Abstract
In the present study, the nano-silver-doped flax fibers (NAgDFF) are prepared in two steps. In the first step, oxidation of the flax fibers is performed by potassium periodate to form dialdehyde cellulose (DAC) and the second step is the reduction of silver ions by DAC. A series of characterization techniques of the photocatalyst NAgDFF was carried out using scanning electron microscopy, Fourier transform infrared spectroscopy, N2 adsorption isotherm, thermogravimetric analysis and energy-dispersive X-ray spectroscopy. The dye degradation potential of NAgDFF for methylene blue (MB), crystal violet (CV) and brilliant green (BG) (individually or mixture) was investigated using batch and column tests. The degradation efficiency was studied under optimized conditions such as pH (5.0), dye initial concentrations (100 ppm for MB and BG, and 150 ppm for CV), contact time (3.0 h), photocatalyst NAgDFF dose (0.08 g) and temperature (25° C). The maximum degradation efficiency of NAgDFF for MB, CV and BG is 64.75, 94.98 and 63.87 (mg/g), respectively. The kinetic studies show that the experimental data match well with the pseudo-second-order kinetic model. Furthermore, equilibrium isotherm data were analyzed according to Langmuir, Freundlich and Dubinin–Radushkevich equations. The thermodynamic parameters for the adsorption processes of cationic dyes on the NAgDFF fibers were also calculated; the negative value of ΔG° indicated the spontaneous nature of sorption. NAgDFF fibers were successfully applied for photodegradation of the investigated cationic dyes from different samples. The study was extended to investigate the biological activity of newly synthesized NAgDFF against various microorganisms.
Similar content being viewed by others
Introduction
Wastewater, generated by various industries, contains multiple contaminants such as dye, heavy metals, antibiotics and pesticides [1, 2]. Effluent generated by the textile, plastic, paper, food, tanneries and pharmaceutical industries contains toxic chemical dyes as one of the major pollutants along with other toxic chemical components [3, 4]. Azo, basic, acidic, cationic, etc., are some of the major dyes that are used by industries to impart color [5]. However, these synthetic dyes are highly toxic, carcinogenic and mutagenic in nature [2, 6]. Industries directly discharge dye-containing wastewater to nearby water bodies such as river, lake and drain [7, 8]. Moreover, in some cases wastewater generated by the various industries is directly used for the irrigational purposes and such practice deteriorates the quality of crops as well as soil [9]. The presence of dye molecule in the water decreases the sunlight penetration into the water bodies affecting aquatic living organism [10]. Hence, proper treatment of dye-containing wastewater is very important before its discharge to nearby water bodies. Degradation of dye molecule to non-toxic component is difficult due to its chemical structure which provides high stability to the dye molecule.
For the last two decades, photocatalytic degradation has been considered a promising method for dye sewage treatment. It has many advantages as it is a solar-driven method; it has no secondary contamination as it can completely mineralize organic dyes into H2O, CO2 and mineral acids. The used material in the photocatalytic degradation process is called a photocatalyst. These materials have a lot of advantages as they are cheap, eco-friendly, effective and can be reused. Semiconductors, metal oxides nanoparticles and metal oxides doped and blended materials are examples of photocatalysts [11,12,13,14,20].
Flax (Linum usitatissimum) is a natural plant fiber. Flax fiber is a cheap, renewable and available material. Flax fiber as other natural fibers can be used in industrial applications such as textiles additives, and in increasing the biodegradability of polymers, etc. Those fibers are used in water treatment due to their availability, cheapness and simplicity. Plants fibers mainly consist of three polymers which are cellulose, hemicellulose and lignin [21,22,23]. In nature, cellulose is considered the most available polymer. It is the main component in plant fiber construction. It is used for the removal of many pollutants like dyes, emulsified organic solvents, heavy metals and oils from water, so it is utilized in water treatment [24].
Nano-silver is a silver oxide material with a large surface area. Nano-silver has both catalytic and antibacterial activities. It can be used as a photocatalyst material in the water treatment from dyes. Its antibacterial activity returns to its positive charge [13, 25,26,27,28,29,30,31,32].
In this work, we report the sorption ability, photocatalytic and antimicrobial properties of novel nano-silver-doped flax fibers (NAgDFF). To the best of our knowledge, sorption, photocatalytic and antimicrobial behaviors of NAgDFF have not yet been explored. Hence, it was of great interest to synthesize these materials which can be used for sorption—photocatalytic degradation of organic pollutants under exposure to light.
For the sorption–photocatalytic degradation studies, common dyes like methylene blue (MB), crystal violet (CV) and brilliant green (BG) were selected. These are triarylmethane/azo dyes which are extensively used in textile and food industries as coloring agents. This is the first report to study the improved degradation potential of chemically synthesized nano-silver-doped flax fiber when used for methylene blue, crystal violet and brilliant green, individually as well as in mixture (simultaneously).
This work can be divided into six major steps: (i) the preparation of NAgDFF; (ii) characterization of NAgDFF using various instrumental performances as Fourier transform infrared spectroscopy (FTIR), energy-dispersive X-ray spectroscopy (EDX), thermal analysis (TGA and DTG), scanning electron microscopy (SEM) and Brunauer–Emmett–Teller (BET); (iii) batch and column photocatalytic degradation experiments utilizing cationic dyes (methylene blue, crystal violet and brilliant green) as pollutants; (iv) studying the optimum parameters like pH, the initial dye concentration, photocatalyst dose, temperature (isotherms and thermodynamics) and contact time; (v) studying the NAgDFF’s biological activity; and (vi) fibers’ regeneration.
Materials and methods
Materials
Flax fibers were obtained from Tanta flax and oil company, Tanta, Egypt. The fibers were treated before using, by washing with bi-distilled water and absolute ethanol. Potassium periodate, HCl, NaOH, methylene blue (MB), crystal violet (CV), brilliant green (BG), AgNO3 and triethylamine were purchased from Sigma-Aldrich.
Preparation of the nano-silver-doped flax fibers (NAgDFF)
In the beginning, 1.0 g of the pretreated flax fiber was shaken with 2.0 g of KPI4 dissolved in 100 ml dist.H2O for 4.0 hours in the presence of sunlight at 45 °C. Then, the obtained oxidized flax fiber was washed several times with dist.H2O and was dried in the oven at 40 °C. Then, dried light oxidized flax fiber was shaken with 50 ml of 1.0 M AgNO3 for 18 hours. At last, fibers were filtrated and washed several times with dist.H2O and dried in an oven at 40 °C. The prepared NAgDFF is yellowish in color, insoluble in water and partially soluble in methanol. Preparation of NAgDFF is schematically represented in Scheme 1.
Instrumentation
Scanning electron microscopy (SEM)
Surface morphologies of the prepared samples were investigated using scanning electron microscopy (SEM, model JSM-T 220A, JEOL, Japan) at an accelerated voltage 20 kv
Energy-dispersive X-ray spectroscopy (EDX)
Energy-dispersive X-ray spectroscopy (EDX) was used in the determination of chemical composition. The samples were pressed onto aluminum foil attached to the sample mount. The analysis was carried out with a Hitachi SEM 3200 electron microscope equipped with an EDS detector.
