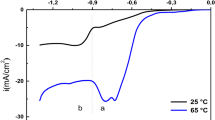
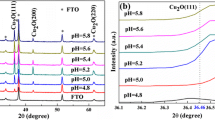
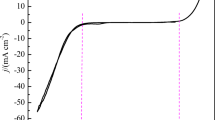
Abstract
In this paper, we report the kinetics, nucleation, and growth mechanism of copper oxide electrodeposited on ITO from copper sulfate and lactic acid solutions at different pH values, using cyclic voltammetry (CV), chronoamperometry (CA), and electrochemical impedance spectroscopy (EIS). According to the cyclic voltammetry, the cathodic peak potential increases with increasing scan rate, indicating that the deposition of Cu2O is an irreversible process influenced by diffusion control. To further investigate the type of Cu2O nucleation, it was found that Cu2O followed diffusion-controlled three-dimensional transient nucleation by comparing the timing current curves with the Scharifker-Hills three-dimensional nucleation model. Furthermore, the three-dimensional transient nucleation was still followed regardless of the deposition potential and pH variation; this result was also confirmed from field emission scanning electron microscopy (FE-SEM). According to the EIS profile, the charge transfer resistance of Cu2O decreased with the increase in deposition potential; the charge transfer resistance was the smallest at pH = 9. In the timing current curve D (diffusion coefficient) showed a decreasing trend with increasing pH values. The nucleation pattern and growth process of nuclei at the early stage of electrodeposition are the basis for the formation of thin films, which is a guideline for obtaining films with better performance.





Similar content being viewed by others
References
Hu C-C, Nian J-N, Teng H (2008) Electrodeposited p-type Cu2O as photocatalyst for H2 evolution from water reduction in the presence of WO3. Sol Energy Mater Sol Cells 92(9):1071–1076
Sui Y, Zeng Y, Fu L et al (2013) Low-temperature synthesis of porous hollow structured Cu2O for photocatalytic activity and gas sensor application. RSC Adv 40(3)
Liu C, Zhou X, Chen S et al (2019) Hydrophobic Cu2O quantum dots enabled by surfactant modification as top hole-transport materials for efficient perovskite solar cells. Adv Sci (Weinh) 6(7):1801169
Rej S, Bisetto M, Naldoni A et al (2021) Well-defined Cu2O photocatalysts for solar fuels and chemicals. J Mater Chem A 9(10):5915–5951
Minami T, Nishi Y, Miyata T (2016) Efficiency enhancement using a Zn1−xGex-O thin film as an n-type window layer in Cu2O-based heterojunction solar cells. Appl Phys Exp 9(5)
Bergerot L, Jiménez C, Chaix-Pluchery O et al (2015) Growth and characterization of Sr-doped Cu2O thin films deposited by metalorganic chemical vapor deposition. Physica Status Solidi (a) 212(8):1735–1741
Ma J, Guo S, Guo X et al (2015) Preparation, characterization and antibacterial activity of core–shell Cu2O@Ag composites. Surf Coat Technol 272:268–272
Minami T, Nishi Y, Miyata T (2013) High-efficiency Cu2O-based heterojunction solar cells fabricated using a Ga2O3 thin film as n-type layer. Appl Phys Exp 6(4)
Yang M, Zhu L, Li Y et al (2013) Asymmetric interface band alignments of Cu2O/ZnO and ZnO/Cu2O heterojunctions. J Alloy Compd 578:143–147
Mittiga A, Salza E, Sarto F et al (2006) Heterojunction solar cell with 2% efficiency based on a Cu2O substrate. Appl Phys Lett 88(16)
Lei J, Yang J-G (2017) Electrochemical mechanism of tin membrane electro-deposition in chloride solutions. J Chem Technol Biotechnol 92(4):861–867
Bouderbala IY, Herbadji A, Mentar L et al (2017) Optical properties of Cu2O electrodeposited on FTO substrates: Effects of Cl concentration. J Electron Mater 47(3):2000–2008
Sun F, Guo Y, Song W et al (2007) Morphological control of Cu2O micro-nanostructure film by electrodeposition. J Cryst Growth 304(2):425–429
Wang LC, De Tacconi NR, Chenthamarakshan CR et al (2007) Electrodeposited copper oxide films: Effect of bath pH on grain orientation and orientation-dependent interfacial behavior. Thin Solid Films 515(5):3090–3095
