Neurological Manifestations of Dengue Infection
- 1Department of Neurology, Jinan Central Hospital Affiliated to Shandong University, Jinan, China
- 2Jinan Infectious Diseases Hospital, Jinan, China
- 3Department of Neurology, Qilu Hospital, Shandong University, Jinan, China
Dengue counts among the most commonly encountered arboviral diseases, representing the fastest spreading tropical illness in the world. It is prevalent in 128 countries, and each year >2.5 billion people are at risk of dengue virus infection worldwide. Neurological signs of dengue infection are increasingly reported. In this review, the main neurological complications of dengue virus infection, such as central nervous system (CNS), peripheral nervous system, and ophthalmic complications were discussed according to clinical features, treatment and possible pathogenesis. In addition, neurological complications in children were assessed due to their atypical clinical features. Finally, dengue infection and Japanese encephalitis were compared for pathogenesis and main clinical manifestations.
Introduction
Dengue counts among the most common arboviral illnesses, representing the fastest spreading tropical disease in the world (Lundberg, 1957). It is considered the second leading cause of acute febrile disease in travelers (Freedman et al., 2006). Four different serotypes (DENV-1,−2,−3, and−4) (Guzman et al., 2010) cause dengue fever, with various infectious outcomes (asymptomatic to severe hemorrhagic fever).
Dengue is prevalent in 128 countries (Brady et al., 2012; Lorenzi et al., 2013; Teixeira et al., 2013), and more than 2.5 billion individuals are in danger each year of contracting dengue virus worldwide. According to some estimates, almost 400 million individuals are infected annually, with ~96 million showing clinical relevance. About 2.5% of all diseased people die (Guzman et al., 2010; Bhatt et al., 2013). In recent years, neurological manifestations of dengue infection have been increasingly reported; however, their precise incidence rates remain undefined.
Neurological signs were first reported in 1976 as atypical symptoms of dengue infection (Sanguansermsri, 1976); their incidence rates varied from 0.5 to 20% in recent years (Murthy, 2010; Carod-Artal et al., 2013; Mamdouh et al., 2013; Sahu et al., 2014; Saini et al., 2017). Neurological manifestations have been reported in 25 countries spanning almost all continents (Qureshi et al., 2012), and involve individuals aged 3 months to 60 years (Qureshi et al., 2012). High body temperature, elevated hematocrit, thrombocytopenia, rash, and liver dysfunction are independent risk factors for neurological complications (Sahu et al., 2014).
Almost 20 years ago, dengue virus neurotropism in the human host was considered an opportunistic characteristic (Ramos et al., 1998). However, more and more evidence strongly supports the notion that the virus is directly neurovirulent (Rosen et al., 1989; Bhoopat et al., 1996; Lum et al., 1996; Miagostovich et al., 1997b; Ramos et al., 1998; Angibaud et al., 2001). Miagostovich et al. detected the dengue virus in the central nervous system (CNS) by assessing viral proteins, ribonucleic acid (RNA), and immunoglobulins (Miagostovich et al., 1997a,b; Araujo et al., 2011; Lima et al., 2011). Salazar et al. (2007) found that the dengue virus is highly neurotropic in Aedesaegypti (Bhoopat et al., 1996). The DENV-2 and DENV-3 serotypes are mostly related to neurological complications (Lum et al., 1996; Thisyakorn et al., 1999; Miagostovich et al., 2006; Soares et al., 2010).
Neuropathogenesis
Neuropathogenesis is likely associated with direct invasion of the CNS by the virus, autoimmune reactions, and metabolic alterations. The dengue virus is considered to be non-neurotropic. However, recent reports associating dengue with neurological complications have changed this view. This virus was described in cerebro-spinal fluid (CSF) more than two decades ago (Lum et al., 1996; Thisyakorn et al., 1999). Chaturvedi et al. demonstrated that the bloodbrain barrier (BBB) is damaged during infection by the dengue virus in experimental animal experiments, indicating viral invasion (Chaturvedi et al., 1991). Meanwhile, immunoreactive neurons, astrocytes, microglia, and endothelial cells were found in cerebral tissues of a fatal case with hemorrhagic dengue fever in 1998 (Ramos et al., 1998). Domingues et al. proposed that dengue virus can actively enter the CNS (Domingues et al., 2008). Data obtained in Vietnam also support direct invasion by dengue virus as pathologically important (Solomon et al., 2000b). Autoimmune reactions and metabolic alterations have been demonstrated in most neurological complications of dengue fever cases (Seet and Lim, 2007; Basu and Chaturvedi, 2008; Jha and Ansari, 2010; Murthy, 2010; Sharma et al., 2011; Verma et al., 2011b; Weeratunga et al., 2014b).
Most neurological manifestations of dengue virus infection have been reported in case reports or short series, and its spectrum is diverse; thus, the classification of neurological manifestations is difficult to apply in practice. The new (2009) World Health Organization (WHO) classification groups dengue infection into three categories, including dengue with no warning signs, disease with warning signs, and severe dengue (WHO, 2009). Different from the traditional system, the revised classification includes CNS involvement as severe dengue. However, neurological complications are not well described and very little is known about these manifestations.
Until 2012, neurological complications of dengue virus infection were classified into three categories based on pathogenesis as proposed by Murthy, Marzia and colleages: (1) metabolic disturbance, e.g., encephalopathy; (2) viral invasion, including encephalitis, meningitis, myositis, and myelitis; (3) autoimmune reactions, including acute disseminated encephalomyelitis, neuromyelitis optica, optic neuritis, myelitis, encephalopathy, and Guillain-Barré syndrome (Murthy, 2010; Puccioni-Sohler et al., 2012). In recent years, Solbrig et al. reported neurological involvements of the CNS and eyes, associated peripheral nervous system (PNS) syndromes, and convalescent or post-dengue immune-mediated syndromes (Solbrig and Perng, 2015; Maurya et al., 2016).
The main neurological complications of dengue virus infection are discussed below.
Central Neurological System Complications
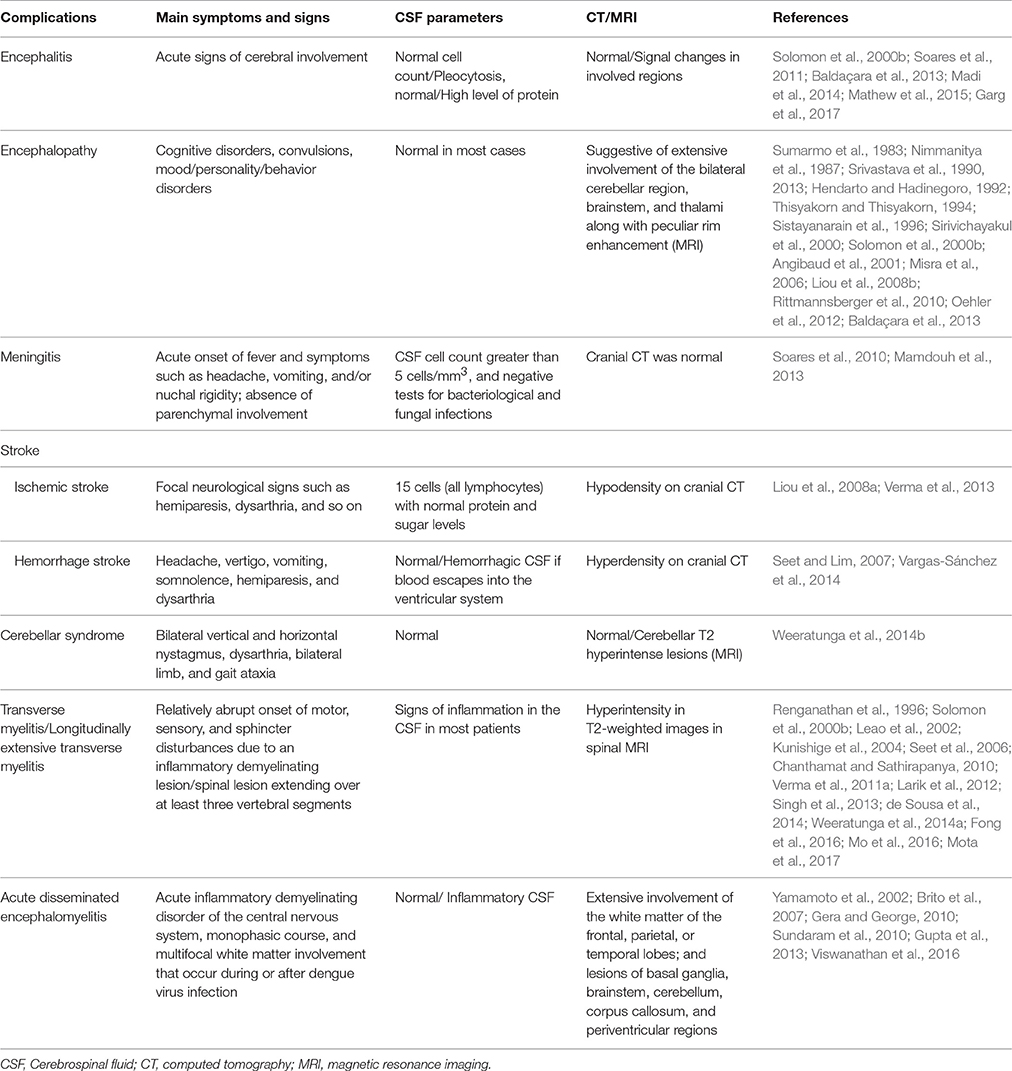
CNS complications are diagnosed by assessing anti-DENV immunoglobulin (Ig)M, detecting viral RNA or non-structural protein 1 (NS1)in the CSF, isolating the virus from the CSF, and after excluding other causative agents of viral brain diseases (Sahu et al., 2014; Solbrig and Perng, 2015). Misra et al. found that CNS involvement reflects severer disease with poorer recovery (Misra et al., 2015). Encephalitis and encephalopathy are the most common neurological presentations of dengue infection (World Health Organization, 1997; Pancharoen and Thisyakorn, 2001; Gupta et al., 2013; Table 1).
Encephalitis
Encephalitis is considered a severe manifestation of dengue virus infection, and found in the three classical disease groups. Diagnosis of dengue encephalitis is based on criteria proposed by Soares and Marzia (2014).
However, normal CSF cellularity cannot exclude dengue encephalitis. According to Soares et al., dengue is the first reason for encephalitis with normal CSF cellularity in 75% of patients with viral meningitis and encephalitis in a dengue endemic region, followed by Herpes Simplex Virus 1 (HSV1) and mild encephalitis (Soares et al., 2011). Neuroimaging of dengue encephalitis yields divergent data, with normal findings in most cases (Baldaçara et al., 2013; Madi et al., 2014). In case of abnormal neuroimaging findings, magnetic resonance imaging (MRI) has advantages over cranial computed tomography (CT) in revealing cerebral lesions in dengue encephalitis. However, changes are usually non-specific (Garg et al., 2017). Decisive characterizations of MRI properties in dengue encephalitis remain undefined (Mathew et al., 2015). Treatment is nonspecific, with mostly symptomatic treatment provided. Most patients have good recovery.