Brunauer, Emmett and Teller (BET) and N2 adsorption isotherm
BET was applied to measure the specific surface area of the samples using (Quantachrome St 3 on NOVA touch 4LX) by gaseous N2 adsorption at − 196 °C.
Fourier Transform Infrared Spectroscopy (FTIR)
Fourier transform infrared spectroscopy (FTIR) analysis of samples was taken on a Spectrum One FTIR spectrometer (Perkin–Bhaskar–Elmer Co., USA). Samples, in powder form, were pulverized and mixed with KBr. The mixture was dried and pressed to form pellets which were used to record the IR spectra.
Thermogravimetric analysis (TGA)
Thermograms of prepared NAgDFF were recorded using thermogravimetric analyzer (TGA-50 Shimadzu), using aluminum pan between a temperature range 30–510 °C at a heating rate of 10 °C using inert atmosphere of N2 at a flow rate of 20 ml/min.
UV- visible spectrophotometry
Concentrations of cationic dyes were determined at λmax 665 nm, 590 nm and 625 nm for MB, CV and BG, respectively, using a Perkin Elmer 550 spectrophotometer in 1 cm quartz cell in ethanol; over a range of 200–900 nm.
Biological activity studies of NAgDFF
The antibacterial activity of NAgDFF was tested against two Gram-positive bacteria (Staphylococcus aureus and Bacillus subtilis) and two Gram-negative bacteria (E. coli and P. aeruginosa). NAgDFF fibers were dissolved in DMSO and a solution of a 1 mg /ml was prepared. Paper disks of Whatman filter paper prepared with standard size (5 mm) were cut and sterilized in an autoclave. The paper disks soaked in the desired concentration of the NAgDFF solution were placed aseptically in petri dishes containing nutrient agar media (agar 20 g + beef extract 3 g + peptone 5 g) seeded with Staphylococcus aureus, Bacillus subtilis, E. coli and P. aeruginosa. Petri dishes were incubated at 36 °C and the diameters of inhibition zones were recorded after complete one-day incubation. Each treatment was replicated three times. Antibacterial activities of a common standard antibiotic amoxicillin were also recorded using the same procedure as above at the same concentration and solvents. The % activity index (A) for the complex was calculated by the formula, Eq. 1. [33,34,35,36].
Photocatalytic degradation procedures
Batch procedure
The data are given on the simple mean of three replicates, whereas those of applications were repeated five times from which the statistical evaluation could be derived.
Prior to dye degradation experiment, dye solution was scanned between 190 and 1100 nm to obtain λmax or absorbance maxima. Dye degradation studies (individually and simultaneously) were performed with three different dyes at concentrations ranging from 10 to 400 mg/L. For dye mixture degradation experiment, the three dyes were mixed: 150 ppm CV, 100 ppm MB and 100 ppm BG. Degradation experiments were carried out in 125 ml transparent stoppered bottles that contained 25 ml of dye solution and photocatalyst NAgDFF dose (0.08 g). Then, the stoppered bottles were shaken at 150 rpm on a thermostated shaker at room temperature, in sunlight. After equilibrium, the contents of bottles were centrifuged. The supernatant liquid that contains the remains of dye was measured. Samples after degradation were scanned regularly between 200 and 900 nm at different time intervals to obtain the lambda maximum.
Various parameters were studied such as contact time (15–300 min), temperature (25–45 °C), photocatalyst NAgDFF dose (0.025–0.2 g), pH (2–8) and initial concentration of dye (25–400 mg/l). The degradation percentage (D %) of dyes in term of percentage was calculated as per Eq. 2. [37, 38].
where Ci is the initial concentration of the dye before the degradation and Cf is the final concentration of the dye after the degradation.
Effect of pH
A 0.08 g of NAgDFF was added to a series of the stoppered transparent bottles that contain 50 mL of the dye solution with initial dye concentration (100 ppm for MB and BG and 150 ppm for CV) in the presence of sunlight. The initial pH was adjusted to particular values (2, 3, 4, 5, 6, 7 and 8) using 0.1 M HCl and/or 0.1 M NaOH. The stoppered bottles were shaken in an equilibrated shaker with shaking speed 150 rpm, in the presence of sunlight and at 25 °C for 4 hours. At the previous conditions, the uptake experiments were performed. The residual of the dyes was measured as presented in the procedures' section.
Effect of initial dye concentration
A 0.08 g of NAgDFF was added to a series of the stoppered transparent bottles containing 50 mL of the dye solution. The initial dye concentration was varied (C0 = 25, 50, 100, 150, 200 and 400 ppm). The stoppered transparent bottles were put in thermostated shaker with shaking speed 150 rpm in the presence of sunlight and at 25°C for 4 hours. By using the mass balance equation (Eq. 3), the equilibrium dye concentration in the solid phase (Qe) was calculated. [39, 40]
where v (L) is volume of dye solution, m (gram) is the photocatalyst mass, Ceq (ppm) is the dye concentration at equilibrium, Co (ppm) is the dye initial concentration, and Qe (mg/g) is the uptake value.
The linear form of the Langmuir, Freundlich and Dubinin–Radushkevich equations are given by (Eq. 4), (Eq. 5) and (Eq. 6) isotherm equations, respectively.
where Ce (ppm) is the concentration of the dye at equilibrium, qe (mg/g) is the capacity of the dye concentration at equilibrium, qm (mg/g) adsorption maximum amount, 1/n, KL, KF and K are heterogeneity factor, Langmuir coefficient (L/mg), Freundlich constant (mg g− 1) and Dubinin–Radushkevich constant, respectively. ε is the adsorption potential and is given by (Eq. 7).
where R (8.314 J/mol K) is the gas constant, and T is the temperature in kelvin.
Effect of the contact time (Degradation kinetics)
The kinetic studies were done by putting 0.08g NAgDFF fibers in a series of stoppered transparent bottles containing 50 ml (100 ppm for MB and BG, and 150 ppm for CV) at optimum pH (5) condition and at 25 °C with shaking time ranging between (1–5 hours) at 150 rpm in the presence of sun light.
To understand the mechanism of the degradation process and determine the degradation rate-limiting step, kinetic studies were performed using the pseudo-first-order (Eq. 8) and the pseudo-second-order (Eq. 9) models.
where qe (mg/g) and qt (mg/g) are degradation efficiency at equilibrium and at time t (min), respectively. K1 is the pseudo-first-order degradation rate constant and K2 is the pseudo-second-order degradation rate constant.
For both models, k and qe were usually calibrated together and close to the experimental degradation data in addition to the use of the correlation coefficient to evaluate the best kinetic model that fits with the experimental work.
Effect of dose of the photocatalyst NAgDFF
To evaluate the impact of the photocatalyst amount, particular masses of NAgDFF fiber were added to each stoppered transparent bottle. Different masses (0.025, 0.05, 0.075, 0.1, 0.15 and 0.2 g) were added to 6 stoppered transparent bottles containing 50 mL of the dye solution. The initial dye concentration was (100 ppm for MB and BG, and 150 ppm for CV) and without pH adjustment. Then, the stoppered transparent bottles were placed into thermostat shaker with shaking speed of 150 rpm and at 25 °C for 4 hours in the presence of the sunlight. The dye’s concentration in the solid phase (Qe) at equilibrium was calculated utilizing (Eq. 3).
