Abstract
Traditional chemical, physical and biological processes for treating wastewater containing textile dye have such disadvantages as high cost, high energy requirement and generation of secondary pollution during treatment process. The advanced oxidation processes technology has been attracting growing attention for the decomposition of organic dyes. Such processes are based on the light-enhanced generation of highly reactive hydroxyl radicals, which oxidize the organic matter in solution and convert it completely into water, CO2 and inorganic compounds. In this presentation, the photocatalytic degradation of dyes in aqueous solution using TiO2 as photocatalyst under solar and UV irradiation has been reviewed. It is observed that the degradation of dyes depends on several parameters such as pH, catalyst concentration, substrate concentration and the presence of oxidants. Reaction temperature and the intensity of light also affect the degradation of dyes. Particle size, BET-surface area and different mineral forms of TiO2 also have influence on the degradation rate.
Similar content being viewed by others
Introduction
Synthetic dyes are widely used in textile industry (Vandevivere et al. 1998; Sakthivel et al. 2003), leather industry (Sakthivel et al. 2003; Tunay et al. 1999), paper production (Vandevivere et al. 1998; Ivanov et al. 1996), food technology (Slampova et al. 2001), medical (Haferlach et al. 2008), agricultural research (Cook and Linden 1997; Kross et al. 1996), light-harvesting arrays (Wagner and Lindsey 1996), photoelectrochemical cells (Wrobel et al. 2001), etc.
At present, 100,000 different types of dyes with annual production rate of 7 × 105 are produced of which about 36,000 tons dye/year are consumed by the textile industries. Up to 20 % of the total world production of dyes is lost during the dyeing process and is released in the textile effluents (Esplugas et al. 2002; Houas et al. 2001; Crini 1996; Weber and Stickney 1993). Synthetic dyes are produced and consumed in large scale and can cause considerable environmental pollution and serious health-risk due to their stability, and toxicity (Shukla and Gupta 1992). A wide range of methods has been developed for the removal of synthetic dyes from waters and wastewaters to decrease their impact on the environment. Conventional water treatment technologies such as solvent extraction, activated carbon adsorption, and chemical treatment process [such as oxidation by ozone (O3)] often produce hazardous by-products and generate large amount of solid wastes, which require costly disposal or regeneration method (Slokar and Marechal 1998; Galindo et al. 2001; Pandurangan et al. 2001).
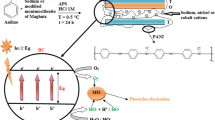
Over the past few years, advanced oxidation processes (AOP) has been proposed as an alternative route for water purification (Legrini et al. 1993). AOP is divided into two categories (heterogeneous and homogenous catalysis). Heterogeneous catalysis has been successfully employed for the degradation of various families of hazardous materials. Titanium dioxide (TiO2), due to its non-toxic, inexpensive, and high reactive nature, has been extensively investigated as a heterogeneous photocatalyst for the remediation of contaminated environment (Hassan et al. 2014; Harbi et al. 2015; Kim et al. 2005). The advantage of TiO2 as photocatalyst is that organic pollutants are usually completely mineralized to non-toxic substances such as CO2, HCl and water and thus the reactions do not suffer the drawbacks of photolysis reactions in terms of the production of intermediate products. Moreover, the reaction can take place at room temperature (Aramendia et al. 2005; Malato et al. 2003; Chatterjee and Dasgupta 2005).
Upon irradiation by photons, electrons on the surface of TiO2 are excited to the conduction band, and positive holes are formed in the valance band. The electrons and holes can either recombine and produce thermal energy or interact with other molecules (Venkatadri and Peters 1993). The holes can react with electron donors in the solution to produce powerful oxidizing free radicals such as hydroxyl radicals, which oxidize the organics on the surface. The holes can also oxidize the substrate by direct electron transfer. In brief, photogeneration of radical species in TiO2/UV system can be described as follows (Kormann et al. 1988):
Parameters effecting the photocatalytic degradation
Photocatalytic degradation of dyes are influenced by several parameters, such as pH, initial concentration of dyes, photocatalyst particle size and its concentration, reaction temperature, light intensity and the presence of electron acceptors.
Effect of oxidizing agent
Inorganic oxidants such as O3 have been proposed to increase the efficiency of TiO2/UV treatments (Wang et al. 2002; Li et al. 2003), H2O2 (Saggioro et al. 2011; Saquib et al. 2008), BrO3 − (Saquib et al. 2008; Muneer and Bahnemann 2002), S2O8 2− (Saquib et al. 2008; Muneer and Bahnemann 2002) and ClO4 − (Pelizzetti et al. 1991). These oxidants increase the number of trapped electrons, which prevents recombination and generates oxidizing radicals, which may, in turn, enhance the photocatalytic degradation of dye.
It has been shown that potassium bromate and ammonium persulfate had a beneficial effect on the degradation rate for the decomposition of Fast Green FCF in the presence of UV 100, whereas in the case of Patent Blue VF, all the electron acceptors were found to enhance the rate noticeably in the presence of P25 (Saquib et al. 2008).
Mahmoodi et al. (2006) worked with Reactive Blue 8 (RB 8) and Reactive Blue 220 (RB 220) and found that when H2O2 concentration changed from 0 to optimal concentration (300 mg/l for RB 8 and 450 mg/l for RB 220). No nonappreciable changes at the decolonization rate were observed when the concentration further increased.
H2O2 increases the formation rate of hydroxyl radical in two ways: (1) the reduction of H2O2 at the conduction band and (2) self-decomposition by illumination (So et al. 2002; Lee et al. 2003). Hydrogen peroxide, in low concentrations, enhances the degradation of compounds due to a more efficient generation of hydroxyl radical and inhibition of electron–hole pair recombination (Saggioro et al. 2011; Prado et al. 2008; Aguedach et al. 2005). Moreover, the solution phase may, at times, be oxygen starved, due to either oxygen consumption or slow oxygen mass transfer. Peroxide addition thereby increases the rate toward what it would have been an adequate oxygen supply (Daneshvar et al. 2004). However, when the concentration of H2O2 increases, the electron acceptor reacts with hydroxyl radicals, and acts as scavenger of the photoproduced holes (Daneshvar et al. 2003; Malato et al. 1998). In addition, H2O2 can modify the TiO2 surface. This fact probably decreases its photocatalytic efficiency (Zhu et al. 2000). In addition, in the presence of high concentration of peroxide, OH· radicals preferentially react with the excess of H2O2. This undesirable reaction competes with the destruction of the dye chromophore (Galindo and Kalt 1999; Coleman et al. 2007). Radical–radical reaction must also be taken into account (Konstantinou and Albanis 2004).