Encephalopathy
Encephalopathy caused by dengue fever can be reflected by reduced sensitivity, cognitive impairment, convulsions, and personality and behavior disorders, including acute mania, depression, emotional lability, anxiety, psychosis, and agoraphobia (Rittmannsberger et al., 2010; Baldaçara et al., 2013; Srivastava et al., 2013). In a review reporting cases of dengue fever associated with neurological disorders in 2012, encephalopathy was considered by far the most encountered complication (Oehler et al., 2012). Most encephalopathy cases occur in children of developing countries, and do not show CSF abnormalities (Angibaud et al., 2001). Dengue associated encephalopathy is generally very serious, with around 50% of the affected patients succumbing (Angibaud et al., 2001).
In the past, encephalopathy was considered to be exclusively associated with Dengue hemorrhagic fever/Dengue shock syndrome (DHF/DSS). Brain edema, anoxia, hemorrhage, intense hyponatraemia, liver or kidney failure, release of toxic substances, metabolic acidosis, and direct organ invasion are commonly reported precursors of encephalopathy in patients with serious DHF/DSS (Sumarmo et al., 1983; Nimmanitya et al., 1987; Srivastava et al., 1990; Hendarto and Hadinegoro, 1992; Thisyakorn and Thisyakorn, 1994; Sirivichayakul et al., 2000; Solbrig and Perng, 2015).
A substitution of alanine by a valine residue at position 173 of the envelope glycoprotein was reported in encephalopathy-associated dengue type 2 virus in 1996 (Sistayanarain et al., 1996). However, whether such mutation is directly involved in envelope-receptor interactions has not been clearly elucidated. Burst suppression, seizures, focal patterns, or epilepsia partialis continua can be observed on EEGs (electroencephalographs) of patients with encephalopathy (Kalita and Misra, 2006; Misra et al., 2006; Liou et al., 2008a,b).
Meningitis
Dengue infection associated meningitis is rarely encountered (Soares et al., 2006). In the related literature, three reports about dengue meningitis are found (Soares et al., 2010, 2011; Mamdouh et al., 2013). Soares et al. described a 24-year-old woman with the symptoms of fever, headache, and nuchal rigidity, in whom diagnosis was confirmed by polymerase chain reaction (PCR) and positive results in CSF evaluation (Soares et al., 2010). Two additional cases of dengue meningitis were reported by Mamdouh in 2013. Both cases presented with fever, headache, neck rigidity, and low platelet count in a dengue endemic area. Diagnosis was confirmed by positive IgM in the CSF in the absence of the typical clinical spectrum of infection. CSF cellularity was normal in one case. Brain CT or MRI scan was normal. Dengue meningitis might cause migraine such as headaches with poor response to anti-migraine therapy and common analgesics. The two patients achieved full recovery after several months without any residual neurological deficit (Mamdouh et al., 2013).
Stroke
Ischemic and hemorrhagic strokes have been reported in a few cases. Liou et al. reported a dengue fever patient who showed thrombocytopenia and ischemic stroke in 2008 (Liou et al., 2008a). In 2013, Rajesh Verma et al. reported a 68-year-old male patient presenting with moderate grade, continuous fever for 15 days; the patient also had sudden onset of weakness of the left half of his body as well as facial asymmetry, 10 days prior to hospital admission. Dengue infection was confirmed and brain MRI revealed acute infarction in the right parietal lobe. After 2 months of medication and physiotherapy, a partial improvement was observed in limb weakness (Verma et al., 2013). In cases with ischemic stroke, meningovasculitis, or a transient hypercoagulable state during dengue infection was postulated as the pathogenetic mechanism (Liou et al., 2008a; Verma et al., 2013).
A summary of intracerebral hemorrhage cases associated with dengue virus infection in various countries from 2001 to 2014 was reported by Vargas-Sánchez et al. (2014). In the above study, patient age ranged from 9 to 68 years, and there were 5 females and 7 males. Outcome was unspecified in one patient, while 7 and 5 patients recovered and died, respectively. Meanwhile, DENV2 was detected in 5 patients; one patient was infected with DENV3 and precision was not provided for the remaining seven. The involved regions included the pontine, basal gangliar, cerebellar, parietal, temporal, and frontal lobes of the brain (Vargas-Sánchez et al., 2014).
Current studies suggest that in dengue virus infection, cytokine overproduction results in immune-mediated endothelial cell damage (Seet and Lim, 2007; Basu and Chaturvedi, 2008). Hemorrhage may be caused by elevated vascular permeability, plasma leakage, and vasculitis due to dengue (Seet and Lim, 2007; Basu and Chaturvedi, 2008).
Cerebellar Syndrome
Cerebellar syndrome was mentioned by Weeratunga et al. (2014b). They described three patients diagnosed with dengue infection based on combined clinical and laboratory findings, fulfilling the WHO criteria in an endemic area. Within 2 weeks of diagnosis, they developed cerebellar symptoms. The manifestations of cerebellar syndrome include bilateral vertical and horizontal nystagmus, dysarthria, bilateral limb, and gait ataxia. Other causes of cerebellar dysfunction were excluded, and all cases were self-limiting. MR brain scans showed cerebellar T2 hyperintense lesions in one case, while the remaining two showed normal signals. The CSF in all cases had normal protein levels and cell count. All cases showed antibodies for dengue in blood and CSF samples. A low-grade inflammatory process was the proposed mechanism (Weeratunga et al., 2014b).
Transversemyelitis(TM)/Longitudinally Extensive Transverse Myelitis (LETM)
The diagnosis of transversemyelitis (TM) is established by a somewhat sudden onset of sensorimotor and sphincter disturbances resulting from inflammatory demyelinating lesions (Scott et al., 2011). In longitudinally extensive transverse myelitis (LETM), a spinal lesion covers three or more vertebral segments (Wolf et al., 2012). According to Jacob and Weinshenker's opinions, TM can be broadly classified into four categories: (1) demyelination (monofocal clinical isolated syndrome) or multifocal demyelination [acute disseminated encephalomyelitis (ADEM) or multiple sclerosis]; (2) combined systemic connective tissue disease; (3) infection; (4) idiopathic illness (Jacob and Weinshenker, 2008).
Positive findings by spinal MRI are crucial in reaching the diagnosis of TM/LETM. Hyperintensity in T2-weighted signals found in spinal MRI scans support transverse myelitis diagnosis. In addition, inflammatory reactions in the CSF are found in most patients. TM/LETM in dengue is rarely encountered, and no more than 12 case reports are available (Renganathan et al., 1996; Leao et al., 2002; Kunishige et al., 2004; Seet et al., 2006; Chanthamat and Sathirapanya, 2010; Verma et al., 2011a; Larik et al., 2012; Singh et al., 2013; Weeratunga et al., 2014a; Fong et al., 2016; Mo et al., 2016; Mota et al., 2017).
However, the spectrum of TM could be broad, and more attention should be paid to atypical cases. For example, Mota reported a patient confirmed with acute TM without paraparesis following a dengue virus infection last year (Mota et al., 2017).
Treatment with intravenous steroids is useful, and individuals with parainfectious-dengue TM show satisfactory recovery (Fong et al., 2016). Fong et al. reported that a previously healthy 12-year-old girl with parainfectious-dengue TM and concomitant spinal epidural haematoma had good clinical recovery without surgical intervention after 6 months (Fong et al., 2016). It was shown that post-infection autoimmune reactions and direct infection are associated with transverse myelitis (Solomon et al., 2000b; Sindic et al., 2001; de Sousa et al., 2014; Mo et al., 2016).
Acute Disseminated Encephalomyelitis (ADEM)
ADEM is characterized by an acute inflammatory demyelinating ailment affecting the CNS, a monophasic course, and multifocal white matter involvement which occurs during or after dengue virus infection (Puccioni-Sohler et al., 2013). PubMed and Scopus combinely reported only 7 cases prior to May 2017 (Yamamoto et al., 2002; Brito et al., 2007; Gera and George, 2010; Sundaram et al., 2010; Gupta et al., 2013; Viswanathan et al., 2016). ADEM onset occurs within a limited time (averaging 5.6 days) after initial dengue signs (Gupta et al., 2013). An abnormal CSF contributes to ADEM diagnosis, while a normal CSF cannotrule out its possibility (Viswanathan et al., 2016).
Few studies reporting radiologically confirmed dengue associated ADEM are available. MRIs show extensive involvement of the white matter of the frontal, parietal, or temporal lobe, basal ganglia, brainstem, cerebellum, corpus callosal, and periventricular lesions (Puccioni-Sohler et al., 2012, 2013; Gupta et al., 2013; Domingues and Kuster, 2014; Mudin, 2015; Viswanathan et al., 2016). Perivenous demyelination, macrophage influx, and perivascular infiltration of lymphocytes with hemorrhagic foci were reported after histological examination of such lesions (Sundaram et al., 2010).
Dengue related ADEM results from immune reactions (Gupta et al., 2013). In Murthy's study, its pathophysiology was considered a transient autoimmune reaction to myelin or unknown self-antigens (Murthy, 2010). There is no established treatment for ADEM, but the use of steroids is effective during its active phase (Carod-Artal et al., 2013; Gupta et al., 2013; Domingues and Kuster, 2014).
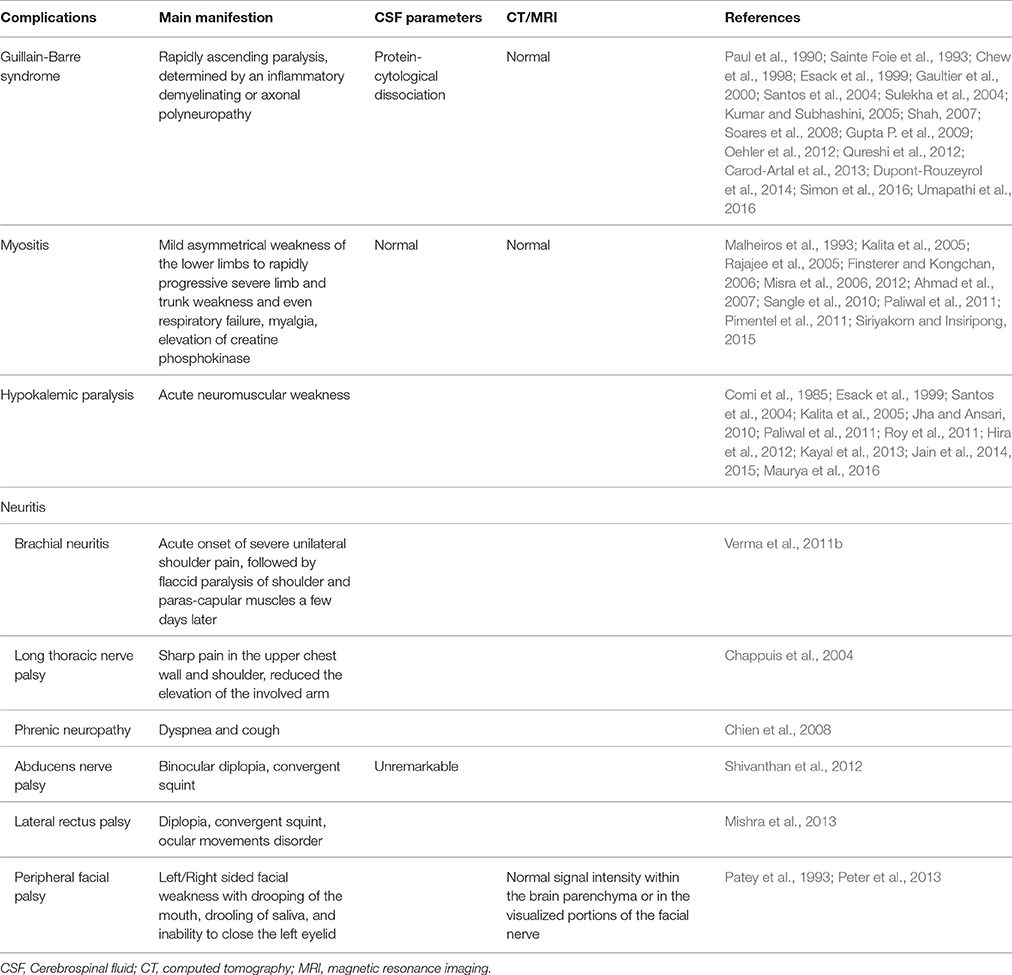
Peripheral Nervous System Complications
Reviews indicated that peripheral nervous system signs comprise 5% of neurological symptoms in dengue fever. They usually occur later than CNS manifestations (Oehler et al., 2012). The associated peripheral syndromes mainly include Guillain-Barre syndrome, hypokalemic quadriparesis or plegia, mononeuritis multiplex, brachial plexitis, diaphragmatic paralysis, and myositis (Jha and Ansari, 2010; Sharma et al., 2011; Verma et al., 2011b; Gutch et al., 2012; Ratnayake et al., 2012; Jain et al., 2014; Table 2).