Yang Y, Han J, Ning X et al (2014) Controllable morphology and conductivity of electrodeposited Cu2O thin film: effect of surfactants. ACS Appl Mater Interfaces 6(24):22534–22543
Han J, Chang J, Wei R et al (2018) Mechanistic investigation on tuning the conductivity type of cuprous oxide (Cu2O) thin films via deposition potential. Int J Hydrogen Energy 43(30):13764–13777
Miao X, Wang S, Sun W et al (2019) Room-temperature electrochemical deposition of ultrathin CuOx film as hole transport layer for perovskite solar cells. Scripta Mater 165:134–139
Yu X, Tang X, Li J et al (2017) Nucleation mechanism and optoelectronic properties of Cu2O onto ITO Electrode in the electrochemical deposition process. J Electrochem Soc 164(14):D999–D1005
Herbadji A, Bouderbala IY, Mentar L et al (2020) Effect of copper sulfate concentration on the electrochemical nucleation process, growth and properties of n-Type Cu2O Thin Films. Russ J Electrochem 55(12):1336–1349
Mech K, Bisztyga-Szklarz M, Szaciłowski K (2020) Brief insights into Cu2O electrodeposition: Detailed progressive voltammetric and electrogravimetric Analysis of a Copper Lactate System. J Electrochem Soc 167(4)
Ait Himi M, El Ghachtouli S, Youbi B et al (2020) Nucleation and growth mechanism of manganese oxide electrodeposited on ITO substrate. Materials Today: Proceedings 30:963–969
Nath P, Pandey R, Das A et al (2017) Effect of deposition potential and copper concentration on the phase transformation mechanism and structural distribution during electrodeposition of Ni-Cu magnetic alloy thin films. Metallic Materials Kovove Mater 55(4):255–265
Achilli E, Vertova A, Visibile A et al (2017) Structure and stability of a copper(II) Lactate Complex in alkaline solution: a Case study by energy-dispersive x-ray absorption spectroscopy. Inorg Chem 56(12):6982–6989
Chen T, Kitada A, Fukami K et al (2019) Determination of stability constants of copper(II)–lactate complexes in Cu2O electrodeposition baths by UV-vis absorption spectra factor analysis. J Electrochem Soc 166(15):D761–D767
Chen T, Kitada A, Seki Y et al (2018) Identification of copper(II)–lactate complexes in Cu2O electrodeposition baths: deprotonation of the α-hydroxyl group in highly concentrated alkaline solution. J Electrochem Soc 165(10):D444–D451
Luo Z, Su Y, Yue S et al (2021) Electrodeposition of copper nanopowder with controllable morphology: influence of pH on the nucleation/growth mechanism. J Solid State Electrochem 25(5):1611–1621
Scharifker B, Hills G (1983) Theoretical and experimental studies of multiple nucleation. Electrochim Acta 28(7):879–889
Hills G, Scharifker B, Montenegro I et al (1982) Electrochemical nucleation: Part I. General considerations. Electroanal Chem Interfac Electrochem 138(2):225–239
Bahar J, Lghazi Y, Youbi B et al (2022) Nucleation and growth mechanism of cuprous oxide electrodeposited on ITO substrate. Materials Today: Proceedings
Tarallo LHA (1999) Theory of the chronoamperometric transient for electrochemical nucleation with diffusion-controlled growth. J Electroanal Chem 470(1):70–76
Zhang J, An M, Chen Q et al (2016) Electrochemical study of the diffusion and nucleation of gallium(III) in [Bmim][TfO] ionic liquid. Electrochim Acta 190:1066–1077
Wadhene R, Assaker IB, Chtourou R (2018) Effect of sulfur quantity in post-treatment on physicals and electrical properties of electrodeposited Kesterite Cu2ZnSnS4 thin films. J Mater Sci: Mater Electron 29(20):17374–17387
Funding
This work was supported by the National Natural Science Foundation of China (no. 52174354).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Min, C., Li, S., Shi, Z. et al. Effect of pH on the electrodeposition nucleation and growth mechanism of cuprous oxide. J Solid State Electrochem 27, 1085–1093 (2023). https://doi.org/10.1007/s10008-023-05408-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-023-05408-x