Abstract
Objective and design
Changes in the immune status of patients with sepsis may have a major impact on their prognosis. Our research focused on changes in various immune cell subsets and T-cell activation during the progression of sepsis.
Methods and subjects
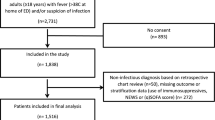
We collected data from 188 sepsis patients at the First Affiliated Hospital of Zhejiang University School of Medicine. The main focus was on the patient’s immunocyte subset typing, T-cell activation/Treg cell analysis, and cytokine assay, which can indicate the immune status of the patient.
Results
The study found that the number of CD4+ T cells, CD8+ T cells, NK cells, and B cells decreased early in the disease, and the decrease in CD4+ and CD8+ T cells was more pronounced in the death group. T lymphocyte activation was inhibited, and the number of Treg cells increased as the disease progressed. T lymphocyte inhibition was more significant in the death group, and the increase in IL-10 was more significant in the death group. Finally, we used patients’ baseline conditions and immunological detection indicators for modeling and found that IL-10, CD4+ Treg cells, CD3+HLA-DR+ T cells, and CD3+CD69+ T cells could predict patients’ prognosis well.
Conclusion
Our study found that immunosuppression occurs in patients early in sepsis. Early monitoring of the patient’s immune status may provide a timely warning of the disease.






Similar content being viewed by others
Data availability
The data supporting the results of this research are available from the corresponding author on reasonable request.
References
Venet F, Monneret G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat Rev Nephrol. 2018;14(2):121–37.
Lu X, Yang YM, Lu YQ. Immunosenescence: a critical factor associated with organ injury after sepsis. Front Immunol. 2022;13: 917293.
van der Poll T, Shankar-Hari M, Wiersinga WJ. The immunology of sepsis. Immunity. 2021;54(11):2450–64.
Ruan WS, et al. Early activation of myeloid-derived suppressor cells participate in sepsis-induced immune suppression via PD-L1/PD-1 Axis. Front Immunol. 2020;11:1299.
Li L, Lu YQ. The regulatory role of high-mobility group protein 1 in sepsis-related immunity. Front Immunol. 2020;11: 601815.
Monneret G, Gossez M, Venet F. Sepsis and immunosenescence: closely associated in a vicious circle. Aging Clin Exp Res. 2021;33(3):729–32.
Yang X, et al. Deregulation of T cell response in sepsis. Front Biosci (Landmark Ed). 2014;19(8):1370–6.
Kumar V. T cells and their immunometabolism: a novel way to understanding sepsis immunopathogenesis and future therapeutics. Eur J Cell Biol. 2018;97(6):379–92.
Wik JA, Skålhegg BS. T cell metabolism in infection. Front Immunol. 2022;13: 840610.
Hohlstein P, et al. Prognostic relevance of altered lymphocyte subpopulations in critical illness and sepsis. J Clin Med. 2019. https://doi.org/10.3390/jcm8030353.
Venet F, et al. Increased circulating regulatory T cells (CD4(+)CD25 (+)CD127 (-)) contribute to lymphocyte anergy in septic shock patients. Intensive Care Med. 2009;35(4):678–86.
Faivre V, et al. Human monocytes differentiate into dendritic cells subsets that induce anergic and regulatory T cells in sepsis. PLoS ONE. 2012;7(10): e47209.
Szeto C, et al. Impact of HLA-DR antigen binding cleft rigidity on T cell recognition. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21197081.
Kar A, Mehrotra S, Chatterjee S. CD38: T Cell immuno-metabolic modulator. Cells. 2020. https://doi.org/10.3390/cells9071716.
Esensten JH, et al. CD28 costimulation: from mechanism to therapy. Immunity. 2016;44(5):973–88.
Cibrián D, Sánchez-Madrid F. CD69: from activation marker to metabolic gatekeeper. Eur J Immunol. 2017;47(6):946–53.
Jarczak D, Nierhaus A. Cytokine storm-definition, causes, and implications. Int J Mol Sci. 2022. https://doi.org/10.3390/ijms231911740.