Guillain-Barré Syndrome
Guillain-Barré syndrome (GBS) features a quickly rising paralysis, reflecting an inflammatory demyelinating or axonal polyneuropathy (Soares et al., 2008). In most cases, a protein-cytological dissociation in the CSF is indicative of GBS (Allos, 1998). Presentations of reduced conduction velocity, conduction blockage in motor nerves, extended distal latency, and prolongation or absence of F responses are often observed by electromyography in GBS patients (Soares et al., 2008).
GBS following dengue virus infection is uncommon (Meena et al., 2011; Qureshi et al., 2012; Chaudhary et al., 2014). In the literature, about 20 case reports of GBS associated with dengue infection are available (Paul et al., 1990; Sainte Foie et al., 1993; Esack et al., 1999; Santos et al., 2004; Sulekha et al., 2004; Kumar and Subhashini, 2005; Soares et al., 2008; Oehler et al., 2012; Carod-Artal et al., 2013; Dupont-Rouzeyrol et al., 2014; Simon et al., 2016). Most cases were pediatric patients, and only few adults were involved (Chew et al., 1998; Gupta P. et al., 2009; Qureshi et al., 2012).
Neurological signs develop at 1–19 days after the onset of dengue. Simon et al. found GBS occurs early in DF with an unusual progression; in their report, average time elapsed from fever to neurological signs was 2 days, ranging between 1 and 3 days (Simon et al., 2016). A patient with Miller Fisher syndrome caused by dengue has been described (Gaultier et al., 2000). Umapat et al. reported that asymptomatic dengue infection may also trigger Guillain-Barré syndrome, and found four GBS cases after dengue diagnosis, including 1 and 3 with acute motor axonal neuropathy and acute inflammatory demyelinating polyneuropathy, respectively (Umapathi et al., 2016).
The exact mechanism of dengue associated GBS remains unclear. It is considered a neurological disease modulated by immunocytes (Chew et al., 1998; Gupta P. et al., 2009). Pro-inflammatory substances such as tumor necrosis factor, interleukins, and the complement may play important roles in GBS pathogenesis (Shah, 2007). Immune reactions induced by dengue virus infection might involve peripheral nerve constituents; for example, myelin or axon may be targeted by the immune response (Carod-Artal et al., 2013).
Plasma exchange seems to be more effective than supportive treatments in the treatment of GBS in randomized clinical trials (Comi et al., 1985; Qureshi et al., 2012). Immunoglobulins administered intravenously show similar effectiveness to plasma exchange, and might be more advantageous (Chew et al., 1998; Gupta P. et al., 2009; Qureshi et al., 2012). Corticosteroids alone do not make a difference, and there is not enough data supporting their usefulness (Comi et al., 1985; Qureshi et al., 2012). Other treatment options, e.g., CSF filtration, are still being assessed (Qureshi et al., 2012).
Myositis
Dengue myositis diagnosis is based on clinical manifestations of dengue virus infection, positive serum IgM for the dengue virus, high creatine phosphokinase levels, normal CSF, and exclusion of other causes (Paliwal et al., 2011). The clinical spectrum is broad, from mild asymmetrical weakness of lower extremities to sudden progressive severe limb and trunk weakness, and even lung failure (Paliwal et al., 2011). However, muscle weakness is distinctly uncommon in dengue virus infection (Malheiros et al., 1993; Sangle et al., 2010). Doctors should pay more attention to patients suffering from early pulmonary impairment, elevated creatine phosphokinase amounts, and serious myalgia (Paliwal et al., 2011).
The pathogenesis of myositis remains unclear. The proposed mechanisms comprise direct muscular invasion by the virus and immunity associated destruction of muscle fibers, particularly by tumor necrosis factor (Malheiros et al., 1993; Paliwal et al., 2011). Dengue myositis is reflected by perivascular infiltration of mononuclear cells, mitochondrial proliferation, fat accumulation, nuclear centralization, fiber-type grouping, and/or myonecrosis foci on muscle biopsy (Malheiros et al., 1993; Misra et al., 2012).
Normal motor unit potentials are of reduced duration and amplitude, and polyphasic on electromyographic examination. Fibrillations, sharp waves, and complex repetitive discharges do not occur (Kalita et al., 2005; Misra et al., 2012).
However, dengue myositis is considered a relatively benign and self-limiting disease in pediatric reports (Rajajee et al., 2005; Misra et al., 2006; Ahmad et al., 2007; Pimentel et al., 2011). In adult patients, dengue myositis is often more severe, even leading to severe rhabdomyolysis (Kalita et al., 2005; Finsterer and Kongchan, 2006; Siriyakorn and Insiripong, 2015). Sangle et al. reported a 16-year-old girl with dengue shock syndrome showing myositis and myocarditis. Symptomatic treatment and rehabilitation were provided, and she had recovered well at discharge after 1 month of hospitalization (Sangle et al., 2010). Finsterer et al. found dengue can cause persistent and serious myositis, which is resolved after administration of corticosteroids (Finsterer and Kongchan, 2006).
Hypokalemic Paralysis
Dengue-associated hypokalemic paralysis was reported by several authors, but most studies were based on isolated case reports and short series (Esack et al., 1999; Santos et al., 2004; Kalita et al., 2005; Jha and Ansari, 2010; Roy et al., 2011; Jain et al., 2014; Maurya et al., 2016). Maurya and colleagues assessed 12 dengue patients with hypokalemic paralysis, who had complete and rapid recovery after potassium supplementation (Maurya et al., 2016).
It has been suggested that elevated creatine phosphokinase amounts result from vasoconstriction and muscular ischemia caused by hypokalemia (Comi et al., 1985). In a study of 12 patients with acute neuromuscular weakness in a dengue epidemic, 10 individuals showed hypokalemia, while Guillain-Barré syndrome and myositis were found in one patient each. Of the latter 2 patients, the one with myositis showed slow improvement and normal serum potassium levels, and the other with Guillain-Barré syndrome recovered within 6 weeks (Hira et al., 2012). In a retrospective study of 7 patients with dengue myositis who had generalized weakness, a mild serum creatin phosphokinase (CPK) elevation was noted in 3 individuals with hypokalemia, whereas three cases with fulminant presentation and respiratory muscle involvement had markedly elevated CPK levels (16,590-117,200 U/L) with normal potassium amounts. All patients completely recovered within 4 weeks (Paliwal et al., 2011).
The severity of weakness may not correlate with potassium levels in Maurya's report (Maurya et al., 2016). Hypokalemia does not always lead to paralysis. In 1,342 patients with dengue fever assessed in China, hypokalemia occurred in up to 28% individuals but weakness was not mentioned (Ying et al., 2007).
Hypokalemic paralysis differs from dengue myositis and idiopathic hypokalemic paralysis according to clinical, biochemical features, and consequences (Maurya et al., 2016). It represents a systemic complication of dengue fever (Murthy, 2010; Jain et al., 2014). The pathogenesis of hypokalemic paralysis in dengue remains obscure. A probable explanation for hypokalemia in dengue is serum potassium redistribution in cells and transient kidney tubular dysfunction increasing urinary potassium elimination (Jha and Ansari, 2010; Jain et al., 2015; Maurya et al., 2016). In addition, infection-related stress may lead to catecholamine or insulin release, which results in an intracellular shift of potassium (Jha and Ansari, 2010; Hira et al., 2012). Supplementation of potassium can achieve satisfactory recovery in patients with hypokalemia paralysis (Jha and Ansari, 2010; Kayal et al., 2013). Jain et al. reported a 30-year-old man with dengue and hypokalemic paralysis alongside hypomagnesemia. Weakness persisted after potassium supplementation, while low serum magnesium levels were detected. However, he completely recovered within 48–72 h, with a normalization of serum potassium and magnesium levels. The possible mechanism may be that muscle Na+ and K+-ATPase activity is inhibited by hypomagnesemia, leading to a reduced ion influx into muscle fibers and secondary kaliuresis (Jain et al., 2015).
Neuritis
Neuritis after dengue infection is believed to rarely occur. However, recent studies have reported more and more dengue fever patients showing uncommon neurological signs. Dengue-associated neuritis, such as brachial neuritis, long thoracic nerve palsy, phrenic nerve palsy, abducens nervepalsy, and peripheral facial palsy have been reported in different areas of the world (Patey et al., 1993; Chappuis et al., 2004; Chien et al., 2008; Verma et al., 2011b; Shivanthan et al., 2012; Mishra et al., 2013; Peter et al., 2013).
Its diagnosis is established, after other reasons for neuritis, such as tumor and demyelinating diseases, infections, trauma, and stroke, are accordingly excluded. Dengue-associated nerve palsy is mostly managed by supportive treatment. Some cases improve even without specific treatment, e.g., with steroids or intravenous immunoglobulins (Peter et al., 2013; Biswas and Pal, 2014), while others have a good response to steroids (Verma et al., 2011b).
The pathogenesis of dengue-associated neuritis is likely related to immune reactions, although the mechanisms remain largely unclear (Carod-Artal et al., 2013). Most patients achieve a remarkable recovery.
Ophthalmic Complications
Ophthalmic complications in dengue fever were previously considered rare events, but more cases have been reported (Carod-Artal et al., 2013). Multiple studies identify maculopathy as the most frequent neuro-ocular sign; less commonly encountered complications are optic neuropathy, retina vasculopathy, and cranial nerve palsy (Yip et al., 2012). Kapoor et al. retrospectively assessed 134 dengue fever patients, among whom up to 40% had ocular manifestations (Kapoor et al., 2006). Ocular complications involve: (1) the anterior segment of the eye, e.g., subconjunctival hemorrhage, uveitis, or a shallow anterior chamber (Cruz-Villegas et al., 2003; Pierre Filho Pde et al., 2008); (2) the posterior segment of the eye, e.g., maculopathy, macular edema, optic neuropathy, or vitreous hemorrhage (Nainiwal et al., 2005; Bacsal et al., 2007; Chang et al., 2007; Tan et al., 2007; Kanungo et al., 2008; Loh et al., 2008; Quek et al., 2009). Presentation of dengue-related ocular signs and symptoms often corresponds to thrombocytopenia (Chan et al., 2006; Teoh et al., 2006). However, some complications, e.g., uveitis, can occur 3–5 months after dengue infection (Gupta A. et al., 2009). The majority of individuals with such complications are spontaneously relieved. Systemic steroids and occasional immunoglobulins are provided to patients with severe vision loss. The prognosis of dengue-related ophthalmic complications is favorable. Almost all patients become normal or improve in vision (Yip et al., 2012).
