Abstract
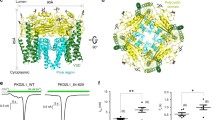
Here we present the structure of the T1 domain derived from the voltage-dependent potassium channel Kv1.3 of Homo sapiens sapiens at 1.2 Å resolution crystallized under near-physiological conditions. The crystals were grown without precipitant in 150 mM KPi, pH 6.25. The crystals show I4 symmetry typical of the natural occurring tetrameric assembly of the single subunits. The obtained structural model is based on the highest resolution currently achieved for tetramerization domains of voltage-gated potassium channels. We identified an identical fold of the monomer but inside the tetramer the single monomers show a significant rotation which leads to a different orientation of the tetramer compared to other known structures. Such a rotational movement inside the tetrameric assembly might influence the gating properties of the channel. In addition we see two distinct side chain configurations for amino acids located in the top layer proximal to the membrane (Tyr109, Arg116, Ser129, Glu140, Met142, Arg146), and amino acids in the bottom layer of the T1-domain distal from the membrane (Val55, Ile56, Leu77, Arg86). The relative populations of these two states are ranging from 50:50 for Val55, Tyr109, Arg116, Ser129, Glu140, 60:40 for Met142, 65:35 for Arg86, 70:30 for Arg146, and 80:20 for Ile56 and Leu77. The data suggest that in solution these amino acids are involved in an equilibrium of conformational states that may be coupled to the functional states of the whole potassium channel.





Similar content being viewed by others
Abbreviations
- NMR:
-
Nuclear magnetic resonance
- NMM:
-
New minimal medium
- TROSY:
-
Transversed relaxation optimized spectroscopy
- HSQC:
-
Heteronuclear single quantum coherence
- DSS:
-
4,4-dimethyl-4-silapentane-1-sulfonic acid
- MR:
-
Molecular replacement
- SLS:
-
Swiss light source
- CCP4:
-
Collaborative Computational Project No. 4
References
Ader C, Schneider R, Hornig S, Velisetty P, Wilson EM, Lange A, Giller K, Ohmert I, Martin-Eauclaire MF, Trauner D, Becker S, Pongs O, Baldus M (2008) Nat Struct Mol Biol 15:605–612
Antz C, Geyer M, Fakler B, Schott MK, Frank R, Guy HR, Ruppersberg JP, Kalbitzer HR (1997) Nature 385:272–275
Bähring R, Covarrubias M (2011) J Physiol 589(3):461–479
Beeton C, Wulff H, Standifer NE, Azam P, Mullen KM, Pennington MW, Kolski-Andreaco A, Wei E, Grino A, Counts DR, Wang PH, LeeHealey CJ, Andrews BS, Sankaranarayanan A, Homerick D, Roeck WW, Tehranzadeh J, Stanhope KL, Zimin P, Havel PJ, Griffey S, Knaus HG, Nepom GT, Gutman GA, Calabresi PA, Chandy KG (2006) Proc Natl Acad Sci USA 103:17414–17419
Bixby KA, Nanao MH, Shen NV, Kreusch A, Bellamy H, Pfaffinger PJ, Choe S (1999) Nat Struct Biol 6:38–43
Budisa N, Steipe B, Demange P, Eckerskorn C, Kellerman J, Huber R (1995) Eur J Biochem 230:788–796
Chen X, Wang Q, Ni F, Ma J (2010) Proc Natl Acad Sci USA 107:11352–11357
Choe S, Kreusch A, Pfaffinger PJ (1999) Trends Biochem Sci 24:345–349
Collaborative Computational Project, Number 4 (1994). Acta Cryst D50:760–763
Covarrubias M, Bhattacharji A, De Santiago-Castillo JA, Dougherty K, Kaulin YA, Na-Phuket TR, Wang G (2008) Neurochem Res 33:1558–1567
Cushman SJ, Nanao MH, Jahng AW, DeRubeis D, Choe S, Pfaffinger PJ (2000) Nat Struct Biol 7:403–407
Diederichs K, Karplus PA (1997) Nat Struct Biol 4:269–275
Doyle DA, Cabral JM, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R (1998) Science 280:69–77
Emsley P, Cowtan K (2004) Acta Cryst D60:2126–2132
Escobar LI, Martínez-Téllez JC, Salas M, Castilla SA, Carrisoza R, Tapia D, Vázquez M, Bargas J, Bolívar JJ (2004) Am J Physiol Cell Physiol 286:C965–C974
Garcia ML (2004) Nature 430:153–155
Gronwald W, Kalbitzer HR (2004) Progr NMR Spectr 44:33–96
Grunnet M, Rasmussen HB, Hay-Schmidt A, Klaerke DA (2003) Biochim Biophys Acta 1616:85–94
Gulbis JM, Zhou M, Mann S, MacKinnon R (2000) Science 289:123–127
Jan LY, Jan YN (1994) Nature 371:119–122
Jan LY, Jan YN (2012) J Physiol 590(11):2591–2599