Effect of temperature
A series of stoppered transparent bottles containing 50 ml of cationic dyes’ solution (100 ppm for MB and BG, and 150 ppm for CV) and 0.08 g of NAgDFF, at pH (5), were shaken for 4 hours in an equilibrated shaker at 150 rpm, while temperature was kept between (25–45) °C in the presence of light. Residual concentration of dye was determined after photocatalytic degradation and filtration.
By using (Eq. 10) and (Eq. 11), the thermodynamic parameters such as free energy (ΔGo), enthalpy (ΔHo) and entropy (ΔSo) have been calculated.
where R (8.314 J/mol K) is the gas constant, the values of ΔHo were calculated from the slope (−ΔHo/R) of ln Kc vs. 1/T, and ΔSo was calculated from the intercept (ΔSo/R) of ln Kc vs. 1/T.
Column procedure
NAgDFF fibers were applied as a filter in the presence of sunlight as a light source. Columns with different diameters (0.7 cm and 0.9 cm) were packed with NAgDFF fibers as a thin layer in the bottom without adjusting the pH. Some parameters affecting the degradation efficiency were studied such as column diameter, fiber weight and flow rate. The degradation percentage was calculated as in (Eq. 2). The concentration of each dye after the filtration process was determined spectrophotometrically, each one at its specific λmax.
Effect of photocatalyst NAgDFF dose
Columns with 0.7 cm diameter were packed with different doses (0.15–0.25 g) of NAgDFF fibers. Cationic dye solutions of 100 ppm of each are allowed to pass through each column with a flow rate (0.166 ml/min) in the presence of sunlight as a UV source.
Effect of column diameter
Columns with different diameters (0.7 cm and 0.9 cm) are packed with a fixed dose (0.2 g) of NAgDFF fibers. Cationic dye solutions of 100 ppm of each are allowed to pass through each column in the presence of sunlight as a UV source with a fast flow rate of 0.277 ml/min for 0.9 cm column diameter and 0.166 ml/min for 0.7 cm column diameter.
Effect of the flow rate
Columns with diameters 0.7cm are packed with a fixed dose (0.2 g) of NAgDFF fibers. Cationic dye solutions of 100 ppm of each are allowed to pass through each column in the presence of sunlight as a light source at different flow rates (fast: 0.1667ml/min and slow: 0.0625ml/min).
Regeneration studies
The regeneration of NAgDFF was examined by five repeated cycles by the batch method. 0.08 g of NAgDFF was shaken with 50 ml of (100 ppm for MB and BG and 150ppm for CV) for 4 hours; then, the photocatalyst NAgDFF was filtered, and then, it was eluted with 0.2 M HNO3. These procedures were repeated for another 4 cycles. The desorption % of dyes from NAgDFF was calculated from eq. 12.
Sample analysis
Surface natural water samples were collected from the Nile River (Damietta branch, Egypt) and seawater samples (Alexandria City, Egypt). Tap water samples were collected (Mansoura University, Egypt). All samples were filtered using a sintered glass G4 filter. All the selected samples were acidified with concentrated nitric acid to pH ~ 2 and then preserved in polyethylene vessels for further use. The organic matter was digested before the separation process; 0.5–1.0 g of K2S2O8 was added to one liter of the selected water sample and the mixture was heated for 30 min at 95 °C. After cooling to room temperature, 80 mg of NAgDFF was added to a series of transparent stoppered bottles containing different concentrations of cationic dyes 0.0, 50 and 100 ppm at 25 °C and optimum pH conditions. The stoppered bottles were shaken at 150 rpm on an equilibrated shaker for 4 h in the presence of sunlight; then filtered. To the filtrate, another 80 mg of NAgDFF was added and the pH was controlled again. The sample was stirred again for 15 min and filtered. Both filtrates were gathered and collected. The residual concentrations of cationic dyes were measured for five cycles.
Results and discussion
Characterization
Scanning electron microscopy (SEM)
The SEM photographs of the natural flax, oxidized flax and NAgDFF fibers are shown in Fig.1. The rough and narrow strips on the surface of oxidized flax fiber may be ascribed to the action of periodate on the flax fibers during the oxidation. Moreover, the increase in the roughness of the NAgDFF surface showed by silver-doped flax fiber may be attributed to the chemical reaction of the silver nitrate with the oxidized flax fibers.
Energy-dispersive X-ray spectroscopy (EDX)
The results obtained from the EDX of native flax fiber and NAgDFF are displayed in Fig. 2 and Table 1. The results indicate that, upon oxidation and subsequent reduction of silver ion by the oxidized flax fiber, new content of silver appeared showing a good percent of silver metal. This result confirms the formation of nano-silver-doped flax fiber.
Brunauer, Emmett and Teller (BET) and N2 adsorption isotherm
The porosity and the surface area of the native flax fibers and NAgDFF were determined using nitrogen adsorption–desorption isotherms. The results of adsorption isotherms are depicted in (Figure S1), while the calculated parameters are summarized in Table 2. The BET surface area of the native flax fibers was found to be 44.215 m2 g− 1, while that of the NAgDFF fiber was found to be 9.3992 m2 g− 1. The reduction in surface area values of NAgDFF may be attributed to the covering of flax fiber pores by nano-silver which results in reduction of N2 molecules adsorption.
Surface functional groups
FTIR spectra of native, oxidized and NAgDFF fibers
The FTIR spectrum of native flax fibers (Fig. 3) showed certain peaks in the range 1070–1150 cm− 1 which are ascribed to the stretching vibrations of C–O, peaks in the range 1250–1420 cm− 1 attributed to the bending vibrations of O–H and peaks at approximately 3500–3200 cm− 1 due to O–H stretching vibrations. The successive preparation steps of NAgDFF were distinguished by using FTIR spectra; the obtained results are shown in Fig. 4. The FTIR spectrum (Fig. 4a) of the oxidized flax fiber showed an evident peak at about 2905 cm− 1 due to O–H stretching vibration and at about 1730 cm− 1 due to C=O stretching vibration, which is correlated to the new designed carboxylic group stretching vibrations. After treatment with silver nitrate, as shown in (Fig. 4b), O–H stretching vibration peak of the carboxylic group that appears at about 2905 cm− 1 is shifted into lower wave number and lower peak intensity at about 2861 cm− 1 that confirms the formation of NAgDFF.
FTIR of NAgDFF fiber after dyes’ degradation
Figure 5(a–c) represents the IR spectra of the investigated cationic dyes after the process of degradation-adsorption using the prepared NAgDFF fibers. As it can be clearly seen, the IR spectra of the NAgDFF-loaded cationic dyes show differences from that of the NAgDFF. In Fig. 5(a–c), the peak that appeared at 1170 cm− 1 is assigned to C = C of the aromatic rings for the three dyes. The new peaks that appeared in the range from 1266 cm− 1 to 1342 cm− 1 represent the C–N stretching peaks of the three dyes. The new peaks that appeared in the range from 1460 to 1591 cm− 1 are assigned to the aromatic ring structure. The appearance of the previous peaks proves the adsorption and degradation of the dyes by NAgDFF.