The mechanisms underlying dengue infection-related ocular signs remain unclear, but could involve immune processes with possible association with dengue serotyping (Chan et al., 2006; Bacsal et al., 2007; Su et al., 2007; Yip et al., 2012).
Neurological Complications in Children
Dengue causes high morbidity and mortality in children living in tropical and subtropical areas of the world. Around 95% of patients with serious disease are below 15 years old (Bhattacharya et al., 2013). All four dengue virus serotypes can be detected in infectious children, and clinical symptoms range from mild fever to fatal dengue shock syndrome (Verhagen and de Groot, 2014).
National surveillance in Asia showed that individuals below 1 year old and those between 4 and 9 years of age are most likely to develop severe dengue infection (Kongsomboon et al., 2004; Huy et al., 2010). It is worth noting that compared with older children, infants often show a higher frequency of plasma leakage and shock in dengue (Nguyen et al., 2004; Hammond et al., 2005). Therefore, the management of infants with dengue infection is important because they sometimes present with unusual manifestations, and early diagnosis is very challenging (Kalayanarooj and Nimmannitya, 2003). Whether severe dengue disease occurs or not largely depends on factors such as virus properties, host immunity, age, and genetic makeup. Female children may be associated with severe dengue (Anders et al., 2011). Prognosis of dengue hemorrhagic fever and dengue shock syndrome is affected by prevention, early detection, and timely therapy; mortality ranges between 2.5 and 5.0% (Alejandria, 2009). In case of shock, mortality can approach 12–44% (Rigau-Pérez et al., 1998). Patients usually have satisfactory recovery after optimal fluid/electrolyte supplementation.
Neurological complications recorded in pediatrics include ADEM (Kamath and Ranjit, 2006), hepatic encephalopathy (Kamath and Ranjit, 2006), acute childhood myositis (Ahmad et al., 2007), hemiconvulsion-hemiplegia-epilepsy (Gastaut et al., 1960; Saini et al., 2017), parkinsonism (Fong et al., 2014), ischemic stroke due to dengue vasculitis (Nanda et al., 2014), sub-arachnoid hemorrhage (Kamath and Ranjit, 2006), and transverse myelitis (Fong et al., 2016). Most cases achieve satisfactory recovery after timely treatment (Kankirawatana et al., 2000; Cam et al., 2001; Kamath and Ranjit, 2006). Children intervened late are harder to resuscitate (Kamath and Ranjit, 2006).
Hospitalization may not be necessary for children with mild dengue infection (Nguyen et al., 2004; Hammond et al., 2005). There are no specific therapeutic agents for dengue, but fluid replacement is immediately required in pediatric cases with haemorrhagic fever or shock syndrome, to expand the plasma volume. Crystalloids are as potent as colloids in children with moderately severe and severe dengue shock syndrome (Alejandria, 2009). For children with suspected DHF, attentive clinical monitoring and supportive care are critical measures for reducing fatality rates (Tantawichien, 2012). Preventive transfusions are not recommended (Tantawichien, 2012), and steroid administration is not considered a beneficial option in dengue shock syndrome (Smart and Safitri, 2009).
Therapy and Prevention
Currently, no definite effective antiviral agents are available for dengue infection treatment. General supportive therapy prevails, emphasizing on intense hematological monitoring, fluid-replacement, and/or blood transfusion if needed. Non-steroidal anti-inflammatory drugs may worsen gastritis or cause bleeding.
A safe and efficacious dengue vaccine is considered a great hope for preventing and controlling this disease. Currently, prevention is only achieved by vector control. However, several vaccine preparations are under investigation (Simmons et al., 2012). Notably, it is especially important for children to avoid Aedes mosquito bites in dengue-endemic regions (Elling et al., 2013).
Comparsion with Japanese Encephalitis Virus
Dengue is considered a non-neurotropic virus. However, neurotropism and neuro-invasion in dengue have been reported (Chardboonchart et al., 1990; Despres et al., 1998; Solomon et al., 2000b; Cam et al., 2001). DF is usually self-limiting, and death is rather uncommon (Tantawichien, 2012). In vitro experiments in 1998 indicated DENV directly infects neurons, which results in permanent damage (Despres et al., 1998). In 2000, Jan and colleages demonstrated that phospholipase A2 (PLA2) activation, cytochrome C release from the mitochondria, superoxide anion production, and nuclear factor-kB translocation could lead to neuronal apoptosis (Jan et al., 2000).
The major pathogenetic mechanisms include endothelial cell disfunction and development of coagulation disorders. Therefore, the clinical signs of dengue fever mostly comprise endothelial cell damage, enhanced vascular permeability, and increased plasma leakage (Halstead and Cohen, 2015). The dengue virus shows tropisms mainly for monocytes, macrophages, and dendritic cells (Jessie et al., 2004; Martina et al., 2009).
Though belonging to the same flavivirus group, Japanese encephalitis virus (JEV) is a proven neurotropic virus, and mainly targets neuronal cells (Kimura-Kuroda et al., 1993). Nearly 75% of symptomatic patients show manifestations of encephalitis, which often leads to various neurological complications or patient fatality (Lee et al., 2012; Sarkari et al., 2012a,b). JEV infection is considered a leading cause of pediatric encephalitis (Thongtan et al., 2012).
Little is known about the mechanism by which the virus spreads to the CNS as well as its brain tropism (Myint et al., 2007). Ghosh Roy et al. assumed that direct spread to the CNS occurs when mosquitos directly bite into blood vessels (Ghosh Roy et al., 2014).
Apoptosis induced by JEV involves the following three related mechanisms: direct neuron infection, infection of other CNS cells (e.g., microglia and astrocytes), and inflammatory reactions (Chen et al., 2004; Raung et al., 2007; Das and Basu, 2008; Swarup et al., 2008). As a result, CNS cell infection by JEV causes considerable neuronal apoptosis (Chen et al., 2004; German et al., 2006; Ghoshal et al., 2007). Serious vascular congestion, cerebral edema, neuronal death, astrocyte activation, and microglial proliferation are found in fatal cases of JEV infection (Misra and Kalita, 2010).
Therefore, it is relatively easy to understand why up to one-third of JE cases hospitalized die while about one-half of all survivors show permanent neurological sequelae, even individuals with apparent recovery (Kumar et al., 1993; Solomon et al., 2000a). However, most patients even with neurological manifestations in dengue infection have an unexpected good recovery with no obvious sequelae.
Dengue is gradually becoming a main public health problem worldwide. A growing number of related studies demands increased awareness and understanding of the neurological complications of dengue infection. Physicians, especially neurologists, will continue to play important roles in its diagnosis and treatment. Pathogenesis, detection, antivirals, vaccines, environmental risk reduction, and vector control still deserve further studies.
Author Contributions
GL: Designed the study, collected data, wrote the manuscript. YL: Designed the study, reviewed the manuscript. XL: Designed the study, reviewed the manuscript. ZN: Collected data and edited the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Dr. Edward C. Minot, Shandong University, for linguistic advice.
References
Ahmad, R., Abdul Latiff, A. K., and Abdul Razak, S. (2007). Myalgia Cruris Epidemica: an unusual presentation of dengue fever. Southeast Asian J. Trop. Med. Public Health 38, 1084–1087.
Alejandria, M. M. (2009). Dengue haemorrhagic fever or dengue shock syndrome in children. BMJ Clin. Evid. 2009:0917.
Allos, B. M. (1998). Campylobacter jejuni infection as a cause of the Guillain Barré syndrome. Emerging Infect. Dis. 12, 173–184. doi: 10.1016/S0891-5520(05)70416-5
Anders, K. L., Nguyet, N. M., Chau, N. V., Hung, N. T., Thuy, T. T., Lien le, B., et al. (2011). Epidemiological factors associated with dengue shock syndrome and mortality in hospitalized dengue patients in Ho Chi Minh City, Vietnam. Am. J. Trop. Med. Hyg. 84, 127–134. doi: 10.4269/ajtmh.2011.10-0476
Angibaud, G., Luaute, J., Laille, M., and Gaultier, C. (2001). Brain involvement in Dengue fever. J. Clin. Neurosci. 8, 63–65. doi: 10.1054/jocn.2000.0735
Araújo, F. M., Brilhante, R. S., Cavalcanti, L. P., Rocha, M. F., Cordeiro, R. A., Perdigao, A. C., et al. (2011). Detection of the dengue non-structural 1 antigen in cerebral spinal fluid samples using a commercially available enzyme-linked immunosorbent assay. J. Virol. Methods. 177, 128–131. doi: 10.1016/j.jviromet.2011.07.003
Bacsal, K. E., Chee, S. P., Cheng, C. L., and Flores, J. V. (2007). Dengue associated maculopathy. Arch. Ophthalmol. 125, 501–510. doi: 10.1001/archopht.125.4.501
Baldaçara, L., Ferreira, J. R., Filho, L. C., Venturini, R. R., Coutinho, O. M., Camarço, W. C., et al. (2013). Behavior disorder after encephalitis caused by dengue. J. Neuropsychiatry Clin. Neurosci. 25:E44. doi: 10.1176/appi.neuropsych.12020040
Basu, A., and Chaturvedi, U. C. (2008). Vascular endothelium: the battlefield of dengue viruses. Immunol. Med. Microbiol. 53, 287–299. doi: 10.1111/j.1574-695X.2008.00420.x
Bhatt, S., Gething, P. W., Brady, O. J., Messina, J. P., Farlow, A. W., Moyes, C. L., et al. (2013). The global distribution and burden of dengue. Nature 496, 504–507. doi: 10.1038/nature12060
Bhattacharya, M. K., Maitra, S., Ganguly, A., Bhattacharya, A., and Sinha, A. (2013). Dengue: a growing menace - a snapshot of recent facts, figures & remedies. Int. J. Biomed. Sci. 9, 61–67.
Bhoopat, L., Bhamarapravati, N., Attasiri, C., Yoksarn, S., Chaiwun, B., Khunamornpong, S., et al. (1996). Immunohistochemical characterization of a new monoclonal antibody reactive with dengue virus infected cells in frozen tissue using immunoperoxidase technique. Asian Pac. J. Allergy Immunol. 14, 107–113.
Biswas, N. M., and Pal, S. (2014). Oculomotor nerve palsy in dengue encephalitis-a rare presentation. Indian J. Med. Res. 140, 793–794.
Brady, O. J., Gething, P. W., Bhatt, S., Messina, J. P., Brownstein, J. S., Hoen, A. G., et al. (2012). Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl. Trop. Dis. 6:e1760. doi: 10.1371/journal.pntd.0001760
Brito, C. A., Sobreira, S., Cordeiro, M. T., and Lucena-Silva, N. (2007). Acute disseminated encephalomyelitis in classic dengue. Rev. Soc. Bras. Med. Trop. 40, 236–238. doi: 10.1590/S0037-86822007000200019
Cam, B. V., Fonsmark, L., Hue, N. B., Phuong, N. T., Poulsen, A., and Heegaard, E. D. (2001). Prospective case - control study of encephalopathy in children with dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 65, 848–851. doi: 10.4269/ajtmh.2001.65.848
Carod-Artal, F. J., Wichmann, O., Farrar, J., and Gascón, J. (2013). Neurological complications of dengue virus infection. Lancet Neurol. 12, 906–919. doi: 10.1016/S1474-4422(13)70150-9
Chan, D. P., Teoh, S. C., Tan, C. S., Nah, G. K., Rajagopalan, R., Prabhakaraguptaf, M. K., et al. (2006). Ophthalmic complications of dengue. Emerging Infect. Dis. 12:285–289. doi: 10.3201/eid1202.050274
Chang, P. E., Cheng, C. L., Asok, K., Fong, K. Y., Chee, S. P., and Tan, C. K. (2007). Visual disturbances in dengue fever: an answer at last? Singapore Med. J. 48, 71–73.