The UV/H2O2 process involves the photolysis of H2O2 by the rupture of the O–O bond and the formation of two OH· radicals (Beltran et al. 1997). Practical difficulties with the UV photolysis of H2O2 are: (1) low wavelength (below 200–400 nm) required to make the process efficient, and (2) turbid waters containing strong UV absorbers such as aromatics organic compounds require higher light concentration, which increases the cost of the process. Dixit et al. (2010) studied photochemical oxidation of phenol and chlorophenol by UV/H2O2/TiO2 process and compared with UV/H2O2 and UV/TiO2 processes. He showed that when compared with the UV/H2O2 and UV/TiO2 processes, the UV/H2O2/TiO2 process reduced the energy consumption by 40–50 %. It was found that application of both oxidation and photocatalysis enhanced the degradation mechanism and hence gives much higher removal.
An investigation on the decomposition of methylene blue by iron oxides at various concentrations of oxalate solutions showed that much higher concentration of hydroxyl radicals was generated by iron oxides compared with those from a commercial TiO2 (ST-01), as determined by the coumarin method. It was also found that the photodecomposition rate increased with increasing the generation of OH· radicals (Gulshan et al. 2010).
Effect of pH
The interpretation of pH effects on the efficiency of the photodegradation process is a very difficult task. This is because three possible reaction mechanisms can contribute to dye degradation, (1) hydroxyl radical attack, (2) direct oxidation by the positive hole, and (3) direct reduction by the electron in the conducting band. The contribution of each one depends on the substrate nature and pH (Tang et al. 1997). The solution pH modifies the electrical double layer of the solid electrolyte interface, and consequently affects the sorption–desorption processes and the separation of the photogenerated electron–hole pairs in the surface of the semiconductor particles. TiO2 shows an amphoteric character so that either a positive or a negative charge can be developed on its surface (Poulios et al. 2000) and thus a pH variation can influence the adsorption of dye molecules onto the TiO2 surfaces (Wang et al. 2008).
Bubacz et al. (2010) observed an increase in the rate of the photocatalytic degradation of methylene blue with an increase in pH. According to Ling et al. (2004), basic pH electrostatic interactions between negative TiO− and methylene blue cation leads to strong adsorption with a corresponding high rate of degradation. Ling et al. (2004) noted that basic pH electrostatic interactions between negative TiO− and methylene blue cation leads to strong adsorption with a corresponding high rate of degradation. The surface charge properties of TiO2 were also found to change with a change of pH value due to the amphoteric behavior of semi conducting TiO2 (Guillard et al. 2003; Zielińska et al. 2003; Senthilkumaar et al. 2006).
Neppolian et al. (2002a) did not find significant influence of acidic pH on the percentage degradation of Reactive Blue 4, whereas the inhibitory effect was found to be more pronounced in the alkaline range (pH 11–13).
In acidic solutions (pH < 5), the photodegradation of the dye is retarded by the high concentration of proton, resulting in lower degradation efficiency. In alkaline medium (pH > 10), on the other hand, the presence of hydroxyl ions neutralizes the acidic end-products that are produced by the photodegradation reaction. A sudden drop of degradation had been observed when the initial pH of the reaction mixture was kept at 11. The reactive blue dye substituents of an electron-donating group such as—NH2 in the α-positions of the carbonyl group form intramolecular hydrogen bonds at high pH values (Rys and Zollinger 1972). Therefore, the dye structure becomes chemically stable at high pH ranges. The chromophores of the dye remain intact after light irradiation and hence reduce the degradation rate of the dye (Tsui and Chu 2001). Hence, the changes in the behavior of the dye molecule may be responsible for the change in the percentage degradation of dye at higher pH.
Baran et al. (2008) investigated degradation of Bromocresol Purple at pH 4.5 and 8.0 and noted that under acidic conditions, and the molecule has a positive charge.
When the solution was acidified from pH 8.0 to pH 4.5, a 6-fold increase in adsorption efficacy was observed. The observed increase was caused by a change in the charge of the Bromocresol Purple. The simultaneous significant increase in dye degradation rate may indicate that pH change also influences dye photodegradation. Unfortunately such an easy way to accelerate photocatalysis is impossible to achieve in the case of anionic dyes. The unavoidable increase in acidity will coagulate the photocatalyst and decrease its activity. Kansal et al. (2009) also found that the degradation of Reactive Black 5 and Reactive Orange 4 dyes was favored in acidic medium with TiO2. Tanaka et al. (2000) found that positively charged TiO2 surface adsorbed more Acid Orange 7 at lower pH value, and more decomposition was achieved. The acid black 1 has a sulfuric group in its structure, which is negatively charged. So the acidic solution favors adsorption of dye onto photocatalyst surface as TiO2 surface is positively charged in acidic solution (Grzechulska and Morawski 2002).
Lachheb et al. (2002) in their work with Crocein Orange G (OG), Methyl Red (MR), Congo Red (CR), Alizarin S (AS) and Methylene Blue (MB) found that the pH had a little influence upon the kinetics of disappearance. Since MB is a cationic dye, it is conceivable that at high pH, its adsorption is favored on a negatively charged surface. By contrast, OG has its adsorption inhibited by high pH because of its negatively charged sulfonate -ϕ-SO3 − function. The other dyes, having several functional groups, were found to have a resulting behavior similar to that of MB.
Kiriakidou et al. (1999) found strong dependence of pH of the solution on the degradation rate of Acid Orange 7 (AO7). They noted that AO7 has a negatively charged sulfonic group and at low pH, attractive forces between the TiO2 surface and the dye favors adsorption. On the other hand, at high pH, the TiO2 surface is negatively charged and repulsive forces leads to decreased adsorption. Finally, at pH values close to the pHzpc, adsorption takes intermediate values. The degradation rate was rather insensitive in the pH range of 2–9; however, it increased significantly upon increasing pH of the solution above 10. It is expected that most of the adsorbed dye molecules are not in direct contact with the photocatalyst surface resulting in decreased degradation rates with decreased pH.
Hu et al. (2003a) studied the changes of adsorption and decolorization of Procion Red MX-5B (MX-5B) and Cationic Blue X-GRL (CBX) with pH. They found that adsorption of MX-5B decreased with increasing pH. However, the photodegradation rate of MX-5B increased under UV irradiation with the decrease in adsorption of MX-5B. It was confirmed that, in the decolorization of MX-5B, the more active bonds including the C–N bonds linked to the benzene ring or the naphthalene ring were hydroxylated first (Hu et al. 2003b). The photodegradation of MX-5B therefore occurred in the bulk aqueous phase. On the contrary, the adsorption of CBX increased with pH, and the decolorization of CBX was also accelerated with the adsorption of CBX. Because of the high dependency on adsorption, the decolorization of CBX occurred mainly on the surface of TiO2.
Hung and Yuan (2000) found that Orange G decomposed more quickly in the more acidic or base conditions and degraded more slowly in neutral conditions. They attributed higher degradation in base condition to fact that more OH− present in the solution may result in formation of more OH· radicals.
