Abstract
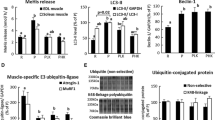
The aim of this study was to elucidate the effects of long-term intake of leucine in dietary protein malnutrition on muscle protein synthesis and degradation. A reduction in muscle mass was suppressed by leucine-supplementation (1.5% leucine) in rats fed protein-free diet for 7 days. Furthermore, the rate of muscle protein degradation was decreased without an increase in muscle protein synthesis. In addition, to elucidate the mechanism involved in the suppressive effect of leucine, we measured the activities of degradation systems in muscle. Proteinase activity (calpain and proteasome) and ubiquitin ligase mRNA (Atrogin-1 and MuRF1) expression were not suppressed in animals fed a leucine-supplemented diet, whereas the autophagy marker, protein light chain 3 active form (LC3-II), expression was significantly decreased. These results suggest that the protein-free diet supplemented with leucine suppresses muscle protein degradation through inhibition of autophagy rather than protein synthesis.







Similar content being viewed by others
References
Aki M, Shimbara N, Takashina M, Akiyama K, Kagawa S, Tamura T, Tanahashi N, Yoshimura T, Tanaka K, Ichihara A (1994) Interferon-gamma induces different subunit organizations and functional diversity of proteasomes. J Biochem (Tokyo) 115:257–269
Anthony JC, Reiter AK, Anthony TG, Crozier SJ, Lang CH, MacLean DA, Kimball SR, Jefferson LS (2002) Orally administered leucine enhances protein synthesis in skeletal muscle of diabetic rats in the absence of increases in 4E-BP1 or S6K1 phosphorylation. Diabetes 51:928–936
Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ (2001) Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294:1704–1708
Buse MG, Reid SS (1975) Leucine. A possible regulator of protein turnover in muscle. J Clin Invest 56:1250–1261
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenolchloroform extraction. Anal Biochem 162:156–159
Debras E, Prod’homme M, Rieu I, Balage M, Dardevet D, Grizard J (2007) Postprandial leucine deficiency failed to alter muscle protein synthesis in growing and adult rats. Nutrition 23:267–276
Fang CH, Li BG, Fischer DR, Wang JJ, Runnels HA, Monaco JJ, Hasselgren PO (2000) Burn injury upregulates the activity and gene expression of the 20 S proteasome in rat skeletal muscle. Clin Sci (Lond) 99:181–187
Fujita T, Kajita M, Sano H (2006) Responses of whole body protein synthesis, nitrogen retention and glucose kinetics to supplemental starch in goats. Comp Biochem Physiol B Biochem Mol Biol 144:180–187
Hamel FG, Upward JL, Siford GL, Duckworth WC (2003) Inhibition of proteasome activity by selected amino acids. Metabolism 52:810–814
Harper AE, Miller RH, Block KP (1984) Branched-chain amino acid metabolism. Annu Rev Nutr 4:409–454
Haverberg LN, Deckelbaum L, Bilmazes C, Munro HN, Young VR (1975) Myofibrillar protein turnover and urinary N τ-methylhistidine output. Response to dietary supply of protein and energy. Biochem J 152:503–510
Higuchi K, Hayashi K, Ohtsuka A, Tomita Y (1996) Calcitonin decreases corticosterone-induced skeletal muscle calpain activity. J Nutr Sci Vitaminol (Tokyo) 42:491–496
Ito Y, Oumi S, Nagasawa T, Nishizawa N (2006) Oxidative stress induces phosphoenolpyruvate carboxykinase expression in H4IIE cells. Biosci Biotechnol Biochem 70:2191–2198
Kadowaki M, Kanazawa T (2003) Amino acids as regulators of proteolysis. J Nutr 133:2052S–2056S
Karim MR, Kanazawa T, Daigaku Y, Fujimura S, Miotto G, Kadowaki M (2007) Cytosolic LC3 ratio as a sensitive index of macroautophagy in isolated rat hepatocytes and H4-II-E cells. Autophagy 3:553–560
Kimball SR, Jefferson LS (2004) Regulation of global and specific mRNA translation by oral administration of branched-chain amino acids. Biochem Biophys Res Commun 313:423–427
Kimball SR, Shantz LM, Horetsky RL, Jefferson LS (1999) Leucine regulates translation of specific mRNAs in L6 myoblasts through mTOR-mediated changes in availability of eIF4E and phosphorylation of ribosomal protein S6. J Biol Chem 274:11647–11652
Li C, Najafi H, Daikhin Y, Nissim IB, Collins HW, Yudkoff M, Matschinsky FM, Stanley CA (2003) Regulation of leucine-stimulated insulin secretion and glutamine metabolism in isolated rat islets. J Biol Chem 278:2853–2858
Matthews DE (2005) Observations of branched-chain amino acid administration in humans. J Nutr 135:1580S–1584S