Ye Q, et al. An imbalance of T cell subgroups exists in children with sepsis. Microbes Infect. 2019;21(8–9):386–92.
Fajgenbaum DC, June CH. Cytokine Storm. N Engl J Med. 2020;383(23):2255–73.
Chousterman BG, Swirski FK, Weber GF. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol. 2017;39(5):517–28.
Singer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10.
Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862–74.
Lindell RB, et al. Impaired lymphocyte responses in pediatric sepsis vary by pathogen type and are associated with features of immunometabolic dysregulation. Shock. 2022;57(6):191–9.
Fabri A, et al. Characterization of circulating IL-10-producing cells in septic shock patients: a proof of concept study. Front Immunol. 2020;11: 615009.
Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348(2):138–50.
Song CY, et al. Immune dysfunction following COVID-19, especially in severe patients. Sci Rep. 2020;10(1):15838.
Carvelli J, et al. Imbalance of circulating innate lymphoid cell subpopulations in patients with septic shock. Front Immunol. 2019;10:2179.
Reizine F, et al. Beneficial effects of citrulline enteral administration on sepsis-induced T cell mitochondrial dysfunction. Proc Natl Acad Sci USA. 2022. https://doi.org/10.1073/pnas.2115139119.
Taylor MD, et al. CD4 and CD8 T cell memory interactions alter innate immunity and organ injury in the CLP sepsis model. Front Immunol. 2020;11: 563402.
Gorabi AM, et al. The pivotal role of CD69 in autoimmunity. J Autoimmun. 2020;111: 102453.
van de Donk N, Richardson PG, Malavasi F. CD38 antibodies in multiple myeloma: back to the future. Blood. 2018;131(1):13–29.
Petersen OH. Is CD38 involved in Ca(2+) signalling elicited by activation of T cell receptors? Cell Calcium. 2022;101: 102524.
Ahmed A, Vyakarnam A. Emerging patterns of regulatory T cell function in tuberculosis. Clin Exp Immunol. 2020;202(3):273–87.
Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(4):227–42.
Franceschi C, et al. Inflammaging and “Garb-aging.” Trends Endocrinol Metab. 2017;28(3):199–212.
Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345(6274):458–60.
Guignant C, et al. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit Care. 2011;15(2):R99.
Lawrence KL, et al. CD4+ lymphocyte adenosine triphosphate determination in sepsis: a cohort study. Crit Care. 2010;14(3):R110.
Millrud CR, Bergenfelz C, Leandersson K. On the origin of myeloid-derived suppressor cells. Oncotarget. 2017;8(2):3649–65.
Arnold CR, et al. Gain and loss of T cell subsets in old age–age-related reshaping of the T cell repertoire. J Clin Immunol. 2011;31(2):137–46.
de Lima MHF, et al. Sepsis-Induced immunosuppression is marked by an expansion of a highly suppressive repertoire of FOXP3+ T-regulatory cells expressing TIGIT. J Infect Dis. 2022;225(3):531–41.
Nascimento DC, et al. IL-33 contributes to sepsis-induced long-term immunosuppression by expanding the regulatory T cell population. Nat Commun. 2017;8:14919.
Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13(3):108–16.
Acknowledgements
This research was supported by the Key Research and Development Program of Zhejiang Province (2019C03076).
Author information
Authors and Affiliations
Contributions
Study conception and design: XL, C-YS, Y-QL; data collection: XL, PW, LL, L-YL, SJ, J-NZ; data analysis: XL, M-XF; drafting of the article: XL; critical revision: Y-MY, Y-QL. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The studies involving human participants were reviewed and approved by the Ethics Committee of The First Affiliated Hospital, Zhejiang University School of Medicine (20230150). This study was in accordance with the national legislation and the institutional requirements. The written informed consent was obtained by the patients/participants. The procedures were in accordance with the Helsinki Declaration of 1964.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, X., Song, CY., Wang, P. et al. The clinical trajectory of peripheral blood immune cell subsets, T-cell activation, and cytokines in septic patients. Inflamm. Res. 73, 145–155 (2024). https://doi.org/10.1007/s00011-023-01825-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-023-01825-w