Effect of dye concentration
Saquib and Muneer (2003) studied the effect of substrate concentration on the degradation of gentian violet at varying concentrations such as 0.18, 0.25, 0.35 and 0.5 mM. The degradation rate increased with increasing the substrate concentration up to 0.25 mM and then declined. Kiriakidou et al. (1999) working with Acid Orange 7 (AO7) got similar result. The experiments were conducted at pH 6 and the initial AO7 concentration was varied between 25 and 600 mg/l the time required for the decolorization of AO7 solutions was found to depend significantly on the initial dye concentration. Complete decolorization of the solutions took place in less than an hour for relatively low C0 values (25–100 mg/l), while this was not the case for higher initial dye concentrations (200–600 mg/l). Other works also revealed similar result (Sakthivel et al. 2003; Saquib and Muneer 2003; Augugliaro et al. 2002; Sohrabi et al. 2009; Sivalingam et al. 2003).
The effect of initial concentration of dyes on the percentage degradation was studied by Neppolian et al. (2002) They varied the initial concentration of Reactive Yellow 17 from 8 × 10−4 to 1.2 × 10−3 M, of Reactive Red 2 from 4.16 × 10−4 to 1.25 × 10−3 M and of Reactive Blue 4 from 1 × 10−4 to 5 × 10−4 M with optimum catalyst loading. They found that the percentage degradation decreases with increasing initial concentration of the dye. This is in agreement with the works of others (Saggioro et al. 2011; Daneshvar et al. 2003; Giwa et al. 2012; Zhang et al. 2002).
The probability of formation of OH· radicals on the catalyst surface and OH· radicals reacting with dye molecules determine the degradation rate. As the initial dye concentrations increase, more dye molecules are available for excitation and energy transfer (Avasarala et al. 2010). This dependence is perhaps related to the formation of several monolayers of adsorbed dye on the TiO2 surface, which is favored at high dye concentrations. Till the critical level is reached, the surface is not completely covered leading to constant reaction rates (Kiriakidou et al. 1999). On the other hand, the decrease in degradation efficiency with the increase in dye concentration occurs due to several reasons. As the initial concentrations of the dye increase more and more, dye molecules are adsorbed on the surface of the catalyst and significant amount of UV is absorbed by the dye molecules rather than the TiO2 particles. Hence, the penetration of light to the surface of the catalyst decreases (Daneshvar et al. 2003; Kiriakidou et al. 1999; Saquib and Muneer 2003; Augugliaro et al. 2002). The generation of hydroxyl radicals decreases since the active sites was occupied by dyes (Daneshvar et al. 2003; Grzechulska and Morawski 2002). The adsorbed dye on the photocatalyst also inhibits the reaction of adsorbed molecules with the photo-induced positive holes or hydroxyl radicals, since there is no direct contact of the semiconductor with them (Daneshvar et al. 2003; Grzechulska and Morawski 2002; Kiriakidou et al. 1999; Poulios and Aetopoulou 1999). High dye concentration also shields the UV light (Saggioro et al. 2011; Segne et al. 2011; Liu et al. 2006). This reduces the path length of the photons entering the solution (Saggioro et al. 2011; Davis et al. 1994). Again, as the initial concentration of the dye increases, the requirement of catalyst surface needed for the degradation also increases. Since illumination time and amount of catalyst are constant, the OH· radical (primary oxidant) formed on the surface of TiO2 is also constant. So the relative number of free radicals attacking the dye molecules decreases with increasing amount of the dye (Mengyue et al. 1995).
Effect of photocatalyst concentration
TiO2 dosage can affect the degradation rate. Wei and Wan (1991) have reported that the catalyst amount has both positive and negative impact on the photodecomposition rate. The initial reaction rates were found to be directly proportional to catalyst concentration indicating the heterogeneous regime. Different concentrations of Degussa P25 in the range 0.5–5 g/l were employed to study the effect of photocatalyst concentration on the degradation kinetics of gentian violet. The degradation rate for the decomposition of the dye was found to increase with an increase in catalyst concentration, (Saquib and Muneer 2003). Qamar et al. (2005) found that the degradation rate for the decomposition of chromotrope 2B and amido black 10B increase with the increase in catalyst concentration. The investigation was done in different concentrations of Degussa P25 varying from 0.5 to 5 g/l. Hung and Yuan (2000) also found that the degradation rate of Orange G is proportional to the TiO2 concentrations. Here TiO2 concentrations ranged from 300 to 2000 mg/l. This is also in agreement with the work of Saggioro et al. (2011).
Zhang et al. (2002) examined the dependence of the photooxidation kinetics of methylene blue on TiO2 loading and found that the rates increase with TiO2 loading up to a limit and then decrease to a constant value. Others (Daneshvar et al. 2003; Sohrabi and Ghavami 2008) also found the same characteristics.
The effect of TiO2 loading on percentage degradation of the dyes Reactive Yellow 17(RY17), Reactive Red 2(RR2), Reactive Blue 4 (RB4) has been examined by varying its amount from 100 to 600 mg/100 ml of the dye solution (Neppolian et al. 2002b). The percentage degradation was found to increase rapidly with an increase in the amount of TiO2 from 100 to 300 mg/100 ml for all the three dyes. In the case of RR2 and RB4, the percentage degradation increased only slightly in the range 300–500 mg of TiO2, while in the case of RY17, percentage degradation was found to be constant with increase in TiO2 loading suggesting that upper level for catalyst effectiveness exists.
Further increase in the amount of TiO2 from 500 to 600 mg in RY17 decreased the percentage degradation. Neppolian et al. (2002a) found that the degradation percentage or Reactive Blue 4 increases linearly with catalyst loading up to 2.5 g/l. Catalyst loading was varied from 1 to 4 g/l of the dye solution (4 × 10−4 M).
An increased loading of catalyst increases the quantity of photons adsorbed and consequently the degradation rates (Herrmann 1995; Muruganandham and Swaminathan 2006). On the other hand, an increase in the catalyst loading increases the solution opacity and leads to a decrease in the penetration of the photon flux in the reactor and thereby decreases the photocatalytic degradation rate (Kamble et al. 2003; Gogate and Pandit 2004). Moreover, a loss in surface area by agglomeration (particle–particle interactions) at high solid concentration is also observed (Kaneco et al. 2004). Excessive light scattering by the suspended particles also has a progressively diminishing influence on the reaction rate. The available number of dye molecules can also be insufficient for adsorption to a greater number of TiO2 particles (Zhang et al. 2002; Sohrabi and Ghavami 2008). This can be rationalized in terms of availability of active sites on TiO2 surface and the light penetration of photoactivating light into the suspension (Neppolian et al. 2002b). An optimum amount of TiO2 has to be added to avoid unnecessary excess catalyst and also to ensure total absorption of light photons for efficient photomineralization. Herrmann (1999) found that the optimum loading of photocatalyst was dependent on the initial solute concentration
Effect of different photocatalysts
Photocatalytic activity of different photocatalysts varies with the differences in the lattice mismatch, and BET-surface and impurities on the catalyst’s surface affect the adsorption behavior of a pollutant and the lifetime and recombination rate of electron–hole pairs. A large surface area can be the determining factor in certain photodegradation reactions, as a large amount of adsorbed organic molecules promote the reaction rate (Watson et al. 2003; Chen et al. 2004; Xiaohong et al. 2003). On the other hand, the predominant way of electron–hole recombination may be different depending on the particle size (Zhang et al. 1998).