Mordier S, Deval C, Bechet D, Tassa A, Ferrara M (2000) Leucine limitation induces autophagy and activation of lysosome-dependent proteolysis in C2C12 myotubes through a mammalian target of rapamycin-independent signaling pathway. J Biol Chem 275:29900–29906
Nagasawa T, Hirano J, Yoshizawa F, Nishizawa N (1998) Myofibrillar protein catabolism is rapidly suppressed following protein feeding. Biosci Biotechnol Biochem 62:1932–1937
Nagasawa T, Kido T, Yoshizawa F, Ito Y, Nishizawa N (2002) Rapid suppression of protein degradation in skeletal muscle after oral feeding of leucine in rats. J Nutr Biochem 13:121–127
Nagasawa T, Kikuchi N, Ito Y, Yoshizawa F, Nishizawa N (2004) Suppression of myofibrillar protein degradation after refeeding in young and adult mice. J Nutr Sci Vitaminol (Tokyo) 50:227–230
Nagasawa T, Sakai T, Onodera R (1991) Simple and sensitive determination of plasma N τ-methylhistidine by high-performance liquid chromatography using pre-column derivative formation with o-phthalaldehyde-2-mercaptoethanol. J Chromatogr 566:223–227
Nagasawa T, Yoshizawa F, Nishizawa N (1996) Plasma N τ-methylhistidine concentration is a sensitive index of myofibrillar protein degradation during starvation in rats. Biosci Biotechnol Biochem 60:501–502
Nakashima K, Ishida A, Yamazaki M, Abe H (2005) Leucine suppresses myofibrillar proteolysis by down-regulating ubiquitin-proteasome pathway in chick skeletal muscles. Biochem Biophys Res Commun 336:660–666
Ono Y, Torii F, Ojima K, Doi N, Yoshioka K, Kawabata Y, Labeit D, Labeit S, Suzuki K, Abe K, Maeda T, Sorimachi H (2006) Suppressed disassembly of autolyzing p94/CAPN3 by N2A connectin/titin in a genetic reporter system. J Biol Chem 281:18519–18531
Rajan VR, Mitch WE (2008) Muscle wasting in chronic kidney disease: the role of the ubiquitin proteasome system and its clinical impact. Pediatr Nephrol 23:527–535
Reeves PG, Nielsen FH, Fahey GC Jr (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951
Rieu I, Sornet C, Bayle G, Prugnaud J, Pouyet C, Balage M, Papet I, Grizard J, Dardevet D (2003) Leucine-supplemented meal feeding for ten days beneficially affects postprandial muscle protein synthesis in old rats. J Nutr 133:1198–1205
Rieu I, Balage M, Sornet C, Debras E, Ripes S, Rochon-Bonhomme C, Pouyet C, Grizard J, Dardevet D (2007) Increased availability of leucine with leucine-rich whey proteins improves postprandial muscle protein synthesis in aging rats. Nutrition 23:323–331
Smith K, Reynolds N, Downie S, Patel A, Rennie MJ (1998) Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am J Physiol 275:E73–E78
Sugawara T, Ito Y, Nishizawa N, Nagasawa T (2007) Supplementation with dietary leucine to a protein-deficient diet suppresses myofibrillar protein degradation in rats. J Nutr Sci Vitaminol (Tokyo) 53:552–555
Sugden PH, Fuller SJ (1991) Regulation of protein turnover in skeletal and cardiac muscle. Biochem J 273:21–37
Thomas DR (2007) Loss of skeletal muscle mass in aging: examining the relationship of starvation, sarcopenia and cachexia. Clin Nutr 26:389–399
Wassner SJ, Schlitzer JL, Li JB (1980) A rapid, sensitive method for the determination of 3-methylhistidine levels in urine and plasma using high-pressure liquid chromatography. Anal Biochem 104:284–289
Welle S, Bhatt K, Pinkert CA (2006) Myofibrillar protein synthesis in myostatin-deficient mice. Am J Physiol Endocrinol Metab 290:E409–E415
Yoshizawa F, Kido T, Nagasawa T (1999) Stimulative effect of dietary protein on the phosphorylation of p70 S6 kinase in the skeletal muscle and liver of food-deprived rats. Biosci Biotechnol Biochem 63:1803–1805
Acknowledgments
We thank Prof. Kadowaki (Niigata University) for discussion about autophagy. This work was supported in part by Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science; the 21st century COE in Iwate University program from the Ministry of Education, Culture, Sports, Science and Technology of Japan; and Ajinomoto Co. Ltd.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sugawara, T., Ito, Y., Nishizawa, N. et al. Regulation of muscle protein degradation, not synthesis, by dietary leucine in rats fed a protein-deficient diet. Amino Acids 37, 609–616 (2009). https://doi.org/10.1007/s00726-008-0180-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-008-0180-0