Jensen MO, Jogini V, Borhani DW, Leffler AE, Dror RO, Shaw DE (2012) Science 336:229–233
Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R (2002) Nature 417:515–522
Jiang Y, Lee A, Chen J, Ruta V, Cadene M, Chait BT, MacKinnon R (2003) Nature 423:33–41
Kosolapov A, Tu L, Wang J, Deutsch C (2004) Neuron 44:295–307
Kreusch A, Pfaffinger PJ, Stevens CF, Choe S (1998) Nature 392:945–948
Kuo A, Gulbis JM, Antcliff JF, Rahman T, Lowe ED, Zimmer J, Cuthbertson J, Ashcroft FM, Ezaki T, Doyle DA (2003) Science 300:1922–1926
Lange A, Giller K, Hornig S, Martin-Eauclaire MF, Pongs O, Becker S, Baldus M (2006) Nature 440:959–962
Live DH, Davis DG, Agosta WC, Cowburn D (1984) J Am Chem Soc 106:1939–1941
Long SB, Campbell EB, MacKinnon R (2005) Science 309:897–903
Long SB, Tao X, Campbell EB, MacKinnon R (2007) Nature 450:376–382
MacKinnon R, Cohen SL, Kuo A, Lee A, Chait BT (1998) Science 280:106–109
McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ (2007) J Appl Cryst 40:658–674
Minor DL Jr, Lin YF, Mobley BC, Avelar A, Jan YN, Jan LY, Berger JM (2000) Cell 102:657–670
Murshudov GN, Vagin AA, Dodson EJ (1997) Acta Crystallogr D53:240–255
Nanao MH, Zhou W, Pfaffinger PJ, Choe S (2003) Proc Natl Acad Sci USA 100:8670–8675
Panyi G, Vámosi G, Bacsó Z, Bagdány M, Bodnár A, Varga Z, Gáspár R, Mátyus L, Damjanovich S (2004) Proc Natl Acad Sci USA 101:1285–1290
Pervushin K, Riek R, Wider G, Wüthrich K (1997) Proc Natl Acad Sci USA 94:12366–12371
Pervushin K (2000) Q Rev Biophys 33:161–197
Pioletti M, Findeisen F, Hura GL, Minor DL Jr (2006) Nat Struct. Mol Biol 13:987–995
Prince-Carter A, Pfaffinger PJ (2009) J Gen Physiol 134:15–34
Roosild TP, Lê KT, Choe S (2004) Trends Biochem Sci 29:39–45
Rus H, Pardo CA, Hu L, Darrah E, Cudrici C, Niculescu T, Niculescu F, Mullen KM, Allie R, Guo L, Wulff H, Beeton C, Judge SIV, Kerr DA, Knaus HG, Chandy KG, Calabresi PA (2005) Proc Natl Acad Sci USA 102:11094–11099
Scannevin RH, Wang K, Jow F, Megules J, Kopsco DC, Edris W, Carroll KC, Lü Q, Xu W, Xu Z, Katz AH, Olland S, Lin L, Taylor M, Stahl M, Malakian K, Somers W, Mosyak L, Bowlby MR, Chanda P, Rhodes KJ (2004) Neuron 41:87–598
Szabó I, Bock J, Grassmé H, Soddemann M, Wilker B, Lang F, Zoratti M, Gulbins E (2008) Proc Natl Acad Sci USA 105:14861–14866
Tao X, Lee A, Limapichat W, Dougherty DA, MacKinnon R (2010) Science 328:67–73
Wang G, Shahidullah M, Rocha CA, Strang C, Pfaffinger PJ, Covarrubias M (2005) J Gen Physiol 126:55–69
Wang G, Covarrubias M (2006) J Gen Physiol 127:391–400
Wang G, Strang C, Pfaffinger PJ, Covarrubias M (2007) J Biol Chem 282:13637–13647
Wang H, Yan Y, Liu Q, Huang Y, Shen Y, Chen L, Chen Y, Yang Q, Hao Q, Wandg K, Chai J (2007) Nat Neurosci 10:32–39
Winklmeier A, Weyand M, Schreier C, Kalbitzer HR, Kremer W (2009) Acta Cryst F65:688–691
Wishart DS, Bigam CG, Yao J, Abildgaard F, Dyson HJ, Oldfield E, Markley JL, Sykes BD (1995) J Biomol NMR 6:135–140
Xu J, Wang P, Li Y, Li G, Kaczmarek LK, Wu Y, Koni PA, Flavell RA, Desir GV (2004) Proc Natl Acad Sci USA 101:3112–3117
Yellen G (2002) Nature 419:35–42
Yellen G (1998) Q Rev Biophys 31:239–295
Yi BA, Minor DL Jr, Lin YF, Jan YN, Jan LY (2001) Proc Natl Acad Sci USA 98:11016–11023
Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R (2001) Nature 414:43–48
Acknowledgments
This work was supported by the European Union, the Fonds der Chemischen Industrie (FCI), and the Deutsche Forschungsgemeinschaft (SFB 699). The crystallographic experiments were performed on the X10SA beamline at the SLS, Paul Scherrer Institut, Villigen, Switzerland.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
The coordinates and structure factors have been deposited in the Protein Data Bank under accession number 4BGC.
Werner Kremer and Michael Weyand have contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kremer, W., Weyand, M., Winklmeier, A. et al. 1.2 Å X-ray Structure of the Renal Potassium Channel Kv1.3 T1 Domain. Protein J 32, 533–542 (2013). https://doi.org/10.1007/s10930-013-9513-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-013-9513-2