Titanium dioxide (TiO2) or titania is a well-known photocatalyst material. Anatase, rutile, brookite and titanium dioxide (B) or TiO2 (B) are the four mineral forms of TiO2. It has been found that anatase TiO2 takes part in photocatalytic reaction more efficiently than the other forms of titanium dioxide (Tanaka et al. 1991). This superiority originates from its band gap, which is 3.2 eV, compared to 3.0 eV of rutile. It means the conductive zone of anatase type is 0.2 eV higher and therefore is more favorable for driving conjugate reactions involving electrons. Moreover, during photooxidation reaction, very stable surface peroxide groups can be formed in the anatase type of this oxide (Baraton and Merhari 2004; Luo and Ollis 1996). Furthermore, there are also studies which claim that a mixture of anatase (70–75 %) and rutile (30–25 %) is more active than pure anatase (Muggli and Ding 2001; Ohno et al. 2001).
Degussa P-25 titanium dioxide is mainly used as the photocatalyst. It is mostly in the anatase form and has a BET-surface area of 50 m2/g corresponding to a mean particle size of 20–30 nm (Rachel et al. 2002; Bickley et al. 1991). UV100 consists of 100 % anatase with a specific BET-surface area >250 m2/g and primary particle size of 5 nm (Lindner et al. 1997). The photocatalyst PC500 has a BET-surface area of >250 m2/g with 100 % anatase and primary particle size of 5–10 nm (Rachel et al. 2002). TiO2-Tytanpol A11 is totally in the anatase form and has a crystalline size of 37.3 nm, specific surface area of 11.4 m2/g, a mean pore diameter of about 7.7 nm and a band gap of 3.31 eV (Zielińska et al. 2003).
Saquib and Muneer (2003) investigated the influence of three different photocatalysts (P25, UV100 and PC500) on the degradation kinetics of gentian violet. They observed that the mineralization and degradation of dye proceeds much more rapidly in the presence of P25 as compared with other photocatalysts. Higher concentration of electrons and holes is available for suitable reactants to initiate the photocatalytic reaction due to slow recombination of (e−/h+) pair of P25.
Qamar et al. (2005) also tested the photocatalytic activity of three different commercially available TiO2 powders (namely P25, UV100 and PC500) on the degradation kinetics of chromotrope 2B and amido black 10B. They observed that the degradation of both dyes proceeds much more rapidly in the presence of Degussa P25 as compared with other TiO2 samples.
Sivalingam et al. (2003) experimented with Degussa P-25 TiO2 and combustion synthesized TiO2 for the degradation of methylene blue under UV exposure. Complete degradation of methylene blue of initial concentration of 200 ppm with the catalyst loading of 1 kg/m3 of combustion synthesized TiO2 occurred in 65 min, while Degussa P-25 showed reduction in concentration up to 100 ppm under identical conditions. The degradation of dyes under solar radiation with an initial concentration of 100 ppm and catalyst loading of 1 kg/m3 was also investigated. Complete degradation occurred in 3 h and 40 min, whereas time required for the complete degradation of MB under similar operating condition was 5 h even for the initial concentration of 20 ppm (Nosaka and Fox 1988) for commercial Degussa P-25 catalyst. This indicates that the photocatalytic activity of the combustion synthesized TiO2 is much higher than commercial Degussa P-25 catalyst for both UV and solar exposure. The higher photocatalytic activity of the combustion synthesized TiO2 over commercial catalysts may be attributed to higher surface area, high crystallinity and pure anatase crystal structure.
Giwa et al. (2012) studied the degradation of Reactive Yellow 81 and Reactive Violet 1 by two commercially available catalysts TiO2-P25 (Degussa) and TiO2 anatase and found Degussa P25 to be more effective for degradation. The high photoreactivity of TiO2-P25 has been attributed to two factors: the slow recombination of the electron–hole pair, and its large surface area (Muruganandham et al. 2006).
Zielińska et al. (2003) worked with TiO2-Tytanpol A11 and TiO2-Degussa P25 for the decomposition of Reactive Red 198 (RB198), Acid Black 1 and Acid Blue 7 (AB7). They found photocatalyst P25 to be more active than A11.
After 100 min of irradiation, new absorption bands that are characteristic of the intermediate by-products appeared in the region of 1700 and 2800–3100 cm−1 for RR198 and AB7. This suggests that created by-products are adsorbed on the photocatalyst. Higher specific surface area and lower band gap energy of P25 prevents by-product formation on active sites and accelerates activity for photocatalysis.
Effect of intensity and wavelength of light
TiO2 has a wide band gap (3.2 eV—anatase, 3.00 eV—rutile and 3.13 eV—brookite), which limits its absorption in the UV region of solar spectrum (Kuang et al. 2009; Khan et al. 2002; Atta et al. 2011).
The wavelength and intensity of the UV light irradiation source affects the degradation of dye in aqueous solution using TiO2 catalyst powder in photocatalytic reactor (Konstantinou and Albanis 2004). The artificial UV irradiation is more reproducible than sunlight and can bring higher efficiency in the degradation of textile dyes. However, solar energy, because of its abundance and non-hazardous nature, is expected to emerge as an alternative cost effective light source (Neppolian et al. 2002b; Muruganandham and Swaminathan 2004). Usually, solar photocatalytic degradation reactions are carried out by using solar illumination directly (Gonçalves et al. 2005) or using parabolic collectors (Malato et al. 2002).
Ollis et al. (1992) stated that:
-
(1)
At low light intensities (0–20 mW/cm2), the rate would increase linearly with increasing light intensity (first order).
-
(2)
At intermediate light intensities beyond a certain value (approximately 25 mW/cm2), the rate would depend on the square root of the light intensity (half order).
-
(3)
At high light intensities the rate is independent of light intensity. There are more photons per unit time and unit area; thus, the chances of photon activation on catalyst surface increase and therefore the photocatalytic power is stronger. However, as the light intensity increases, the number of activation sites remains the same thus the reaction rate only reaches a certain level even when the light intensity continues to increase.
The photocatalytic degradation of Reactive Yellow 17, Reactive Red 2, Reactive Blue 4 dyes using TiO2 as photocatalyst and solar/UV irradiation as light source has been carried out by Neppolian et al. (2002b). They found UV irradiation to be more effective than solar irradiation. The difference in the rate of degradation is attributed to difference in the input energy. The energy of UV irradiation is large compared to band gap energy of the catalysts. Hence, the problem of electron–hole recombination is largely avoided with UV source. But in sunlight only 5 % of the total radiation possesses the optimum energy for the band gap excitation of electrons (Fatin et al. 2012). Hence, the percentage degradation is found to be less in solar radiation of textile dyes. It was also found that the percentage degradation of all the three dyes increased with increasing solar light intensity. Under the higher intensity of light irradiation, the enhancement was considerably higher because the electron–hole formation is predominant, and hence, electron–hole recombination is negligible. However, at lower light intensity, electron–hole pair separation competes with recombination which in turn decreases the formation of free radicals, thereby causing less effect on the percentage degradation of the dyes (Bahnemann 1999).