Thermogravimetric analysis (TGA)
Thermogravimetric analyses for the oxidized and modified flax fibers were carried out in the temperature range of 30–800 °C to give information about their thermal stability, Figs. 6 and 7. The thermograms demonstrated that each compound undergoes a series of different degradation steps. The thermal degradation of oxidized flax fibers and NAgDFF was studied under the same conditions and the total residues were determined as following 4.1% and 7.67%, respectively. The higher value of total residues of NAgDFF compared to the parent one (oxidized flax fibers) suggests that silver ions show a catalytic degradation effect during their complexation with the oxidized flax fibers.
Biological activity studies of nano-silver-doped flax fibers
The antibacterial activity of NAgDFF was studied against E. coli, P. aeruginosa, S. aureus and Bacillus subtilis bacteria by comparing its inhibition zones with the inhibition zones of Amoxicillin (AMX). As presented in Table 3, NAgDFF showed stronger activity than AMX antibiotic against P. aeruginosa and Bacillus subtilis bacteria.
Mechanism of the antibacterial activity of NAgDFF
Nanoparticles have antibacterial activity against Gram-positive and –Gram-negative bacteria. Its antibacterial activity returns to its positive charge [30]. There are three imaginations for the mechanism of antibacterial activity of NAgDFF:
-
1.
Silver nanoparticles have the ability for penetrating the cell membrane of the bacteria resulting in inactivation of the enzymes and the generation of H2O2 which cause the death of the bacterial cell. Silver nanoparticles interact with the sulfur and phosphorus containing compounds like RNA and DNA [41–43].
-
2.
Production of the free radicals. These free radicals do damage for the bacterial membrane and finally resulting in cell death [44].
-
3.
Silver nanoparticles attach to the cell membrane and disrupt membrane functions like respiration [45, 46].
The proposed mechanism of antimicrobial activity of NAgDFF is represented in Scheme 2
Photocatalytic degradation of cationic dyes using NAgDFF
Digital photographs
Digital photographs of the native cellulose, oxidized cellulose, NAgDFF and the NAgDFF after sorption–photocatalytic degradation of dyes are shown in Fig. 8(a–f). An obvious change in the color of the native cellulose, oxidized and NAgDFF fibers before and after sorption–degradation of the three dyes can be clearly noticed. The yellow-colored native cellulose (Fig. 8a) is changed into brown dialdehyde cellulose (DAC) after oxidation and doping of the nano-silver on the oxidized flax fibers (Fig. 8b). After sorption–degradation of dyes, the color of the NAgDFF was changed from brown to blue in the case of MB (Fig. 8c), to violet in the case of CV (Fig. 8d), to green in the case of BG (Fig. 8e) and to bluish violet color in case of dye mixture (Fig. 8f). These results indicate the tendency of the NAgDFF toward the adsorption of the investigated cationic dyes.
Photocatalytic activity of nano-silver-doped flax fiber
The maximum absorbance (λ max) for CV (590 nm), MB (665 nm), BG (625 nm) and (CV + MB + BG) mixture is presented in (Fig. S2 supplementary material). All the degradation studies were examined at their maximum absorbance.
Figure 9(a–c) represent the degradation of CV, MB and BG, respectively, in the presence of NAgDFF as photocatalytic agent. It was observed that with time the absorbance peaks decrease, highlighting that NAgDFF was able to degrade the three dyes. For crystal violet (CV), the maximum removal (99.56%) was observed at 150 ppm within the time period of 4 h, whereas the minimum removal (70.25%) was observed at 150 ppm within the time period of 15 min. For methylene blue (MB), the maximum removal (98.8) was observed at 100 ppm within the time period 4 h, whereas the minimum removal (10.5%) was observed at 100 ppm within the time period of 15 min. For brilliant green (BG), the maximum removal (98.2) was observed at 100 ppm within the time period 4 h, whereas the minimum removal (57.675%) was observed at 100 ppm within the time period of 15 min.
As actual wastewater contains multiple dyes as contaminants, it is important to examine the dye degradation capability of synthesized NAgDFF in mixtures. For the dye mixture, capability of NAgDFF for dye degradation was tested at 100 ppm MB, 150 ppm CV and 100 ppm BG, and was investigated. Figure 9d shows that when the three dyes were mixed, overlapping of the peaks occurred and two new peaks appeared at 560 nm and 640 nm. It was observed that at time two hours, the peak at 560 nm was disappeared and the absorbance of peak at 640 nm was decreased from 1.9 to 1. After 4 h, the absorbance was decreased to 0.5.
Optimization studies using batch test
Surface chemistry of NAgDFF
Surface chemistry of NAgDFF was examined with the help of point of zero charge (PZC) studies. The point of zero charge (PZC) is important for the characterization of the sorbent as well as illustration of the affinity of the sorbate to sorbent surface. The PZC of NAgDFF was evaluated according to the reported study [47]. The value of pH varied from 2 to 8 and the PZC was determined. Adsorbent surface is neutral near the pH that corresponds to PZC, while it is negatively and positively charged above and below it, respectively. The value of PZC for the NAgDFF was found to be at pH 5. This result indicated that at a pH below 5, the surface of the NAgDFF displayed positive charges, while at a pH above 5, the surface displayed negative charges. This indicates that NAgDFF has affinity for the investigated cationic dyes near pH 5.0 (Fig. S3 supplementary material).
Effect of pH on the cationic dyes’ degradation
Solution pH is an important parameter for reactions taking place on the surface of a particulate, as is the case of NAgDFF. A change in pH can alter the surface charge on the nanosorbent as well as the degree of dissociation of the dye molecule. The pH effect on the degradation of the dyes using NAgDFF was studied in the pH range 2–8; the obtained results are explored in Fig. 10. It was noticed that the sorption–photocatalytic degradation ability of NAgDFF toward the investigated dyes increases by increasing the pH value until pH 5 is reached and then decreases. The high removal % is due to the strong electrostatic interaction between the NAgDFF and cationic dyes’ molecules. The lower dye removal at acidic pH is probably due to the presence of excess H+ ions competing with the positively charged cationic dyes’ molecules for the sorption sites of NAgDFF. The photocatalytic degradation for the three dyes without adjusting pH is the same value at pH 5.
Effect of initial concentration of cationic dyes
The effect of initial concentration on the sorption–photodegradation of dyes was studied at normal dye pH; the results are graphed in Fig. 11. It is seen that the equilibrium sorption capacities of the NAgDFF increase with an increase in initial dye concentration and the adsorption at different concentrations is rapid in the initial stages and gradually decreases with the progress of adsorption until the equilibrium is reached. This may be attributed to the fact that, the higher the initial dye concentration, the greater the driving force of the concentration gradient at solid–liquid interface which cause an increase of the amount of dye adsorbed on the adsorbent. As expected the adsorption capacity increased with the initial dye concentration. With more dyes present in solution, larger fraction of the active sites is involved in the adsorption process. At higher dye concentrations, the adsorption capacity reached a plateau indicating saturation of the available binding sites on the adsorbent. High adsorption on the photocatalyst surface could enhance the photodegradation process. However, if the increase in dye concentration was too high, the nanocomposite would be saturated because the adsorption capacity was exceeded. In addition, if the dye concentration is too high, the photodegradation could be reduced because abundant dyes could block the penetration of light into NAgDFF.