Chanthamat, N., and Sathirapanya, P. (2010). Acute transverse myelitis associated with dengue viral infection. J. Spinal Cord Med. 33, 425–427. doi: 10.1080/10790268.2010.11689722
Chappuis, F., Justafré, J. C., Duchunstang, L., Loutan, L., and Taylor, W. R. (2004). Dengue fever and long thoracic nerve palsy in a traveler Returning from Thailand. J. Travel Med. 11, 112–114. doi: 10.2310/7060.2004.16983
Chardboonchart, V., Khemarunmanus, M., Yoksan, S., and Bhamarapravati, N. (1990). Dengue virus serotypic identification using suckling mouse and Western blot technique. Southeast Asian J. Trop. Med. Public Health 21, 614–620.
Chaturvedi, U. C., Dhawan, R., Khanna, M., and Mathur, A. (1991). Breakdown of the blood—brain barrier during dengue infection of mice. J. Gen. Virol. 72, 859–866. doi: 10.1099/0022-1317-72-4-859
Chaudhary, R., Gupta, B. S., Gupta, R., Garg, A., and Khedar, R. S. (2014). Guillain–Barre syndrome: an unusual complication of dengue fever. Sch. J. Med. Case Rep. 2, 133–135.
Chen, C. J., Chen, J. H., Chen, S. Y., Liao, S. L., and Raung, S. L. (2004). Upregulation of RANTES gene expression in neuroglia by Japanese encephalitis virus infection. J. Virol. 78, 12107–12119. doi: 10.1128/JVI.78.22.12107-12119.2004
Chew, N. K., Goh, K. J., Omar, S., and Tan, C. T. (1998). Guillain–Barre syndrome with antecedent dengue infection: a report of two cases. Neurol. J. Southeast Asia 3, 85–86.
Chien, J., Ong, A., and Low, S. Y. (2008). An unusual complication of dengue fever. Singapore Med. J. 49, e340–e342.
Comi, G., Testa, D., Cornelio, F., Comola, M., and Canal, N. (1985). Potassium depletion myopathy: a clinical and morphological study of six cases. Musc. Nerve 8, 17–21. doi: 10.1002/mus.880080104
Cruz-Villegas, V., Berrocal, A. M., and Davis, J. L. (2003). Bilateral choroidal effusions associated with dengue fever. Retina 23, 576–578. doi: 10.1097/00006982-200308000-00031
Das, S., and Basu, A. (2008). Japanese encephalitis virus infects neural progenitor cells and decreases their proliferation. J. Neurochem. 106, 1624–1636. doi: 10.1111/j.1471-4159.2008.05511.x
de Sousa, A. M., Alvarenga, M. P., and Alvarenga, R. M. (2014). A cluster of transverse myelitis following dengue virus infection in the Brazilian Amazon Region. Trop. Med. Health 42, 115–120. doi: 10.2149/tmh.2014-06
Desprès, P., Frenkiel, M. P., Ceccaldi, P. E., Duarte Dos Santos, C., and Deubel, V. (1998). Apoptosis in the mouse central nervous system in response to infection with mouse neurovirulent dengue viruses. J. Virol. 72, 823–829.
Domingues, R. B., and Kuster, G. W. (2014). Diagnosis and management neurologic manifestations associated with acute dengue virus infection. J. Neuroinfect. Dis. 5:138. doi: 10.4172/2314-7326.1000138
Domingues, R. B., Kuster, G. W., Onuki-Castro, F. L., Souza, V. A., Levi, J. E., and Pannuti, C. S. (2008). Involvement of the central nervous system in patients with dengue virus infection. J. Neurol. Sci. 267, 36–40. doi: 10.1016/j.jns.2007.09.040
Dupont-Rouzeyrol, M., Aubry, M., Connor, O. O., Roche, C., Gourinat, A. C., Guigon, A., et al. (2014). Epidemiological and molecular features of dengue virus type-1 in New Caledoni, South Pacific, 2001–2013. Virol. J. 11:61. doi: 10.1186/1743-422X-11-61
Elling, R., Henneke, P., Hatz, C., and Hufnagel, M. (2013). Children in fever dengue where are we now? Pediatr. Infect. Dis. J. 32, 1020–1022. doi: 10.1097/INF.0b013e31829fd0e9
Esack, A., Teelucksingh, S., and Singh, N. (1999). The Guillain Barré syndrome following dengue fever. West Indian Med. J. 48, 36–37.
Finsterer, J., and Kongchan, K. (2006). Severe, persisting, steroid-responsive dengue myositis. J. Clin. Virol. 35, 426–428. doi: 10.1016/j.jcv.2005.11.010
Fong, C. Y., Hlaing, C. S., Tay, C. G., and Ong, L. C. (2014). Post-dengue encephalopathy and Parkinsonism. Pediatr Infect Dis. J. 33, 1092–1094. doi: 10.1097/INF.0000000000000382
Fong, C. Y., Hlaing, C. S., Tay, C. G., Kadir, K. A., Goh, K. J., and Ong, L. C. (2016). Longitudinal extensive transverse myelitis with cervical epidural haematoma following dengue virus infection. Eur. J. Paediatr. Neurol. 20, 449–453. doi: 10.1016/j.ejpn.2016.01.012
Freedman, D. O., Weld, L. H., Kozarsky, P. E., Fisk, T., Robins, R., von Sonnenburg, F., et al. (2006). Spectrum of disease and relation to place of exposure among ill returned travelers. N. Engl. J. Med. 354, 119–130. doi: 10.1056/NEJMoa051331
Garg, R. K., Rizvi, I., Ingole, R., Jain, A., Malhotra, H. S., Kumar, N., et al. (2017). Cortical laminar necrosis in dengue encephalitis—a case report. BMC Neurol. 17:79. doi: 10.1186/s12883-017-0855-9
Gastaut, H. P. F., Payan, H., Salomon, G., Toga, M., and Vigouroux, M. (1960). HHE syndrome: hemiconvulsions–hemiplegia–epilepsy. Epilepsia 1, 418–417. doi: 10.1111/j.1528-1157.1959.tb04278.x
Gaultier, C., Angibaud, G., Laille, M., and Lacassin, F. (2000). Probable syndrome de Miller Fisher au cours d'une dengue de type 2. Rev. Neurol. 156, 169–171.
Gera, C., and George, U. (2010). Acute disseminating encephalomyelitis with hemorrhage following dengue. Neurol. India 58, 595–596. doi: 10.4103/0028-3886.68661
German, A. C., Myint, K. S., Mai, N. T., Pomeroy, I., Phu, N. H., Tzartos, J., et al. (2006). A preliminary neuropathological study of Japanese encephalitis in humans and a mouse model. Trans. R. Soc. Trop. Med. Hyg. 100, 1135–1145. doi: 10.1016/j.trstmh.2006.02.008
Ghosh Roy, S., Sadigh, B., Datan, E., Lockshin, R. A., and Zakeri, Z. (2014). Regulation of cell survival and death during Flavivirus infections. World J. Biol. Chem. 5, 93–105. doi: 10.4331/wjbc.v5.i2.93
Ghoshal, A., Das, S., Ghosh, S., Mishra, M. K., Sharma, V., Koli, P., et al. (2007). Proinflammatory mediators released by activated microglia induces neuronal death in Japanese encephalitis. Glia 55, 483–496. doi: 10.1002/glia.20474
Gupta, A., Srinivasan, R., Setia, S., Soundravally, R., and Pandian, D. G. (2009). Uveitis following dengue fever. Eye 23, 873–876. doi: 10.1038/eye.2008.124
Gupta, M., Nayak, R., Khwaja, G. A., and Chowdhury, D. (2013). Acute disseminated encephalomyelitis associated with dengue infection: a case report with literature review. J. Neurol. Sci. 335, 216–218. doi: 10.1016/j.jns.2013.08.029
Gupta, P., Jain, V., Chatterjee, S., and Agarwal, A. K. (2009). Acute inflammatory motor axonopathy associated with dengue fever. JIACM 10, 58–59.
Gutch, M., Agarwal, A., and Amar, A. (2012). Hypokalemic quadriparesis: an unusual manifestation of dengue fever. J. Nat. Sci. Biol. Med. 3, 81–83. doi: 10.4103/0976-9668.95976
Guzman, M. G., Halstead, S. B., Artsob, H., Buchy, P., Farrar, J., Gubler, D. J., et al. (2010). Dengue: a continuing global threat. Nat. Rev. Microbiol. 8(12 Suppl.), S7–S16. doi: 10.1038/nrmicro2460
Halstead, S. B., and Cohen, S. N. (2015). Dengue hemorrhagic fever at 60 years: early evolution of concepts of causation and treatment. Microbiol. Mol. Biol. Rev. 79, 281–291. doi: 10.1128/MMBR.00009-15
Hammond, S. N., Balmaseda, A., Pérez, L., Tellez, Y., Saborío, S. I., Mercado, J. C., et al. (2005). Differences in dengue severity in infants, children, and adults in a 3-year hospital-based study in Nicaragua. Am. J. Trop. Med. Hyg. 73, 1063–1070.
Hendarto, S. K., and Hadinegoro, S. K. (1992). Dengue encephalopathy. Acta Paediatr. Jpn. 34, 350–357. doi: 10.1111/j.1442-200X.1992.tb00971.x
Hira, H. S., Kaur, A., and Shukla, A. (2012). Acute neuromuscular weakness associated with dengue infection. J. Neurosci. Rural Pract. 3, 36–39. doi: 10.4103/0976-3147.91928
Huy, R., Buchy, P., Conan, A., Ngan, C., Ong, S., Ali, R., et al. (2010). National dengue surveillance in Cambodia 1980-2008: epidemiological and virological trends and the impact of vector control. Bull. World Health Organ. 88, 650–657. doi: 10.2471/BLT.09.073908
Jacob, A., and Weinshenker, B. G. (2008). An approach to the diagnosis of acute transverse myelitis. Semin. Neurol. 8, 105–120. doi: 10.1055/s-2007-1019132
Jain, R. S., Gupta, P. K., Agrawal, R., Kumar, S., and Khandelwal, K. (2015). An unusual case of dengue infection presenting with hypokalemic paralysis with hypomagnesemia. J. Clin. Virol. 69, 197–199. doi: 10.1016/j.jcv.2015.06.098
Jain, R. S., Handa, R., Prakash, S., Nagpal, K., and Gupta, P. (2014). Acute hypokalemic quadriparesis: an atypical neurological manifestation of dengue virus. J. Neurovirol. 20, 103–104. doi: 10.1007/s13365-014-0232-z
Jan, J. T., Chen, B. H., Ma, S. H., Liu, C. I., Tsai, H. P., Wu, H. C., et al. (2000). Potential dengue virus-triggered apoptotic pathway in human neuroblastoma cells: arachidonic acid, superoxide anion, and NF-kappaB are sequentially involved. J. Virol. 74, 8680–8691. doi: 10.1128/JVI.74.18.8680-8691.2000
Jessie, K., Fong, M. Y., Devi, S., Lam, S. K., and Wong, K. T. (2004). Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J. Infect. Dis. 189, 1411–1418. doi: 10.1086/383043
Jha, S., and Ansari, M. K. (2010). Dengue infection causing acute hypokalemic quadriparesis. Neurol. India 58, 592–594. doi: 10.4103/0028-3886.68657
Kalayanarooj, S., and Nimmannitya, S. (2003). Clinical presentations of dengue hemorrhagic fever in infants compared to children. J. Med. Assoc. Thai. 86(Suppl. 3), S673–S680.