Hung and Yuan (2000) studied the effect of light intensity for the degradation of Orange G. The light intensity ranged from 215 to 586 μW/cm2. The reaction rates for photolysis of Orange G increased with increasing light intensity.
Chanathaworn et al. (2012) varied the irradiation intensity of black light lamp in the range of 0–114 W/m2 and examined the effects of irradiation intensity on the decolorization of the Rhodamine B. The results indicated that an increase in the irradiation intensity of black light lamps enhanced the dye decolorization.
Liu et al. (2006) conducted the experiment in three different light intensities (1.24 mW/cm2, 2.04 mW/m2, 3.15 mW/m2) and found that the decolorization of Acid Yellow 17 increases with increasing light intensity. Enhancement of the decolorization rate with increasing light intensity was also observed (Sakthivel et al. 2003; So et al. 2002).
Rao et al. (2004) found that the rate of photocatalytic decomposition of acid orange 7 (AO7) is approximately 1.5 times higher in full sunlight than in artificial UV light. Though in the case of AO7, the direct photolysis plays a minor role in sunlight, but it is not completely negligible due to the strong absorption of the dye in the visible range.
The wavelength of the irradiation can affect the efficiency of photolysis. It is believed that shorter wavelength of irradiation can promote the electron hole generation and consequently enhance the efficiency of the catalyst (Nguyen et al. 2014). This has been observed in the CO2 photoreduction over Ag/TiO2 or TiO2, the photodegradation of 4-chlorophenol over TiO2 and the photodecomposition of organic contaminants over CaBi2O4. Many organic compounds absorb in the UV (e.g., higher energy region). Irradiating with too low a wavelength may result in unintended side reactions. Lower wavelength means more energy not more photons.
Effect of reaction temperature
Mozia et al. (2009) observed that in case of Acid Red 18, an increase in the reaction temperature resulted in an increase in the photodegradation rate constant. The effect was observed especially in case of the TiO2 loadings equal to 0.3 and 0.5 g/dm3. The increase in solution temperature from 313 to 323 K resulted in an 11 and 13 % increase in the rate constant for TiO2 loadings of 0.3 and 0.5 g/dm3, respectively. Rising the temperature up to 333 K resulted in the increase in the rate constant k for additional 10 % for both catalyst loadings. The decrease in temperature favors adsorption of the reactant which is a spontaneous exothermic phenomenon. In addition, the lowering in temperature also favors the adsorption of the final reaction product, whose desorption tends to inhibit the reaction. On the contrary, when temperature tends to the boiling point of water, the exothermic adsorption of reactant becomes disfavored and tends to limit the reaction (Mehrotra et al. 2005).
The effect of temperature on the reaction was also studied by Soares et al. (2007). The optimum range of operational temperatures was found to be in the range 40–50 °C. At low temperature, desorption of the products formed limits the reaction because it is slower than the degradation on the surface and the adsorption of the reactants. On the other hand, at a higher temperature, the limiting stage becomes the adsorption of the dye on TiO2. Zhou and Ray (2003) postulated that the reduction of the adsorptive capacity associated with the organics and dissolved oxygen at higher temperatures decreases the rate constant. Therefore, the optimum temperature is generally comprised between 293 and 353 K (Mehrotra et al. 2005).
Summary
Effective destruction of dyes is possible by photocatalysis in the presence of TiO2 suspensions. Various operational parameters affect the activities of TiO2 photocatalysts. Since the influence of the parameters has been in some cases controversial, it is therefore necessary to study the nature of the sample to be degraded, as this will provide a clue on the type of photocatalyst to be used in its degradation. The better understanding of the photocatalytic process and the operative conditions could give great opportunities for its application for the destruction of dyes.
References
Aguedach A, Brosillon S, Morvan J, Lhadi EK (2005) Photocatalytic degradation of azo-dyes reactive black 5 and reactive yellow 145 in water over a newly deposited titanium dioxide. Appl Catal B 57:55–62
Al-Harbi LM, Kosa SA, Abd El Maksod IH, Hegazy EZ (2015) The photocatalytic activity of TiO2-Zeolite composite for degradation of dye using synthetic UV and Jeddah sunlight. J Nanomater 15:1–6
Aramendia MA, Marinas A, Marinas JM, Moreno JM, Urbano FJ (2005) Photocatalytic degradation of herbicide fluroxypyr in aqueous suspension of TiO2. Catal Today 101:187–193
Atta NF, Amin HMA, Khalil MW, Galal A (2011) Nanotube arrays as photoanodes for dye sensitized solar cells using metal phthalocyanine dyes. Int J Electrochem Sci 6:3316–3332
Augugliaro V, Baiocchi C, Prevot AB, García-López E, Loddo V, Malato S et al (2002) Azo-dyes photocatalytic degradation in aqueous suspension of TiO2 under solar irradiation. Chemosphere 49:1223–1230
Avasarala BK, Tirukkovalluri SR, Bojja S (2010) Synthesis, characterization and photocatalytic activity of alkaline earth metal doped titania. Indian J Chem 49A:1189–1196
Bahnemann D (1999) Photocatalytic detoxification of polluted waters. In: Boule P (ed) The handbook of environmental chemistry 2. Part L: environmental photochemistry. Springer, Berlin, pp 285–351
Baran W, Makowski A, Wardas W (2008) The effect of UV radiation absorption of cationic and anionic dye solutions on their photocatalytic degradation in the presence TiO2. Dyes Pigments 76:226–230
Baraton MI, Merhari L (2004) Surface chemistry of TiO2 nanoparticles: influence on electrical and gas sensing properties. J Eur Ceram Soc 24:1399–1404
Beltran FJ, Gonzalez M, Alvarez P (1997) Tratamiento del agua mediante ozonación avanzada (I): Procesos con peróxido de hidrógeno. Ingeneria Quimica 331:161–168
Bickley RI, Carreno TG, Lees JS, Palmisano L, Tilley RJD (1991) A structural investigation of titanium dioxide photocatalysts. J Solid State Chem 92:178–190