Effect of contact time
Contact time is an important parameter of adsorption which can reflect the adsorption kinetics of NAgDFF for the three dyes. The effect of contact time on the adsorption capacity of NAgDFF for MB, CV and BG is shown in Figure S4 (supplementary material). It is clear that the adsorption capacity of NAgDFF increased rapidly with the increase in contact time from 1 to 3 h and more than 90% of the equilibrium adsorption capacity for the three dyes occurred within 3 h. At 4 h, the adsorption capacity became constant and the adsorption reached equilibrium. From the curves, it can be noticed that CV exhibited the highest adsorption rates while BG was the lowest.
As shown, the adsorption process was divided into three stages: (1) an initial stage with adsorption occurring instantly; (2) subsequently slow adsorption and (3) a final stage with adsorption reaching equilibrium and remaining constant. The first stage can be attributed to the rapid attachment of the dyes to the surface of NAgDFF by surface mass transfer. At this stage, more than 80% of adsorption was found in all cases. The second stage was slower, possibly because many of the available external sites were already occupied and because of the slow diffusion of dye molecules into the network of NAgDFF.
Effect of catalyst dose
Analysis of the effect of catalyst dose revealed that the percentage degradation of the three dyes increased with increase in weight of NAgDFF fibers from 0.025 to 0.08 g (Fig. S5 supplementary material). However, the amount of the dyes adsorbed (mgg− 1) was found to decrease with further increase in adsorbent dosage
Effect of temperature on the cationic dyes’ degradation (thermodynamic studies)
The free energy (ΔGo), enthalpy (ΔHo) and entropy (ΔSo) thermodynamic parameters of the MB, CV and BG dyes degradation by NAgDFF in the presence of light were anticipated by examining cationic dyes degradation in a temperature range between 298 K and 318 K. The thermodynamic equilibrium constant (Kc) in addition to the rest of the thermodynamic parameters was calculated as in the (Eq. 10) and (Eq. 11). The obtained values and all the estimated thermodynamic parameters listed in Table 4 and the plotted curves are presented in Fig. 6 S. As can be observed, the exhibited negative ΔGo values reveal the thermodynamic feasibility of the photocatalytic degradation under the studied temperature range (spontaneous process). Moreover, the observed decrease in the degradation by increasing temperature indicates that CV, MB and BG degradation from solution is favorable at lower temperature.
The enthalpy of degradation is taken as a measurement of the energy barrier that must be crossed by the reacting molecules; the calculated negative ΔHo values confirm the exothermic nature of the degradation process and subsequently suggest that some amount of heat is lost to the degradation solution system upon dyes degradation. The values of ΔHo are higher than 80 Kjoule/mole which indicates that the adsorption process was chemisorption [48]. In addition, the measured negative ΔSo values indicated the lower randomness and higher alignment degree as a result of dyes degradation [49, 50].
The degradation efficiency of the three dyes decreased with the increase in the temperature. Useful information about the nature of the surface may be provided from the magnitude of the heat of the degradation process.
Sorption–photodegradation kinetics
Quantitative kinetics analyses are essential for best degradation designing system and well understanding of the degradation mechanism in addition to identifying the photocatalytic degradation rate-limiting step. The pseudo-first-order equation (Eq. 8) and the pseudo-second-order equation (Eq. 9) are the most frequently used in the kinetic analysis.
The kinetics of the degradation and adsorption of the studied dyes by NAgDFF fibers are presented in (Fig. S7 and Fig. S8 supplementary material).
The parameters of pseudo-first and pseudo-second-order kinetic models obtained for MB, CV and MG adsorption by NAgDFF fibers are given in Table 5. It can be seen that for the adsorption of the three dyes, the regression coefficient (R2) values are between 0.8517–0.9984 and 0.9778–0.9993 for the pseudo-first- and pseudo-second-order kinetic models, respectively. The higher R2 values indicate that the pseudo-second-order kinetic model fits quite well with the experimental data. The values of the experimental maximum capacity for the three dyes are closer to qe2 more than qe1. Regarding rate constants (K1 and K2) calculated in each model, it can be straightly observed that rate constants calculated from first order model were high which means a slow adsorption rate which in turn does not agree with experimental results. However, rate constants derived from the second order model were much smaller which makes more sense and correlate with experimental results. It can be also concluded that chemisorption is the main dominant process and it is suggested to be as well the limiting step, where s and n are the sample standard deviation and replicate measurements (n = 5), respectively.
Sorption–photodegradation isotherms
The adsorption isotherms of NAgDFF for all the three dyes have been tested with the Langmuir and Freundlich models, in order to establish the correlation for the equilibrium data in the design of the sorption system. This indicates the distribution of the adsorbed molecules between the liquid phase and the solid phase when the adsorption process reaches equilibrium state. A better regression coefficient (R2) value gives an indication of the applicability of the isotherm models. The Langmuir isotherm equation is based on the assumption that monolayer adsorption of solute molecules occurs on a fixed number of localized surface sites present on the sorbent. In Freundlich isotherm equation, non-ideal sorption takes place by heterogeneous surface energy systems. The linear forms of the Langmuir isotherm, Freundlich and Dubinin–Radushkevich isotherm models are given by (Eqs. 4–6), respectively.
An essential characteristic of Langmuir isotherm can be expressed by a dimensionless constant called equilibrium parameter, RL, defined by:
where C0 is the highest initial dye concentration (mg/L), and RL values indicate the type of isotherm to be either unfavorable (RL > 1), linear (RL = 1), favorable (0 < RL < 1) or irreversible (RL = 0).
In Dubinin–Radushkevich isotherm mode, the energy (E) in Kjoule/mole is calculated as presents in (Eq. 14).
The Langmuir, Freundlich and Dubinin–Radushkevich plots corresponding to the adsorption of all the three dyes are shown in Fig. S9, S10 and S11, respectively. The model parameters obtained by applying Langmuir, Freundlich and Dubinin–Radushkevich models to the experimental data are given in Tables 6. It can be found that the regression coefficients R2 obtained from Langmuir model are closer to 1 than that of the Freundlich and Dubinin–Radushkevich models, suggesting that the Langmuir isotherm fits better with the adsorption of MB, CV and BG on NAgDFF. In addition, the RL values obtained are in all cases between > 1, thereby confirming that the adsorption is a favorable process. The maximum degradation efficiency for MB, CV and BG for NAgDFF fibers is 64.75, 94.98 and 63.87 (mg/ g), respectively. It can be concluded that adsorption of dyes by NAgDFF followed the Langmuir isotherm model suggested that each pollutant of CV, BG and MB was specifically bound to the NAgDFF surface in monolayer and homogenous dispersion. The Dubinin–Radushkevich energy (E) values indicate that the adsorption process was physical which conflicts with what has been proven from Langmuir and ΔHo values that the adsorption process was chemisorption.