Kalita, J., and Misra, U. K. (2006). EEG in dengue virus infection with neurological manifestations: a clinical and CT/MRI correlation. Clin. Neurophysiol. 117, 2252–2256. doi: 10.1016/j.clinph.2006.07.132
Kalita, J., Misra, U. K., Mahadevan, A., and Shankar, S. K. (2005). Acute pure motor quadriparesis: is it dengue myositis? Electromyogr. Clin. Neurophysiol. 45, 357–361.
Kamath, S. R., and Ranjit, S. (2006). Clinical Features Complications and Atypical Manifestations of Children with Severe forms of Dengue Hemorrhagic Fever in South India. Indian J. Pediatr. 73, 889–895. doi: 10.1007/BF02859281
Kankirawatana, P., Chokephaibulkit, K., Puthavathana, P., Yoksan, S., Apintanapong, S., and Pongthapisit, V. (2000). Dengue infections presenting with central nervous system manifestations. J. Child Neurol. 15, 544–547. doi: 10.1177/088307380001500809
Kanungo, S., Shukla, D., and Kim, R. (2008). Branch retinal artery occlusion secondary to dengue fever. Indian J. Ophthalmol. 56, 73–74. doi: 10.4103/0301-4738.37606
Kapoor, H. K., Bhai, S., John, M., and Xavier, J. (2006). Ocular manifestations of dengue fever in an East Indian epidemic. Can. J. Ophthalmol. 41, 741–746. doi: 10.3129/i06-069
Kayal, A. K., Goswami, M., Das, M., and Jain, R. (2013). Clinical and biochemical spectrum of hypokalemic paralysis in North: East India. Ann. Indian Acad. Neurol. 16, 211–217. doi: 10.4103/0972-2327.112469
Kimura-Kuroda, J., Ichikawa, M., Ogata, A., Nagashima, K., and Yasui, K. (1993). Specific tropism of Japanese encephalitis virus for developing neurons in primary rat brain culture. Arch. Virol. 130, 477–484. doi: 10.1007/BF01309676
Kongsomboon, K., Singhasivanon, P., Kaewkungwal, J., Nimmannitya, S., Mammen, Jr. M. P., Nisalak, A., et al. (2004). Temporal trends of dengue fever/dengue hemorrhagic fever in Bangkok, Thailand from 1981 to 2000: an age-period-cohort analysis. Southeast Asian J. Trop. Med. Public Health 35, 913–917.
Kumar, R., Mathur, A., Singh, K. B., Sitholey, P., Prasad, M., Shukla, R., et al. (1993). Clinical sequelae of Japanese encephalitis in children. Indian J. Med. Res. 97, 9–13.
Kumar, S., and Subhashini, P. (2005). Guillain Barré syndrome occurring in the course of dengue fever. Neurol. India 53, 250–251. doi: 10.4103/0028-3886.16437
Kunishige, M., Mitsui, T., Tan, B. H., Leong, H. N., Takasaki, T., Kurane, I., et al. (2004). Preferential gray matter involvement in dengue myelitis. Neurology 63, 1980–1981. doi: 10.1212/01.WNL.0000144194.29643.D0
Larik, A., Chiong, Y., Lee, L. C., and Ng, Y. S. (2012). Longitudinally extensive transverse myelitis associated with dengue fever. BMJ Case Rep. 2012:5378. doi: 10.1136/bcr.12.2011.5378
Leão, R. N., Oikawa, T., Rosa, E. S., Yamaki, J. T., Rodrigues, S. G., Vasconcelos, H. B., et al. (2002). Isolation of dengue 2 virus from a patient with central nervous system involvement (transverse myelitis). Rev. Soc. Bras. Med. Trop. 35, 401–404. doi: 10.1590/S0037-86822002000400018
Lee, D. W., Choe, Y. J., Kim, J. H., Song, K. M., Cho, H., Bae, G. R., et al. (2012). Epidemiology of Japanese encephalitis in South Korea, 2007-2010. Int. J. Infect. Dis. 16, e448–e452. doi: 10.1016/j.ijid.2012.02.006
Lima, D. M., de Paula, S. O., França, R. F., Palma, P. V., Morais, F. R., Gomes-Ruiz, A. C., et al. (2011). A DNA vaccine candidate encoding the structural prM/E proteins elicits a strong immune response and protects mice against dengue-4 virus infection. Vaccine 29, 831–838. doi: 10.1016/j.vaccine.2010.10.078
Liou, L. M., Lan, S. H., and Lai, C. L. (2008a). Dengue fever with ischemic stroke: a case report. Neurologist 14, 40–42. doi: 10.1097/NRL.0b013e3180d0a391
Liou, L. M., Lan, S. H., and Lai, C. L. (2008b). Electroencephalography burst suppression in a patient with dengue encephalopathy: a case report. Clin. Neurophysiol. 119, 2205–2208. doi: 10.1016/j.clinph.2008.03.001
Loh, B. K., Bacsal, K., Chee, S. P., Cheng, B. C., and Wong, D. (2008). Foveolitis associated with dengue fever: a case series. Ophthalmologica 222, 317–320. doi: 10.1159/000144074
Lorenzi, O. D., Gregory, C. J., Santiago, L. M., Acosta, H., Galarza, I. E., Hunsperger, E., et al. (2013). Acute febrile illness surveillance in a tertiary hospital emergency department: comparison of influenza and dengue virus infections. Am. J. Trop. Med. Hyg. 88, 472–480. doi: 10.4269/ajtmh.12-0373
Lum, L. C., Lam, S. K., Choy, Y. S., George, R., and Harun, F. (1996). Dengue encephalitis: a true entity? Am. J. Trop. Med. Hyg. 54, 256–259. doi: 10.4269/ajtmh.1996.54.256
Lundberg, A. (1957). Myalgia cruris epidemica. Acta Paediatr. 46, 18–31. doi: 10.1111/j.1651-2227.1957.tb08627.x
Madi, D., Achappa, B., Ramapuram, J. T., Chowta, N., Laxman, M., and Mahalingam, S. (2014). Dengue encephalitis-A rare manifestation of dengue fever. Asian Pac. J. Trop. Biomed. 4(Suppl 1), S70–S72. doi: 10.12980/APJTB.4.2014C1006
Malheiros, S. M., Oliveira, A. S., Schmidt, B., Lima, J. G., and Gabbai, A. A. (1993). Dengue. Muscle biopsy findings in 15 patients. Arq. Neuropsiquiatr. 51, 159–164. doi: 10.1590/S0004-282X1993000200001
Mamdouh, K. H., Mroog, K. M., Hani, N. H., and Nabil, E. M. (2013). Atypical dengue meningitis in Makkah, Saudi Arabia with slow resolving, prominent migraine like headache, phobia, and arrhythmia. J. Glob. Infect. Dis. 5, 183–186. doi: 10.4103/0974-777X.122021
Martina, B. E., Koraka, P., and Osterhaus, A. D. (2009). Dengue virus pathogenesis: an integrated view. Clin. Microbiol. Rev. 22, 564–581. doi: 10.1128/CMR.00035-09
Mathew, T., Badachi, S., Sarma, G. R., and Nadig, R. (2015). “Dot sign” in dengue encephalitis. Ann. Indian Acad. Neurol. 18, 77–79. doi: 10.4103/0972-2327.165475
Maurya, P. K., Kulshreshtha, D., Singh, A. K., and Thacker, A. K. (2016). Rapidly resolving weakness related to hypokalemia in patients infected with dengue virus. J. Clin. Neuromuscul. Dis. 18, 72–78. doi: 10.1097/CND.0000000000000140
Meena, A. K., Khadilkar, S. V., and Murthy, J. M. K. (2011). Treatement guidelines for Guillain–Barre syndrome. Ann. Indian Acad. Neurol. 14(Suppl. 1), S73–s81. doi: 10.4103/0972-2327.83087
Miagostovich, M. P., dos Santos, F. B., de Araujo, E. S., Dias, J., Schatzmayr, H. G., and Nogueira, R. M. (1997a). Diagnosis of dengue by using reverse transcriptase-polymerase chain reaction. Mem. Inst. Oswaldo Cruz 92, 595–599. doi: 10.1590/S0074-02761997000500006
Miagostovich, M. P., dos Santos, F. B., Fumian, T. M., Guimarães, F. R., da Costa, E. V., Tavares, F. N., et al. (2006). Complete genetic characterization of a Brazilian dengue virus type 3 strain isolated from a fatal outcome. Mem. Inst. Oswaldo Cruz 101, 307–313. doi: 10.1590/S0074-02762006000300015
Miagostovich, M. P., Ramos, R. G., Nicol, A. F., Nogueira, R. M., Cuzzi-Maya, T., Oliveira, A. V., et al. (1997b). Retrospective study on dengue fatal cases. Clin. Neuropathol. 16, 204–208.
Mishra, A., Shukla, S., Aggarwal, S., and Chaudhary, B. (2013). Lateral rectus palsy in a case of dengue fever. Med. J. Armed Forces India 1, 10–12. doi: 10.1016/j.mjafi.2013.05.010
Misra, U. K., and Kalita, J. (2010). Overview: Japanese encephalitis. Prog. Neurobiol. 91, 108–120. doi: 10.1016/j.pneurobio.2010.01.008
Misra, U. K., Kalita, J., Mani, V. E., Chauhan, P. S., and Kumar, P. (2015). Central nervous system and muscle involvement in dengue patients: a study from a tertiary care center. J. Clin. Virol. 72, 146–151. doi: 10.1016/j.jcv.2015.08.021
Misra, U. K., Kalita, J., Maurya, P. K., Kumar, P., Shankar, S. K., and Mahadevan, A. (2012). Dengue-associated transient muscle dysfunction: clinical, electromyography and histopathological changes. Infection 40, 125–130. doi: 10.1007/s15010-011-0203-8
Misra, U. K., Kalita, J., Syam, U. K., and Dhole, T. N. (2006). Neurological manifestations of dengue virus infection. J. Neurol. Sci. 244, 117–122. doi: 10.1016/j.jns.2006.01.011
Mo, Z., Dong, Y., Chen, X., Yao, H., and Zhang, B. (2016). Acute transverse myelitis and subacute thyroiditis associated with dengue viral infection: a case report and literature review. Exp. Ther. Med. 12, 2331–2335. doi: 10.3892/etm.2016.3604
Mota, M. T., Estofolete, C. F., Zini, N., Terzian, A. C., Gongora, D. V., Maia, I. L., et al. (2017). Transverse myelitis as an unusual complication of dengue fever. Am. J. Trop. Med. Hyg. 96, 380–381. doi: 10.4269/ajtmh.16-0284
Mudin, N. R. (2015). Dengue incidence and the prevention and control program in Malaysia. Int. Med. J. Malaysia 14, 5–9.