Bubacz K, Choina J, Dolat D, Morawski AW (2010) Methylene blue and phenol photocatalytic degradation on nanoparticles of anatase TiO2. Pol J Environ Stud 19:685–691
Chanathaworn J, Bunyakan C, Wiyaratn W, Chungsiriporn J (2012) Photocatalytic decolorization of basic dye by TiO2 nanoparticle in photoreactor. Songklanakarin J Sci Technol 34:203–210
Chatterjee D, Dasgupta S (2005) Visible light induced photocatalytic degradation of organic pollutants. J Photochem Photobiol C 6:186–205
Chen Y, Wang K, Lou L (2004) Photodegradation of dye pollutants on silica gel supported TiO2 particles under visible light irradiation. J Photochem Photobiol A 163:281–287
Coleman HM, Vimonses V, Leslie G, Amal R (2007) Degradation of 1,4-dioxane in water using TiO2 based photocatalytic and H2O2/UV processes. J Hazard Mater 146:496–501
Cook SMF, Linden DR (1997) Use of Rhodamine WT to facilitate dilution and analysis of atrazine samples in short-term transport studies. J Environ Qual 26:1438–1441
Crini C (1996) Non-conventional low-cost adsorbents for dye removal: a review. Bioresour Technol 97:1061–1085
Daneshvar N, Salari D, Khataee AR (2003) Photocatalytic degradation of azo dye acid red 14 in water: investigation of the effect of operational parameters. J Photochem Photobiol A 157:111–116
Daneshvar N, Salari D, Khataee AR (2004) Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J Photochem Photobiol A 162:317–322
Davis RJ, Gainer JL, O’Neal G, Wu IW (1994) Photocatalytic decolorization of wastewater dyes. Water Environ Res 66:50–53
Dixit A, Mungray AK, Chakraborty M (2010) Photochemical oxidation of phenol and chlorophenol by UV/H2O2/TiO2 process: a kinetic study. Int J Chem Eng Appl 1(3):247–250
Esplugas S, Giménez J, Contreras S, Pascual E, Rodríguez M (2002) Comparison of different advanced oxidation processes for phenol degradation. Water Res 36:1034–1042
Fatin SO, Lim HN, Tan WT, Huang NM (2012) Comparison of photocatalytic activity and cyclic voltammetry of zinc oxide and titanium dioxide nanoparticles toward degradation of methylene blue. Int J Electrochem Sci 7:9074–9084
Galindo C, Kalt A (1999) UV–H2O2 oxidation of monoazo dyes in aqueous media: a kinetic study. Dyes Pigments 999(40):27–35
Galindo C, Jacques P, Kalt A (2001) Photooxidation of the phenylazonaphthol AO20 on TIO2: kinetic and mechanistic investigations. Chemosphere 45:997–1005
Giwa A, Nkeonye PO, Bello KA, Kolawole EG (2012) Solar photocatalytic degradation of reactive yellow 81 and reactive violet 1 in aqueous solution containing semiconductor oxides. Int J Appl Sci Technol 2:90–105
Gogate PR, Pandit AB (2004) A review of imperative technologies for wastewater treatment I: oxidation technologies at ambient conditions. Adv Environ Res 8:501–551
Gonçalves MST, Pinto EMS, Nkeonye P, Oliveira-Campos AMF (2005) Degradation of C.I. reactive orange 4 and its simulated dyebath wastewater by heterogeneous photocatalysis. Dyes Pigments 64:135–139
Grzechulska J, Morawski AW (2002) Photocatalytic decomposition of azo-dye acid black 1 in water over modified titanium dioxide. Appl Catal B 36:45–51
Guillard C, Lachheb H, Houas A, Ksibi M, Elaloui E, Herrmann JM (2003) Influence of chemical structure of dyes, of pH and of inorganic salts on their photocatalytic degradation by TiO2 comparison of the efficiency of powder and supported TiO2. J Photochem Photobiol A 158:27–36
Gulshan F, Sayaka Y, Yoshikazu K, Toshihiro I, Akira N, Kiyoshi O (2010) Photodecomposition of methylene blue by iron-oxides in an oxalate solution. J Water Res 44:2876–2884
Haferlach T, Bacher U, Kern W, Schnittger S, Haferlach C (2008) The diagnosis of BCR/ABL-negative chronic myeloproliferative diseases (CMPD): a comprehensive approach based on morphology, cytogenetics, and molecular markers. Ann Hematol 87:1–10
Hassan ME, Chen J, Liu G, Zhu D, Cai J (2014) Enhanced photocatalytic degradation of methyl orange dye under the daylight irradiation over CN-TiO2 modified with OMS-2. Materials 7:8024–8036
Herrmann JM (1995) Heterogeneous photocatalysis: an emerging discipline involving multiphase systems. Catal Today 24:157–164
Herrmann JM (1999) Heterogeneous photocatalysis: fundamentals and applications to the removal of various types of aqueous pollutants. Catal Today 53:115–129
Houas A, Lachheb H, Ksibi M, Elaloui E, Guillard C, Herrmann JM (2001) Photocatalytic degradation pathway of methylene blue in water. Appl Catal B 31:145–157
Hu C, Yu JC, Hao Z, Wong PK (2003a) Effects of acidity and inorganic ions on the photocatalytic degradation of different azo dyes. Appl Catal B 46:35–47
Hu C, Tang Y, Yu JC, Wong PK (2003b) Photocatalytic degradation of cationic blue X-GRL adsorbed on TiO2/SiO2 photocatalyst. Appl Catal B 40:131–140
Hung CH, Yuan C (2000) Reduction of Azo-dye via TiO2–photocatalysis. J Chin Inst Environ Eng 10:209–216
Ivanov K, Gruber E, Schempp W, Kirov D (1996) Possibilities of using zeolite as filler and carrier for dye stuffs in paper. Das Papier 50:456–460
Kamble SP, Sawant SB, Pangarkar VG (2003) Batch and continuous photocatalytic degradation of benzenesulfonic acid using concentrated solar radiation. Ind Eng Chem Res 42:6705–6713
Kaneco S, Rahman MA, Suzuki T, Katsumata H, Ohta K (2004) Optimization of solar photocatalytic degradation conditions of bisphenol A in water using titanium dioxide. J Photochem Photobiol A 163:419–424
Kansal SK, Kaur N, Singh S (2009) Photocatalytic degradation of two commercial reactive dyes in aqueous phase using nanophotocatalysts. Nanoscale Res Lett 4:709–716
Khan SUM, Al-Shahry M, Ingler WB Jr (2002) Efficient photochemical water splitting by a chemically modified n-TiO2. Science 297:2243–2245
Kim S, Hwang SJ, Choi W (2005) Visible Light Active Platinum-Ion-Doped TiO2 Photocatalyst. J Phys Chem B 109:24260–24267
Kiriakidou F, Kondarides DI, Verykios XE (1999) The effect of operational parameters and TiO2-doping on the photocatalytic degradation of azo-dyes. Catal Today 54:119–130
Konstantinou IK, Albanis TA (2004) TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations: a review. Appl Catal B 49:1–14
Kormann C, Bahnemann DW, Hoffman MR (1988) Photocatalytic production of H2O2 and organic peroxides in aqueous suspensions of TiO2, ZnO and desert sand. Environ Sci Technol 22:798–806