Optimization studies using column test
Removal of dissolved dyes from water was evaluated through analysis of column experiments. The usage of NAgDFF fibers as filter was examined and the results presented in Table 7 showed that MB, CV and BG dyes were removed with percent higher than 95% using 0.25 g of the NAgDFF fiber, column with diameter 0.7cm at slow flow rate for the three dyes. The maximum removal efficiency was in the order of MB < CV < BG.
Mechanism of photocatalytic degradation of MB, CV and BG dyes
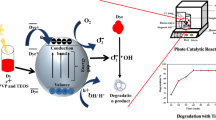
The photocatalytic degradation process can be proposed according to the presented mechanism in Schemes 3 and 4. Briefly, the electron–hole pairs production formed due to the migration of the electrons from valance band to conduction band as a result of the irradiation by the sun light. Active species include hydroxyl radical (HO) and anion radicals (O2−) from H2O and O2, respectively. These active species are produced as a result of the positive holes and the negative electrons. These active species together with the positive holes (h+) are responsible for the breakdown of the MB, CV and BG molecules into smaller molecules, CO2 and H2O.
Mechanism of sorption
The adsorption process usually supports the mixed mechanisms of physisorption and chemisorption mechanism. The physisorption mechanism is always considered the adsorption process done through electrostatic transactions between positive charges of cationic dyes and negative surface of adsorbents which are usually full of donating electrons groups such as hydroxyl group and amine group carbonyl group. On the other hand, the chemisorption mechanism is explained as ion exchange or chelation interaction one.
In the present study, sorption of the cationic dyes is proposed to mixed physisorption and chemisorption mechanism. The physisorption mechanism is supported by the results of point of zero charge (PZC) studies. The value of PZC for the NAgDFF was found to be at pH 5. This result indicated that at a pH below 5, the surface of the NAgDFF displayed positive charges, while at a pH above 5, the surface displayed negative charges. This indicates that NAgDFF has affinity for the investigated cationic dyes near pH 5.0 (Fig. 1S supplementary material). MB, CV and BG are all cationic dyes, and hence, they get attracted to this negative surface charge on the NAgDFF.
On the other hand, the chemisorption mechanism is supported by the following findings:
-
1.
The data obtained from kinetic section clarify that NAgDFF adsorbents obeyed pseudo-second-order model which improved the chemisorption pathway.
-
2.
These data agree with results of isotherm studies which indicated complete following of NAgDFF adsorbents to the monolayer Langmuir model against other model; the arrangement of adsorbates as only one layer completely supports the chemisorption interaction suggestion. RL values (between 0 and 1) indicate a favorable adsorption process.
-
3.
By the same way, the appearance of new functional groups, at FTIR chart, was formed from the chemical treatment for NAgDFF push in the way of chelating interaction between cationic dyes and NAgDFF adsorbent. Therefore, based on all previous results, adsorption process is suggested to take place by means of chelation where the cationic dye replaces the hydrogen ion from the adsorption site and be located to the adsorbent forming an adsorbent dye complex.
Sorbent reusability
For inspecting the reusability of NAgDFF, five sorption–desorption sequences were done under the best conditions and the obtained data are offered in Table 8. It is noticeable that the degradation efficacy of NAgDFF was only slightly decreased after the five cycles; the adsorbent maintained about 95% of its initial efficiency.
Applications
The optimized experimental conditions were applied to real samples to evaluate the efficiency of NAgDFF for the sorption–photocatalytic degradation of cationic dyes. The calibration curves were prepared with the standard solutions. The standard solutions (1.0 L) were treated under the experimental conditions optimized above. The analytical samples were tap water in our laboratory in Mansoura University, Nile river water in Mansoura City and seawater in Alexandria City. The analytical results are as shown in Table 9. All dyes were not found in all of the samples. The recoveries were examined in the samples in which given amounts of each dye were spiked. The recoveries obtained were in the range of 98.00–99.42%. These results indicate that NAgDFF method could be successfully applied to the determination of cationic dyes in real water.
Comparison with previously published studies
By comparing the organic dyes’ maximum adsorption capacity of NAgDFF with other natural materials, it has been found that NAgDFF is comparable or superior than some other reported agro-waste-based materials (Table 10).
Conclusion
Nanotechnology can be applied in solving many problems associated with environment, agriculture, food, etc. Recently, nanotechnology utilization in treatment of the wastewater has provided effective results. Our study employed the synthesis of nano-silver-doped flax fibers (NAgDFF), which were further characterized using UV–visible, SEM, EDX, FTIR, N2 adsorption isotherm and thermogravimetric analysis (TGA). We observed the efficient activity of NAgDFF in the dye degradation as in methylene blue, crystal violet and brilliant green degradation (individually and in mixture) in both batch and column techniques. Further, as most of the previously published studies used single dye with few studies mentioned the nanoparticles potential for a mixture of dyes so that our study could be an indicator for the other researchers to identify other nanoparticles potential for dyes treatment (individual and in mixture).
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary files].
References
Bansal M, Patnala PK, Dugmore T (2020) Adsorption of Eriochrome Black-T (EBT) using tea waste as a low cost adsorbent by batch studies: a green approach for dye effluent treatments. Curr Res Green Sustain Chem 3:100036. https://doi.org/10.1016/j.crgsc.2020.100036
Samuchiwal S, Gola D, Malik A (2021) Decolourization of textile effluent using native microbial consortium enriched from textile industry effluent. Hazardous Materials 402:123835. https://doi.org/10.1016/j.jhazmat.2020.123835
Ikram M, Khan MI, Raza A, Imran M, Ul-Hamid A, Ali S (2020) Outstanding performance of silver-decorated MoS2 nanopetals used as nanocatalyst for synthetic dye degradation. Physica E 124:114246. https://doi.org/10.1016/j.physe.2020.114246