Murthy, J. M. (2010). Neurological complications of dengue infection. Neurol. India. 58(4):581–584. doi: 10.4103/0028-3886.68654
Myint, K. S., Gibbons, R. V., Perng, G. C., and Solomon, T. (2007). Unravelling the neuropathogenesis of Japanese encephalitis. Trans. R. Soc. Trop. Med. Hyg. 101:955–956. doi: 10.1016/j.trstmh.2007.04.004
Nainiwal, S., Garg, S. P., Prakash, G., and Nainiwal, N. (2005). Bilateral vitreous haemorrhage associated with dengue fever. Eye 19, 1012–1013. doi: 10.1038/sj.eye.6701704
Nanda, S. K., Jayalakshmi, S., and Mohandas, S. (2014). Pediatric ischemic stroke due to dengue vasculitis. Pediatr. Neurol. 51, 570–572. doi: 10.1016/j.pediatrneurol.2014.06.019
Nguyen, T. H., Lei, H. Y., Nguyen, T. L., Lin, Y. S., Huang, K. J., Le, B. L., et al. (2004). Dengue hemorrhagic fever in infants: a study of clinical and cytokine profiles. J. Infect. Dis. 189, 221–232. doi: 10.1086/380762
Nimmanitya, S., Thisyakorn, U., and Hemsrichart, V. (1987). Dengue haemorrhagic fever with unusual manifestations. Southeast Asian J. Trop. Med. Public Health 18:398–406.
Oehler, E., Le Hénaff, O., and Ghawche, F. (2012). Neurological manifestations of dengue. Presse Med. 41, e547–e552. doi: 10.1016/j.lpm.2012.03.010
Paliwal, V. K., Garg, R. K., Juyal, R., Husain, N., Verma, R., Sharma, P. K., et al. (2011). Acute dengue virus myositis: a report of seven patients of varying clinical severity including two cases with severe fulminant myositis. J. Neurol. Sci. 300, 14–18. doi: 10.1016/j.jns.2010.10.022
Pancharoen, C., and Thisyakorn, U. (2001). Neurological manifestation in dengue patients. Southeast Asian J. Trop. Med. Public Health 32, 341–345.
Patey, O., Ollivaud, L., Breuil, J., and Lafaix, C. (1993). Unusual neurologic manifestations occurring during dengue fever infection. Am. J. Trop. Med. Hyg. 48, 793–802. doi: 10.4269/ajtmh.1993.48.793
Paul, C., Dupont, B., and Pialoux, G. (1990). Polyradiculonévrite aiguë secondaire à une dengue. Presse Med 19:1503.
Peter, S., Malhotra, N., Peter, P., and Sood, R. (2013). Isolated Bell's palsy-an unusual presentation of dengue infection. Asian Pac. J. Trop. Med. 6, 82–84. doi: 10.1016/S1995-7645(12)60207-7
Pierre Filho Pde, T., Carvalho Filho, J. P., and Pierre, E. T. (2008). Bilateral acute angle closure glaucoma in a patient with dengue fever: case report. Arq. Bras. Oftalmol. 71, 265–268. doi: 10.1590/S0004-27492008000200025
Pimentel, L. H., de Oliveira, G. R., do Vale, O. C., and Gondim Fde, A. (2011). On the spectrum of acute dengue virus myositis. J. Neurol. Sci. 307, 178–179. doi: 10.1016/j.jns.2011.05.018
Puccioni-Sohler, M., Orsini, M., and Soares, C. N. (2012). Dengue: a new challenge for neurology. Neurol Int. 4:e15. doi: 10.4081/ni.2012.e15
Puccioni-Sohler, M., Rosadas, C., and Cabral-Castro, M. J. (2013). Neurological complications in dengue infection: a review for clinical practice. Arq. Neuropsiquiatr.. 71, 667–671. doi: 10.1590/0004-282X20130147
Quek, D. T., Barkham, T., and Teoh, S. C. (2009). Recurrent bilateral dengue maculopathy following sequential infections with two serotypes of dengue virus. Eye 23, 1471–1472. doi: 10.1038/eye.2008.149
Qureshi, N. K., Begum, A., Saha, P. R., and Hossain, M. I. (2012). Guillain–Barre syndrome following dengue fever in adult patient. J. Med. 13, 246–249. doi: 10.3329/jom.v13i2.12772
Rajajee, S., Ezhilarasi, S., and Rajarajan, K. (2005). Benign acute childhood myositis. Indian J. Pediatr. 72, 399–400. doi: 10.1007/BF02731735
Ramos, C., Sánchez, G., Pando, R. H., Baquera, J., Hernández, D., Mota, J., et al. (1998). Dengue virus in the brain of a fatal case of hemorrhagic dengue fever. J. Neurovirol. 4, 465–468. doi: 10.3109/13550289809114548
Ratnayake, E. C., Shivanthan, C., and Wijesiriwardena, B. C. (2012). Diaphragmatic paralysis: a rare consequence of dengue fever. BMC Infect. Dis. 12:46. doi: 10.1186/1471-2334-12-46
Raung, S. L., Chen, S. Y., Liao, S. L., Chen, J. H., and Chen, C. J. (2007). Japanese encephalitis virus infection stimulates Srctyrosine kinase in neuron/glia. Neurosci. Lett. 419, 263–268. doi: 10.1016/j.neulet.2007.04.036
Renganathan, A., Ng, W. K., and Tan, C. T. (1996). Transverse myelitis in association with dengue infection. Neurol. J. Southeast Asia 1, 61–63.
Rigau-Pérez, J. G., Clark, G. G., Gubler, D. J., Reiter, P., Sanders, E. J., and Vorndam, A. V. (1998). Dengue and dengue hemorrhagic fever. Lancet 352, 971–977. doi: 10.1016/S0140-6736(97)12483-7
Rittmannsberger, H., Foff, C., Doppler, S., and Pichler, R. (2010). Psychiatric manifestation of a dengue-encephalopathy [German]. Wien. Klin. Wochenschr. 122, 87–90. doi: 10.1007/s00508-010-1460-8
Rosen, L., and Khin, M. M. U. T. (1989). Recovery of virus from the liver of children with fatal dengue: reflections on the pathogenesis of the disease and its possible analogy with that of yellow fever. Res. Virol. 140, 351–360. doi: 10.1016/S0923-2516(89)80115-3
Roy, A., Tripathi, A. K., Verma, S. P., Reddy, H., and Jain, N. (2011). Acute hypokalaemic quadriparesis in dengue fever. BMJ Case Rep. 2011:3514. doi: 10.1136/bcr.11.2010.3514
Sahu, R., Verma, R., Jain, A., Garg, R. K., Singh, M. K., Malhotra, H. S., et al. (2014). Neurologic complications in dengue virus infection: a prospective cohort study. Neurology 83, 1601–1609. doi: 10.1212/WNL.0000000000000935
Saini, L., Chakrabarty, B., Pastel, H., Israni, A., Kumar, A., and Gulati, S. (2017). Dengue fever triggering hemiconvulsion hemiplegia epilepsy in a child. Neurol. India 65, 636–638. doi: 10.4103/neuroindia.NI_1367_15
Sainte Foie, S., Niel, L., Moreau, J. P., Ast, R., and Chippaux, A. (1993). Un cas de polyradiculonévrite associé a une dengue chez une patiente originaire de la Guyane Française. Bull. Soc. Pathol. Exot. 86, 117–118.
Salazar, M. I., Richardson, J. H., Sánchez-Vargas, I., Olson, K. E., and Beaty, B. J. (2007). Dengue virus type 2: replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol. 7:9. doi: 10.1186/1471-2180-7-9
Sangle, S. A., Dasgupta, A., Ratnalikar, S. D., and Kulkarni, R. V. (2010). Dengue myositis and myocarditis. Neurol. India 58, 598–599. doi: 10.4103/0028-3886.68664
Sanguansermsri, T., Poneprasert, B., and Phornphutkul, B. (1976). Acute Encephalopathy Associated with Dengue Infection. Bangkok: SEAMEO TROPMED, 10–11.
Santos, N. Q., Azoubel, A. C., Lopes, A. A., Costa, G., and Bacellar, A. (2004). Guillain-Barré syndrome in the course of dengue: case report. Arq. Neuropsiquiatr. 62, 144–146. doi: 10.1590/S0004-282X2004000100025
Sarkari, N. B., Thacker, A. K., Barthwal, S. P., Mishra, V. K., Prapann, S., Srivastava, D., et al. (2012a). Japanese encephalitis (JE) part II: 14 years' follow-up of survivors. J. Neurol. 259, 58–69. doi: 10.1007/s00415-011-6131-9
Sarkari, N. B., Thacker, A. K., Barthwal, S. P., Mishra, V. K., Prapann, S., Srivastava, D., et al. (2012b). Japanese encephalitis (JE). Part I: clinical profile of 1,282 adult acute cases of four epidemics. J. Neurol. 259, 47–57. doi: 10.1007/s00415-011-6118-6
Scott, T. F., Frohman, E. M., Seze, J. D., Gronseth, G. S., and Weinshenker, B. G. (2011). Evidence-based guideline: clinical evaluation and treatment of transverse myelitis. Report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology. Neurology 7, 2128–2134. doi: 10.1212/WNL.0b013e31823dc535
Seet, R. C. S., and Lim, E. C. H. (2007). Dysarthria clumsy hand syndrome associated with dengue type-2 infection. J. Neurol. 254, 1129–1130. doi: 10.1007/s00415-006-0458-7
Seet, R. C., Lim, E. C., and Wilder-Smith, E. P. (2006). Acute transverse myelitis following dengue virus infection. J. Clin. Virol. 35, 310–312. doi: 10.1016/j.jcv.2005.08.006
Sharma, C. M., Kumawat, B. L., Ralot, T., Tripathi, G., and Dixit, S. (2011). Guillain Barre syndrome occurring during dengue fever. J. Indian Med. Assoc. 109, 675–682.
Shivanthan, M. C., Ratnayake, E. C., Wijesiriwardena, B. C., Somaratna, K. C., and Gamagedara, L. K. (2012). Paralytic squint due to abducens nerve palsy: a rare consequence of dengue fever. BMC Infect. Dis. 12:156. doi: 10.1186/1471-2334-12-156
Simmons, C. P., Farrar, J. J., Nguyen v, V., and Wills, B. (2012). Dengue. N. Engl. J. Med. 366, 1423–1432. doi: 10.1056/NEJMra1110265
Simon, O., Billot, S. D., Guyon, D., Daures, M., Descloux, E., Gourinat, A. C., et al. (2016). Early Guillain–Barré Syndrome associated with acute dengue fever. J. Clin. Virol. 77, 29–31. doi: 10.1016/j.jcv.2016.01.016
Sindic, C. J., Van Antwerpen, M. P., and Goffette, S. (2001). The intrathecal humoral immune response: laboratory analysis and clinical relevance. Clin. Chem. Lab. Med. 39, 333–340. doi: 10.1515/CCLM.2001.052
Singh, M., Garg, K., Bisht, A., Sharma, B. S., Singh, P. K., Pandia, M., et al. (2013). Spinal epidural hematoma with myelitis and brainstem hemorrhage: an unusual complication of dengue fever. Neurol. India 61, 541–543. doi: 10.4103/0028-3886.121946
Sirivichayakul, C., Sabcharoen, A., Chanthavanich, P., Pengsaa, K., Chokejindachai, W., and Prarinyanupharb, V. (2000). Dengue infection with unusual manifestations: a case report. J. Med. Assoc. Thai. 83, 325–329.