Kross BC, Nicholson HF, Ogilvie LK (1996) Methods development study for measuring pesticide exposure to golf course workers using video imaging techniques. Appl Occup Environ Hyg 11:1346–1350
Kuang S, Yang L, Luo S, Cai Q (2009) Fabrication, characterization and photoelectrochemical properties of Fe2O3 modified TiO2 nanotube arrays. Appl Surf Sci 255:7385–7388
Lachheb H, Puzenat E, Houas A, Ksibi M, Elaloui E, Guillard C et al (2002) Photocatalytic degradation of various types of dyes (Alizarin S, Crocein Orange G, Methyl Red, Congo Red, Methylene Blue) in water by UV-irradiated titania. Appl Catal B 39:75–90
Lee JM, Kim MS, Hwang B, Bae W, Kim BW (2003) Photodegradation of acid red 114 dissolved using a photo-Fenton process with TiO2. Dyes Pigments 56:59–67
Legrini O, Oliveros E, Braun AM (1993) Photochemical processes for water treatment. Chem Rev 93:671–698
Li L, Zhu W, Zhang P, Chen Z, Han W (2003) Photocatalytic oxidation and ozonation of catechol over carbon-black-modified nano-TiO2 thin films supported on Al sheet. Water Res 37:3646–3651
Lindner M, Bahnemann DW, Hirthe B, Griebler WD (1997) Solar water detoxification: novel TiO2 powders as highly active photocatalysts. J Sol Energy Eng 119:120–125
Ling CM, Mohamed AR, Bhatia S (2004) Performance of photocatalytic reactors using immobilized TiO2 film for the degradation of phenol and methylene blue dye present in water stream. Chemosphere 57:547–554
Liu CC, Hsieh YH, Lai PF, Li CH, Kao CL (2006) Photodegradation treatment of azo dye wastewater by UV/TiO2 process. Dyes Pigments 68:191–195
Luo Y, Ollis DF (1996) Heterogeneous photocatalytic oxidation of trichloroethylene and toluene mixtures in air: kinetic promotion and inhibition, time-dependent catalyst activity. J Catal 163:1–11
Mahmoodi NM, Arami M, Limaee NY, Tabrizi NS (2006) Kinetics of heterogeneous photocatalytic degradation of reactive dyes in an immobilized TiO2 photocatalytic reactor. J Colloid Interface Sci 295:159–164
Malato S, Blanco J, Richter C, Braun B, Maldonado MI (1998) Enhancement of the rate of solar photocatalytic mineralization of organic pollutants by inorganic oxidizing species. Appl Catal B 17:347–356
Malato S, Blanco J, Vidal A, Richter C (2002) Photocatalysis with solar energy at a pilot-plant scale: an overview. Appl Catal B 37:1–15
Malato S, Blanco J, Compos A, Caceres J, Guillard C, Herrmann JM et al (2003) Effect of operating parameters on the testing of new industrial titania catalysts at solar pilot plant scale. Appl Catal B: Environ 42:349–357
Mehrotra K, Yablonsky GS, Ray AK (2005) Macro kinetic studies for photocatalytic degradation of benzoic acid in immobilized systems. Chemosphere 60:1427–1436
Mengyue Z, Shifu C, Yaowu T (1995) Photocatalytic degradation of organophosphorus pesticides using thin films of TiO2. J Chem Technol Biotechnol 64:339–344
Mozia S, Morawski AW, Toyoda M, Inagaki M (2009) Application of anatase-phase TiO2 for decomposition of azo dye in a photocatalytic membrane reactor. Desalination 241:97–105
Muggli DS, Ding L (2001) Photocatalytic performance of sulfated TiO2 and Degussa P-25 TiO2 during oxidation of organics. Appl Catal B 32:181–194
Muneer M, Bahnemann D (2002) Semiconductor-mediated photocatalyzed degradation of two selected pesticide derivatives, terbacil and 2,4,5-tribromoimidazole, in aqueous suspension. Appl Catal B 36:95–111
Muruganandham M, Swaminathan M (2004) Solar photocatalytic degradation of a reactive azo dye in TiO2-suspension. Sol Energy Mater Sol Cells 81:439–457
Muruganandham M, Swaminathan M (2006) Photocatalytic decolourisation and degradation of reactive orange 4 by TiO2-UV process. Dyes Pigments 68:133–142
Muruganandham M, Shobana N, Swaminathan M (2006) Optimization of solar photocatalytic degradation conditions of reactive yellow 14 azo dye in aqueous TiO2. J Mol Catal A: Chem 246:154–161
Neppolian B, Choi HS, Sakthivel S, Arabindoo B, Murugesan V (2002a) Solar light induced and TiO2 assisted degradation of textile dye reactive blue 4. Chemosphere 46:1173–1181
Neppolian B, Choi HC, Sakthivel S, Arabindoo B, Murugesan V (2002b) Solar/UV-induced photocatalytic degradation of three commercial textile dyes. J Hazard Mater B89:303–317
Nguyen VH, Shawn DL, Wu JCS, Bai H (2014) Artificial sunlight and ultraviolet light induced photo-epoxidation of propylene over V-Ti/MCM-41 photocatalyst. J Nanotechnol 5:566–576
Nosaka Y, Fox MA (1988) Kinetics for electron transfer from laser-pulse irradiated colloidal semiconductors to adsorbed methylviologen: dependence of the quantum yield on incident pulse width. J Phys Chem 92:1893–1897
Ohno T, Sarukawa K, Tokieda K, Matsumura M (2001) Morphology of a TiO2 photocatalyst (Degussa, P-25) consisting of anatase and rutile crystalline phases. J Catal 203:82–86
Ollis DF, Pelizzetti E, Serpone N (1992) Photocatalyzed destruction of water contaminants. Environ Sci Technol 25:1522–1529
Pandurangan A, Kamala P, Uma S, Palanichamy M, Murugesan V (2001) Degradation of basic yellow auramine O-A textile dye by semiconductor photocatalysis. Indian J Chem Technol 8:496–499
Pelizzetti E, Carlin V, Minero C, Grätzel M (1991) Enhancement of the rate of photocatalytic degradation on TiO2 of 2-chlorophenol, 2,7-dichlorodibenzodioxin and atrazine by inorganic oxidizing species. New J Chem 15:351–359
Poulios I, Aetopoulou I (1999) Photocatalytic degradation of the textile dye reactive orange 16 in the presence of TiO2 suspensions. Environ Technol 20:479–487
Poulios I, Avranas A, Rekliti E, Zouboulis A (2000) Photocatalytic oxidation of Auramine O in the presence of semiconducting oxides. J Chem Technol Biotechnol 75:205–212
Prado AGS, Bolzon LB, Pedroso CP, Moura AO, Costa LL (2008) Nb2O5 as efficient and recyclable photocatalyst for indigo carmine degradation. Appl Catal B 82:219–224
Qamar M, Saquib M, Muneer M (2005) Photocatalytic degradation of two selected dye derivatives, chromotrope 2B and amido black 10B, in aqueous suspensions of titanium dioxide. Dyes Pigments 65:1–9
Rachel A, Sarakha M, Subrahmanyam M, Boule P (2002) Comparison of several titanium dioxides for the photocatalytic degradation of benzenesulfonic acids. Appl Catal B 37:293–300