Stone C, Windsor FM, Munday M, Durance I (2020) Natural or synthetic–how global trends in textile usage threaten freshwater environments. Sci Total Environ 718:134689. https://doi.org/10.1016/j.scitotenv.2019.134689
Mathur M, Vijayalakshmi KS, Gola D, Singh K, Chaudhary S, Kaushik P, Malik A (2015) Decolourization of textile dyes by aspergillus lentulus. Basic Appl Eng Res 2:1469–1473
Samrot AV, Ali HH, Selvarani J, Faradjeva E, Raji P, Prakash P (2021) Adsorption efficiency of chemically synthesized superparamagnetic iron oxide nanoparticles (SPIONs) on crystal violet dye. Curr Res Green Sustain Chem 4:100066. https://doi.org/10.1016/j.crgsc.2021.100066
Gola D, Bhattacharya A, Dey P, Malik A, Ahammad SZ (2020) Assessment of drain water used for irrigation in the Delhi region. J Health Pollut. https://doi.org/10.5696/2156-9614-10.26.200610
Gola D, Malik A, Shaikh ZA, Sreekrishnan TR (2016) Impact of heavy metal containing wastewater on agricultural soil and produce: relevance of biological treatment. Environ Process 3(4):1063–1080. https://doi.org/10.1007/s40710-016-0176-9
Khan S, Malik A (2018) Toxicity evaluation of textile effluents and role of native soil bacterium in biodegradation of a textile dye. Environ Sci Pollut Res 25(5):4446–4458. https://doi.org/10.1007/s11356-017-0783-7
Fatima B, Siddiqui SI, Ahmed R, Chaudhry SA (2019) Green synthesis of f-CdWO4 for photocatalytic degradation and adsorptive removal of bismarck brown R dye from water. Water Resour Ind 22:100119. https://doi.org/10.1016/j.wri.2019.100119
Bharati B, Sonkar AK, Singh N, Dash D, Rath C (2017) Enhanced photocatalytic degradation of dyes under sunlight using biocompatible TiO2 nanoparticles. Mater Res Express 4(8):085503. https://doi.org/10.1088/2053-1591/aa6a36
Chen X, Wu Z, Liu D, Gao Z (2017) Preparation of ZnO photocatalyst for the efficient and rapid photocatalytic degradation of azo dyes. Nanoscale Res Lett 12(1):4–13. https://doi.org/10.1186/s11671-017-1904-4
Guo Y, Cao F, Li Y (2018) Solid phase synthesis of nitrogen and phosphor co-doped carbon quantum dots for sensing Fe3+ and the enhanced photocatalytic degradation of dyes. Sens Actuators B Chem 255:1105–1111. https://doi.org/10.1016/j.snb.2017.08.104
Rajeswari A, Vismaiya S, Pius A (2017) Preparation, characterization of nano ZnO-blended cellulose acetate-polyurethane membrane for photocatalytic degradation of dyes from water. Chem Eng J 313:928–937. https://doi.org/10.1016/j.cej.2016.10.124
Reza KM, Kurny ASW, Gulshan F (2017) Parameters affecting the photocatalytic degradation of dyes using TiO2: a review. Appl Water Sci 7(4):1569–1578. https://doi.org/10.1007/s13201-015-0367-y
Shinde DR, Tambade PS, Chaskar MG, Gadave KM (2017) Photocatalytic degradation of dyes in water by analytical reagent grades ZnO, TiO2 and SnO 2: a comparative study. Drink Water Eng Sci 10(2):109–117. https://doi.org/10.5194/dwes-10-109-2017
Samadi-Maybodi A, Sadeghi-Maleki MR (2016) In-situ synthesis of high stable CdS quantum dots and their application for photocatalytic degradation of dyes. Spectrochim Acta Part A Mol Biomol Spectrosc 152:156–164. https://doi.org/10.1016/j.saa.2015.07.052
Saravanan R, Sacari E, Gracia F, Khan MM, Mosquera E, Gupta VK (2016) Conducting PANI stimulated ZnO system for visible light photocatalytic degradation of coloured dyes. J Mol Liq 221:1029–1033. https://doi.org/10.1016/j.molliq.2016.06.074
Sudrajat H, Babel S, Sakai H, Takizawa S (2016) Rapid enhanced photocatalytic degradation of dyes using novel N-doped ZrO2. J Environ Manag 165:224–234. https://doi.org/10.1016/j.jenvman.2015.09.036
Sudrajat H, Babel S (2017) A novel visible light active N-doped ZnO for photocatalytic degradation of dyes. J water process eng 16:309–318. https://doi.org/10.1016/j.jwpe.2016.11.006
Abutaleb A, Tayeb AM, Mahmoud MA, Daher AM, Desouky OA, Bakather OY, Farouq R (2020) Removal and recovery of U (VI) from aqueous effluents by flax fiber: adsorption, desorption and batch adsorber proposal. J Adv Res 22:153–162. https://doi.org/10.1016/j.jare.2019.10.011
Dizbay-Onat M, Vaidya UK, Balanay JAG, Lungu CT (2018) Preparation and characterization of flax, hemp and sisal fiber-derived mesoporous activated carbon adsorbents. Adsorpt Sci Technol 36(1–2):441–457. https://doi.org/10.1177/0263617417700635
Kyzas GZ, Christodoulou E, Bikiaris DN (2018) Basic dye removal with sorption onto low-cost natural textile fibers. Processes 6(9):166. https://doi.org/10.3390/pr6090166
Hokkanen S, Bhatnagar A, Sillanpää M (2016) A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res 91:156–173. https://doi.org/10.1016/j.watres.2016.01.008
Aravind M, Ahmad A, Ahmad I, Amalanathan M, Naseem K, Mary SMM, Zuber M (2021) Critical green routing synthesis of silver NPs using jasmine flower extract for biological activities and photocatalytical degradation of methylene blue. J Environ Chem Eng 9(1):104877. https://doi.org/10.1016/j.jece.2020.104877
Aravind M, Amalanathan M, Mary M, Parvathiraja C, Alothman AA, Wabaidur SM, Islam MA (2022) Enhanced photocatalytic and biological observations of green synthesized activated carbon, activated carbon doped silver and activated carbon/silver/titanium dioxide nanocomposites. J Inorg Organomet Polym Mater 32(5):1–13. https://doi.org/10.1007/s10904-021-02096-w
Chen X, Schluesener HJ (2008) Nanosilver: a nanoproduct in medical application. Toxicol Lett 176(1):1–12. https://doi.org/10.1016/j.toxlet.2007.10.004
Garibo D, Borbón-Nuñez HA, de León JND, García Mendoza E, Estrada I, Toledano-Magaña Y, Susarrey-Arce A (2020) Green synthesis of silver nanoparticles using lysiloma acapulcensis exhibit high-antimicrobial activity. Sci Rep 10(1):1–11. https://doi.org/10.1038/s41598-020-69606-7
Herbin HB, Aravind M, Amalanathan M, Mary M, Lenin MM, Parvathiraja C, Islam MA (2022) Synthesis of silver nanoparticles using syzygium malaccense fruit extract and evaluation of their catalytic activity and antibacterial properties. J Inorg Organomet Polym Mater 32:1103–1115. https://doi.org/10.1007/s10904-021-02210-y
Kumari RM, Thapa N, Gupta N, Kumar A, Nimesh S (2016) Antibacterial and photocatalytic degradation efficacy of silver nanoparticles biosynthesized using cordia dichotoma leaf extract. Adv Nat Sci Nanosci Nanotechnol 7(4):045009. https://doi.org/10.1088/2043-6262/7/4/045009
Liu H, Tang X, Liu Q (2014) A novel point-of-use water treatment method by antimicrobial nanosilver textile material. J Water Health 12(4):670–677. https://doi.org/10.2166/wh.2014.197
Rao SS, Saptami K, Venkatesan J, Rekha PD (2020) Microwave-assisted rapid synthesis of silver nanoparticles using fucoidan: characterization with assessment of biocompatibility and antimicrobial activity. Int J Biol Macromol 163:745–755. https://doi.org/10.1016/j.ijbiomac.2020.06.230