Siriyakorn, N., and Insiripong, S. (2015). Fatal rhabdomyolysis in dengue hemorrhagic fever: a case report. Southeast Asian J. Trop. Med. Public Health. 46(Suppl. 1), 149–152.
Sistayanarain, A., Maneekarn, N., Polprasert, B., Sirisanthana, V., Makino, Y., Fukunaga, T., et al. (1996). Primary sequence of the envelope glycoprotein of a dengue type 2 virus isolated from patient with dengue hemorrhagic fever and encephalopathy. Southeast Asian J. Trop. Med. Public Health 27, 221–227.
Smart, K., and Safitri, I. (2009). Evidence behind the WHO Guidelines: Hospital care for children: what treatments are effective for the management of shock in severe dengue? J. Trop. Pediatr. 55, 145–148. doi: 10.1093/tropej/fmp046
Soares, C. N., and Marzia, P. S. (2014). Diagnosis criteria of dengue encephalitis. Arq. Neuropsiquiatr. 72:263. doi: 10.1590/0004-282X20130251
Soares, C. N., Cabral-Castro, M. J., Peralta, J. M., de Freitas, M. R., Zalis, M., and Puccioni-Sohler, M. (2011). Review of the etiologies of viral meningitis and encephalitis in a dengue endemic region. J. Neurol. Sci. 303, 75–79. doi: 10.1016/j.jns.2011.01.012
Soares, C. N., Cabral-Castro, M. J., Peralta, J. M., Freitas, M. R., and Puccioni-Sohler, M. (2010). Meningitis determined by oligosymptomatic dengue virus type 3 infection: report of a case. Int. J. Infect. Dis. 14, e150–e152. doi: 10.1016/j.ijid.2009.03.016
Soares, C. N., Cabral-Castro, M., Oliveira, C., Faria, L. C., Peralta, J. M., Freitas, M. R., et al. (2008). Oligosymptomatic dengue infection:potential cause of Guillain Barré syndrome. Arq. Neuropsiquiatr. 66, 234–237. doi: 10.1590/S0004-282X2008000200018
Soares, C. N., Faria, L. C., Peralta, J. M., de Freitas, M. R., and Puccioni-Sohler, M. (2006). Dengue infection: neurological manifestations and cerebrospinal fluid (CSF) analysis. J. Neurol. Sci. 249, 19–24. doi: 10.1016/j.jns.2006.05.068
Solbrig, M. V., and Perng, G. C. (2015). Current neurological observations and complications of dengue virus infection. Curr. Neurol. Neurosci. Rep. 15:29. doi: 10.1007/s11910-015-0550-4
Solomon, T., Dung, N. M., Kneen, R., Gainsborough, M. W., Vaughn, D., and Khanh, V. T. (2000a). Japanese encephalitis. J. Neurol. Neurosurg. Psychiatry 68, 405–415. doi: 10.1136/jnnp.68.4.405
Solomon, T., Minh Dung, N., Vaughn, D. W., Kneen, R., Thao, L. T., Raengsakulrach, B., et al. (2000b). Neurological manifestations of dengue infection. Lancet 355, 1053–1059. doi: 10.1016/S0140-6736(00)02036-5
Srivastava, S., Bhatia, M. S., and Jhanjee, A. (2013). Organic mania in dengue. J. Clin. Diagn. Res. 7, 566–567. doi: 10.7860/JCDR/2013/4891.2827
Srivastava, V. K., Suri, S., Bhasin, A., Srivastava, L., and Bharadwaj, M. (1990). An epidemic of dengue haemorrhagic fever and dengue shock syndrome in Delhi: a clinical study. Ann. Trop. Paed. 10, 329–334. doi: 10.1080/02724936.1990.11747453
Su, D. H., Bacsal, K., Chee, S. P., Flores, J. V., Lim, W. K., Cheng, B. C., et al. (2007). Prevalence of dengue maculopathy in patients hospitalized for dengue fever. Ophthalmology 114, 1743–1747. doi: 10.1016/j.ophtha.2007.03.054
Sulekha, C., Kumar, S., and Philip, J. (2004). Guillain Barré syndrome following dengue fever: report of 3 cases. Indian Pediatr. 41, 948–950.
Sumarmo, W. H., Jahja, E., Gubler, D. J., Suharyono, W., and Sorensen, K. (1983). Clinical observation on virologically confirmed fatal dengue infections in Jakarta, Indonesia. Bull. World Health Organ. 61, 693–701.
Sundaram, C., Shantveer, G., Uppin, S. G., Dakshinamurthy, K. V., and Borgahain, R. (2010). Acute disseminated encephalomyelitis following dengue hemorrhagic fever. Neurol. India 58, 599–601. doi: 10.4103/0028-3886.68666
Swarup, V., Ghosh, J., Das, S., and Basu, A. (2008). Tumor necrosis factor receptor-associated death domain mediated neuronal death contributes to the glial activation and subsequent neuroinflammation in Japanese encephalitis. Neurochem Int. 52, 1310–1321. doi: 10.1016/j.neuint.2008.01.014
Tan, C. S., Teoh, S. C., Chan, D. P., Wong, I. B., and Lim, T. H. (2007). Dengue retinopathy manifesting with bilateral vasculitis and macular oedema. Eye 21, 875–877. doi: 10.1038/sj.eye.6702748
Tantawichien, T. (2012). Dengue fever and dengue haemorrhagic fever in adolescents and adults. Paediatr. Int. Child Health 32(Suppl. 1), 22–27. doi: 10.1179/2046904712Z.00000000049
Teixeira, M. G., Siqueira, Jr. J. B., Ferreira, G. L., Bricks, L., and Joint, G. (2013). Epidemiological trends of dengue disease in Brazil (2000–2010): a systematic literature search and analysis. PLoS Negl. Trop. Dis. 7:e2520. doi: 10.1371/journal.pntd.0002520
Teoh, S. C., Chan, D. P., Nah, G. K., and Rajesh, R. (2006). A re-look at ocular complications in dengue fever and dengue haemorrhagic fever. Dengue Bull. 30, 184–193.
Thisyakorn, U., and Thisyakorn, C. (1994). Dengue infection with unusual manifestations. J. Med. Assoc. Thai. 77, 410–413.
Thisyakorn, U., Thisyakorn, C., Limpitikul, W., and Nisalak, A. (1999). Dengue infection with central nervous system manifestations. Southeast Asian J. Trop. Med. Public Health 30, 504–506.
Thongtan, T., Thepparit, C., and Smith, D. R. (2012). The Involvement of Microglial Cells in Japanese Encephalitis Infections. Clin. Dev. Immunol. 2012:890586. doi: 10.1155/2012/890586
Umapathi, T., Lim, C. S., Ooi, E. E., Zhang, S. L., Goh, E. J., Tan, H. C., et al. (2016). Asymptomatic dengue infection may trigger Guillain-Barré syndrome. J. Peripher. Nerv. Syst. 21, 375–377. doi: 10.1111/jns.12190
Vargas-Sánchez, A., Chiquete, E., Gutiérrez-Plascencia, P., Casta-eda-Moreno, V., Alfaro-Castellanos, D., Paredes-Casillas, P., et al. (2014). Cerebellar hemorrhage in a patient during the convalescent phase of dengue fever. J. Stroke 16, 202–204. doi: 10.5853/jos.2014.16.3.202
Verhagen, L. M., and de Groot, R. (2014). Dengue in children. J. Infect. 69(Suppl. 1), S77–S86. doi: 10.1016/j.jinf.2014.07.020
Verma, R., Sahu, R., Singh, A. S., and Atam, V. (2013). Dengue infection presenting as ischemic stroke: an uncommon neurological manifestation. Neurol. India 61, 317–318. doi: 10.4103/0028-3886.115083
Verma, R., Sharma, P., Khurana, N., and Sharma, L. N. (2011b). Neuralgic amyotrophy associated with dengue fever: case series of three patients. J. Postgrad. Med. 57, 329–331. doi: 10.4103/0022-3859.90086
Verma, S. P., Himanshu, D., Tripathi, A. K., Vaish, A. K., and Nirdesh, J. (2011a). An atypical case of dengue haemorrhagic fever presenting as quadriparesis due to compressive myelopathy. BMJ Case Rep. 2011:3421. doi: 10.1136/bcr.10.2010.3421
Viswanathan, S., Botross, N., Rusli, B. N., and Riad, A. (2016). Acute disseminated encephalomyelitis complicating dengue infection with neuroimaging mimicking multiple sclerosis: a report of two cases. Mult. Scler. Relat. Disord. 10, 112–115. doi: 10.1016/j.msard.2016.10.001
Weeratunga, P. N., Caldera, H. P., Gooneratne, I. K., Gamage, R., Perera, W. S., Ranasinghe, G. V., et al. (2014b). Spontaneously resolving cerebellar syndrome as a sequelae of dengue viral infection: a case series from Sri Lanka. Pract. Neurol. 14, 176–178. doi: 10.1136/practneurol-2013-000571
Weeratunga, P. N., Caldera, M. C., Goonerate, I. K., Gamage, R., and Perera, P. (2014a). Neurological manifestations of dengue: a cross sectional study. Travel Med. Infect. Dis. 12, 189–193. doi: 10.1016/j.tmaid.2013.11.001
WHO (2009). Dengue Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva: World Health Organization.
Wolf, V. L., Lupo, P. J., and Lotze, T. E. (2012). Pediatric acute transverse myelitis overview and differential diagnosis. J. Child Neurol. 27, 1426–1436. doi: 10.1177/0883073812452916
World Health Organization (1997). Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control, 2nd Edition. Geneva: WHO.
Yamamoto, Y., Takasaki, T., Yamada, K., Kimura, M., Washizaki, K., Yoshikawa, K., et al. (2002). Acute disseminated encephalomyelitis following dengue fever. J. Infect. Chemother. 8, 175–177. doi: 10.1007/s101560200030
Ying, R. S., Tang, X. P., Zhang, F. C., Cai, W. P., Chen, Y. Q., Wang, J., et al. (2007). Clinical characteristics of the patients with dengue fever seen from 2002 to 2006 in Guangzhou [in Chinese]. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 21, 123–125.
Keywords: dengue, neurological complications, neuropathogenesis, in children, manifestations, treatment, prevention
Citation: Li G-H, Ning Z-J, Liu Y-M and Li X-H (2017) Neurological Manifestations of Dengue Infection. Front. Cell. Infect. Microbiol. 7:449. doi: 10.3389/fcimb.2017.00449
Received: 18 August 2017; Accepted: 04 October 2017;
Published: 25 October 2017.
Edited by:
Gong Cheng, Tsinghua University, ChinaReviewed by:
Xin Zhao, Institute of Microbiology (CAS), ChinaLong Yang, New York Medical College, United States
Jianfeng Dai, Soochow University, China
Copyright © 2017 Li, Ning, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi-Ming Liu, liuym@sdu.edu.cn
Xiao-Hong Li, xiaohong-li@sdu.edu.cn
 Guo-Hong Li
Guo-Hong Li Zhi-Jie Ning
Zhi-Jie Ning Yi-Ming Liu
Yi-Ming Liu Xiao-Hong Li
Xiao-Hong Li