Rao KVS, Subrahmanyam M, Boule P (2004) Immobilized TiO2 photocatalyst during long-term use: decrease of its activity. Appl Catal B 49:239–249
Rys P, Zollinger H (1972) Fundamentals of the chemistry and application of dyes, 1st edn. Wiley, New York
Saggioro EM, Oliveira AS, Pavesi T, Maia CG, Ferreira LFV, Moreira JC (2011) Use of titanium dioxide photocatalysis on the remediation of model textile wastewaters containing azo dyes. Molecules 16:10370–10386
Sakthivel S, Neppolian B, Shankar MV, Arabindoo B, Palanichamy M, Murugesan V (2003) Solar photocatalytic degradation of azo dye: comparison of photocatalytic efficiency of ZnO and TiO2. Sol Energy Mater Sol Cells 77:65–82
Saquib M, Muneer M (2003) TiO2-mediated photocatalytic degradation of a triphenylmethane dye (gentian violet), in aqueous suspensions. Dyes Pigments 56:37–49
Saquib M, Tariq MA, Faisal M, Muneer M (2008) Photocatalytic degradation of two selected dye derivatives in aqueous suspensions of titanium dioxide. Desalination 219:301–311
Segne TA, Tirukkovalluri SR, Challapalli S (2011) Studies on characterization and photocatalytic activities of visible light sensitive TiO2 nano catalysts co-doped with magnesium and copper. Int Res J Pure Appl Chem 1:84–103
Senthilkumaar S, Porkodi K, Gomathi R, Geetha Maheswari A, Manonmani N (2006) Sol–gel derived silver doped nanocrystalline titania catalysed photodegradation of methylene blue from aqueous solution. Dyes Pigments 69:22–30
Shukla SP, Gupta GS (1992) Toxic effects of omega chrome red ME and its treatment by adsorption. Ecotoxicol Environ Saf 24:155–163
Sivalingam G, Nagaveni K, Hegde MS, Madras G (2003) Photocatalytic degradation of various dyes by combustion synthesized nano anatase TiO2. Appl Catal B 45:23–38
Slampova A, Smela D, Vondrackova A, Jancarova I, Kuban V (2001) Determination of synthetic colorants in foodstuffs. Chem Listy 95:163–168
Slokar YM, Marechal AML (1998) Methods of decoloration of textile wastewaters. Dyes Pigments 37:335–356
So CM, Cheng MY, Yu JC, Wong PK (2002) Degradation of azo dye procion red MX-5B by photocatalytic oxidation. Chemosphere 46:905–912
Soares ET, Lansarin MA, Moro CC (2007) A study of process variables for the photocatalytic degradation of Rhodamine B. Braz J Chem Eng 24:29–36
Sohrabi MR, Ghavami M (2008) Photocatalytic degradation of direct red 23 dye using UV/TiO2: effect of operational parameters. J Hazard Mater 153:1235–1239
Sohrabi MR, Davallo M, Miri M (2009) Influence of operational parameters on eliminating azo dyes from wastewater by advanced oxidation technology. Int J ChemTech Res 1:446–451
Tanaka K, Capule MFV, Hisanaga T (1991) Effect of crystallinity of TiO2 on its photocatalytic action. Chem Phys Lett 187:73–76
Tanaka K, Padermpole K, Hisanaga T (2000) Photocatalytic degradation of commercial azo dyes. Water Res 34:327–333
Tang WZ, Zhang Z, An H, Quintana MO, Torres DF (1997) TiO2/UV photodegradation of azo dyes in aqueous solutions. Environ Technol 18:1–12
Tsui SM, Chu W (2001) Quantum yield study of the photodegradation of hydrophobic dyes in the presence of acetone sensitizer. Chemosphere 44:17–22
Tunay O, Kabdasli I, Ohron D, Cansever G (1999) Use and minimization of water in leather tanning processes. Water Sci Technol 40:237–244
Vandevivere PC, Bianchi R, Verstraete W (1998) Treatment and reuse of wastewater from the textile wet-processing industry: review of emerging technologies. J Chem Technol Biotechnol 72:289–302
Venkatadri R, Peters RW (1993) Chemical oxidation technologies: ultraviolet light/hydrogen peroxide, Fenton’s reagent, and titanium dioxide-assisted photocatalysis. Hazard Waste Hazard Mater 10:107–149
Wagner RW, Lindsey JS (1996) Boron-dipyrromethane dyes for incorporation in synthetic multi-pigment light-harvesting arrays. Pure Appl Chem 68:1373–1380
Wang S, Shiraishi F, Nakano K (2002) A synergistic effect of photocatalysis and ozonation on decomposition of formic acid in an aqueous solution. Chem Eng J 87:261–271
Wang N, Li J, Zhu L, Dong Y, Tang H (2008) Highly photocatalytic activity of metallic hydroxide/titanium dioxide nanoparticles prepared via a modified wet precipitation process. J Photochem Photobiol A 198:282–287
Watson SS, Beydoun D, Scott JA, Amal R (2003) The effect of preparation method on the photoactivity of crystalline titanium dioxide particles. Chem Eng J 95:213–220
Weber EJ, Stickney VC (1993) Hydrolysis kinetics of reactive blue 19-vinyl sulfone. Water Res 27:63–67
Wei TY, Wan CC (1991) Heterogeneous photocatalytic oxidation of phenol with titanium dioxide powders. Ind Eng Chem Res 30:1293–1300
Wrobel D, Boguta A, Ion RM (2001) Mixtures of synthetic organic dyes in a photoelectronic cell. J Photochem Photobiol A 138:7–22
Xiaohong W, Zhaohua J, Huiling L, Shigang X, Xinguo H (2003) Photo-catalytic activity of titanium dioxide thin films prepared by micro-plasma oxidation method. Thin Solid Films 441:130–134
Zhang Z, Wang CC, Zakaria R, Ying JY (1998) role of particle size in nanocrystalline TiO2-based photocatalysts. J Phys Chem B 102:10871–10878
Zhang T, Oyama T, Horikoshi S, Hidaka H, Zhao J, Serpone N (2002) Photocatalyzed N-demethylation and degradation of methylene blue in titania dispersions exposed to concentrated sunlight. Sol Energy Mater Sol Cells 73:287–303
Zhou S, Ray AK (2003) Kinetic studies for photocatalytic degradation of eosin B on a thin film of titanium dioxide. Ind Eng Chem Res 42:6020–6033
Zhu C, Wang L, Kong L, Yang X, Wang L, Zheng S et al (2000) Photocatalytic degradation of AZO dyes by supported TiO2 + UV in aqueous solution. Chemosphere 41:303–309
Zielińska B, Grzechulska J, Kaleńczuk RJ, Morawski AW (2003a) The pH influence on photocatalytic decomposition of organic dyes over A11 and P25 titanium dioxide. Appl Catal B 45:293–300
Zielińska B, Grzechulska J, Morawski AW (2003b) Photocatalytic decomposition of textile dyes on TiO2-Tytanpol A11 and TiO2-Degussa P25. J Photochem Photobiol A 157:65–70
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Reza, K.M., Kurny, A. & Gulshan, F. Parameters affecting the photocatalytic degradation of dyes using TiO2: a review. Appl Water Sci 7, 1569–1578 (2017). https://doi.org/10.1007/s13201-015-0367-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-015-0367-y