Qureshi F, Khuhawar MY, Jahangir TM, Channar AH (2021) Synthesis and characterization of new thermally stable, antimicrobial and red-light-emitting poly (azomethine-ester) s. Polym Bull 78:5055–5074. https://doi.org/10.1007/s00289-020-03357-3
Qureshi F, Khuhawar MY, Jahangir TM (2019) New fluorescent, thermally stable and film forming polyimines containing naphthyl rings. Acta Chim Slov 66:899–912. https://doi.org/10.17344/acsi.2019.5100
Qureshi F, Khuhawar MY, Jahangir TM (2018) Synthesis and characterization of new photo-responsive, ortho and para oriented azomethine polymers. Acta Chim Slov 65(3):718–729. https://doi.org/10.17344/acsi.2018.4419
Sharma N, Sharma H, Khatri P, Shirkot P (2018) Biosynthesis characterization and antibacterial activity of bioiron nanoparticles synthesized by Pseudomonas putida strain LUA 15.1. Int J Chem Stud 6(1):691–699
Ali N, Said A, Ali F, Raziq F, Ali Z, Bilal M, Iqbal H (2020) Photocatalytic degradation of congo red dye from aqueous environment using cobalt ferrite nanostructures: development, characterization, and photocatalytic performance. Water Air Soil Pollut 231(2):1–16
Dawoud TM, Pavitra V, Ahmad P, Syed A, Nagaraju G (2020) Photocatalytic degradation of an organic dye using Ag doped ZrO2 nanoparticles: milk powder facilitated eco-friendly synthesis. J King Saud Univ Sci 32(3):1872–1878. https://doi.org/10.1016/j.jksus.2020.01.040
Qureshi F, Memon SQ, Khuhawar MY, Jahangir TM (2021) Removal of Co2+, Cu2+ and Au3+ ions from contaminated wastewater by using new fluorescent and antibacterial polymer as sorbent. Polym Bull 78:1505–1533. https://doi.org/10.1007/s00289-020-03170-y
Qureshi F, Memon SQ, Khuhawar MY, Jahangir TM, Channar AH (2021) Synthesis and application of fluorescent and thermally stable polyazomethine as adsorbent in the remediation of Ni (II), Cu (II) and Co (II) from wastewater systems. J Polym Res 28:259. https://doi.org/10.1007/s10965-021-02582-2
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, Yacaman MJ (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16(10):2346–2353. https://doi.org/10.1088/0957-4484/16/10/059
Raffi M, Hussain F, Bhatti TM, Akhter JI, Hameed A, Hasan MM (2008) Antibacterial characterization of silver nanoparticles against E. coli ATCC-15224. J mater sci technol 24(2):192–196
Shockman GD, Barren JF (1983) Structure, function, and assembly of cell walls of gram-positive bacteria. Annu Rev Microbiol 37(1):501–527. https://doi.org/10.1146/annurev.mi.37.100183.002441
Kim JS, Kuk E, Yu KN, Kim J-H, Park SJ, Lee HJ, Cho M-H (2007) Antimicrobial effects of silver nanoparticles. Nanomedicine Nanotechnol Biol Med 3(1):95–101. https://doi.org/10.1016/j.nano.2006.12.001
Archana D, Singh BK, Dutta J, Dutta PK (2015) Chitosan-PVP-nano silver oxide wound dressing: in vitro and in vivo evaluation. Int J Biol Macromol 73:49–57. https://doi.org/10.1016/j.ijbiomac.2014.10.05
Murray RGE, Steed P, Elson HE (1965) The location of the mucopeptide in sections of the cell wall of Escherichia coli and other gram-negative bacteria. Can J Microbiol 11(3):547–560
Ram B, Chauhan SG (2018) New spherical nanocellulose and thiol-based adsorbent for rapid and selective removal of mercuric ions. Chem Eng J 331:587–596. https://doi.org/10.1016/j.cej.2017.01.133
Inglezakis VJ, Zorpas AA (2012) Heat of adsorption, adsorption energy and activation energy in adsorption and ion exchange systems. Water Treat 39(1–3):149–157. https://doi.org/10.1080/19443994.2012.669169
Shamsipur M, Fasihi J, Khanchi A, Hassani R, Alizadeh K, Shamsipur H (2007) A stoichiometric imprinted chelating resin for selective recognition of copper (II) ions in aqueous media. Anal Chim Acta 599(2):294–301. https://doi.org/10.1016/j.aca.2007.08.013
Monier M, Abdel-Latif DA (2013) Synthesis and characterization of ion-imprinted chelating fibers based on PET for selective removal of Hg2+. Chem Eng J 221:452–460. https://doi.org/10.1016/j.cej.2013.02.003
Aygün A, Yenisoy-Karakaş S, Duman I (2003) Production of granular activated carbon from fruit stones and nutshells and evaluation of their physical, chemical and adsorption properties. Microporous Mesoporous Mater 66(2–3):189–195. https://doi.org/10.1016/j.micromeso.2003.08.028
Gong R, Sun Y, Chen J, Liu H, Yang C (2005) Effect of chemical modification on dye adsorption capacity of peanut hull. Dyes Pigm 67(3):175–181. https://doi.org/10.1016/j.dyepig.2004.12.003
Senthilkumaar S, Kalaamani P, Subburaam CV (2006) Liquid phase adsorption of crystal violet onto activated carbons derived from male flowers of coconut tree. J Hazard Mater 136(3):800–808. https://doi.org/10.1016/j.jhazmat.2006.01.045
Porkodi K, Kumar KV (2007) Equilibrium, kinetics and mechanism modeling and simulation of basic and acid dyes sorption onto jute fiber carbon: eosin yellow, malachite green and crystal violet single component systems. J Hazard Mater 143(1–2):311–327. https://doi.org/10.1016/j.jhazmat.2006.09.029
Lakshmi UR, Srivastava VC, Mall ID, Lataye DH (2009) Rice husk ash as an effective adsorbent: evaluation of adsorptive characteristics for Indigo carmine dye. J Environ Manag 90(2):710–720. https://doi.org/10.1016/j.jenvman.2008.01.002
Khattri SD, Singh MK (2009) Removal of malachite green from dye wastewater using neem sawdust by adsorption. J Hazard Mater 167(1–3):1089–1094. https://doi.org/10.1016/j.jhazmat.2009.01.101
Arulkumar M, Sathishkumar P, Palvannan T (2011) Optimization of orange G dye adsorption by activated carbon of thespesia populnea pods using response surface methodology. J Hazard Mater 186(1):827–834. https://doi.org/10.1016/j.jhazmat.2010.11.067
Mahmoud DK, Salleh MAM, Karim WAWA, Idris A, Abidin ZZ (2012) Batch adsorption of basic dye using acid treated kenaf fibre char: equilibrium, kinetic and thermodynamic studies. Chem Eng J 181:449–457. https://doi.org/10.1016/j.cej.2011.11.116
Acknowledgements
Not applicable.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
MAA was involved in conceptualization, methodology, investigation, writing—original draft, review, supervision. MAH helped in conceptualization, methodology, investigation, writing—original draft, review, supervision. AGM contributed to methodology, investigation, writing—original draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable to this study.
Consent for publication
Not applicable to this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Akl, M.A., Hashem, M.A. & Mostafa, A.G. Synthesis, characterization, antimicrobial and photocatalytic properties of nano-silver-doped flax fibers. Polym. Bull. 80, 9745–9777 (2023). https://doi.org/10.1007/s00289-022-04531-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04531-5