and Affiliations
Contributions
SG conceived the theme of the review; SM, and SG performed the literature search and constructed the manuscript and figures. The authors also acknowledge Biorender.com as the platform used for the illustration of the figures.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest and the sponsors had no role in the design, execution, interpretation, or writing of the study.
Ethical Approval
Not applicable.
Informed Consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mondal, S., Ghosh, S. Liposome-Mediated Anti-Viral Drug Delivery Across Blood–Brain Barrier: Can Lipid Droplet Target Be Game Changers?. Cell Mol Neurobiol 44, 9 (2024). https://doi.org/10.1007/s10571-023-01443-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10571-023-01443-4