Abstract
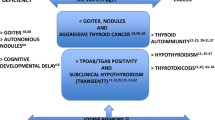
Iodine deficiency is still the main cause of preventable thyroid disorders, worldwide. To optimize iodine intake, programs of voluntary or mandatory iodization of salt have been implemented in several iodine-deficient countries and iodine sufficiency has been achieved in many. Despite the clear beneficial effects on thyroid health, some concerns have been raised on the presumed detriment of iodine prophylaxis on thyroid autoimmunity. Very recent studies aimed at evaluating the long-term consequences of iodine supplementation on thyroid autoimmunity and related dysfunction, have clearly demonstrated that the early post-iodization increase in thyroid antibody positivity is largely transient and not clinically relevant, since the prevalence of overt thyroid dysfunction has remained reassuring low over two decades. The recommended iodine intake is therefore safe with regard to thyroid autoimmunity, the benefits largely outweighing the risks in a population with a stable median iodine concentration not exceeding 300 μg/L. Thus, a possible increase in thyroid autoimmunity should not represent a limitation to promoting iodine supplementation in the general population, also taking into account the steady rise in prevalence of autoimmune disorders which has occurred in the last few decades because of environmental factors other than iodine.

Similar content being viewed by others
References
Sevringhaus EL, Barbour JH (1941) Adequacy of iodised salt for goiter prevention. J ClinEndocrinol 1:850–851. https://doi.org/10.1210/jcem-1-10-85
Vermiglio F, Finocchiaro MD, Lo Presti VP, La Torre N, Nucifora M, Trimarchi F (1989) Partial beneficial effects of the so called “silent iodine prophylaxis” on iodine deficiency disorders (IDD) in northeastern Sicily endemia. J Endocrinol Invest 12:123–126. https://doi.org/10.1007/BF03349938
Völzke H, Caron P, Dahl L et al (2016) Ensuring effective prevention of iodine deficiency disorders. Thyroid 26:189–196. https://doi.org/10.1089/thy.2015.0543.Erratum.In:Thyroid.2016;26:1148
Schaffner M, Rochau U, Stojkov I, Rushaj VQ, Völzke H, Marckmann LJH, Oberaigner W, Siebert U (2020) Barriers against prevention programs for iodine deficiency disorders in Europe: a delphi study affiliations expand. Thyroid. https://doi.org/10.1089/thy.2020.0065
Laurberg P, Cerqueira C, Ovesen L, Rasmussen LB, Perrild H, Andersen S, Bulow Pedersen IB, Carlè A (2010) Iodine intake as a determinant of thyroid disorders in populations. Best Pract Res ClinEndocrinolMetab 24:13–27
Zimmermann MB, Andersson M (2012) Assessment of iodine nutrition in populations: past, present, and future. Nutr Rev 70:553–570
Lazarus JH (2021) Monitoring iodine nutritional status: adults or schoolchildren? J Endocrinol Invest 44:383–385
Farebrother J, Zimmermann MB, Andersson M (2019) Excess iodine intake: sources, assessment, and effect on thyroid function. Ann N Y AcadSci 1446:44–65. https://doi.org/10.1111/nyas.14041
Olivieri A, Trimarchi F, Vitti P (2020) Global iodine nutrition 2020: Italy is an iodine sufficient country. J Endocrinol Invest 43(11):1671–1672. https://doi.org/10.1007/s40618-020-01402-6
Laurberg P, Pedersen KM, Hreidarsson A, Sigfusson N, Iversen E, Knudsen PR (1998) Iodine intake and the pattern of thyroid disorders: a comparative epidemiological study of thyroid abnormalities in the elderly in Iceland and in Jutland, Denmark. J ClinEndocrinolMetab 83:765–769. https://doi.org/10.1210/jcem.83.3.4624
Bulow Pedersen IB, Laurberg P, Arnfred T, Knudsen N, Jørgensen T, Perrild H, Ovesen L (2002) Surveillance of disease frequency in a population by linkage to diagnostic laboratory databases. a system for monitoring the incidences of hyper- and hypothyroidism as part of the Danish iodine supplementation program. Comput Methods Programs Biomed 67:209–216
Bulow Pedersen IB, Laurberg P, Knudsen N et al (2006) Increase in incidence of hyperthyroidism predominantly occurs in young people after iodine fortification of salt in Denmark. J ClinEndocrinolMetab 91:3830–3834
Cerqueira C, Knudsen N, Ovesen L, Perrild H, Rasmussen LB, Laurberg P, Jørgensen T (2009) Association of iodine fortification with incident use of anti-thyroid medication—a Danish nationwide study. J ClinEndocrinolMetab 94:2400–2405. https://doi.org/10.1210/jc.2009-0123
Laurberg P, Jørgensen T, Perrild H, Ovesen L, Knudsen N, Pedersen IB, Rasmussen LB, Carlé A, Vejbjerg P (2006) The Danish investigation on iodine intake and thyroid disease, DanThyr: status and perspectives. Eur J Endocrinol 155:219–228. https://doi.org/10.1530/eje.1.02210.Erratum.In:EurJEndocrinol.2006,155:643
Pedersen IB, Laurberg P, Knudsen N, Jørgensen T, Perrild H, Ovesen L, Rasmussen LB (2007) An increased incidence of overt hypothyroidism after iodine fortification of salt in Denmark: a prospective population study. J ClinEndocrinolMetab 92:3122–3127
Vejbjerg P, Knudsen N, Perrild H, Carle A, Laurberg P, Pedersen IB, Rasmussen LB, Ovesen L, Jorgensen T (2007) Effect of a mandatory iodization program on thyroid gland volume based on individuals’ age, gender, and preceding severity of dietary iodine deficiency: a prospective, population-based study. J ClinEndocrinolMetab 92:1397–1401
Peteresen M, Pedersen IB, Knudsen M, Andersen S, Jørgensen T, Perrild H, Ovesen L, Rasmussen LB, Thuesen BH, Carlé A (2019) Changes in subtypes of overt thyrotoxicosis and hypothyroidism following iodine fortification. ClinEndocrinol 91:652–659. https://doi.org/10.1111/cen.14072
Baltisberger BL, Minder CE, Burgi H (1995) Decrease of incidence of toxic nodular goitre in a region of Switzerland after full correction of mild iodine deficiency. Eur J Endocrinol 132:546–549
Mostbeck A, Galvan G, Bauer P et al (1990) The incidence of hyperthyroidism in Austria from 1987 to 1995 before and after an increase in salt iodization in 1990. Eur J Nucl Med 25:367–374
Aghini Lombardi F, Fiore E, Tonacchera M, Antonangeli M, Rago T, Frigeri M, Provenzale AM, Montanelli L, Grasso L, Pinchera A, Vitti P (2010) The effect of voluntary iodine prophylaxis in a small rural community: the Pescopagano survey 15 years later. J ClinEndocrinolMetab 98:1031–1039. https://doi.org/10.1210/jc.2012-2960
Ippolito S, Cusini C, Lasalvia P, Gianfagna F, Veronesi G, Gallo D, Masiello E, Premoli P, Sabatino J, Mercuriali A, Lai A, Piantanida E, Tanda ML, Bartalena L (2020) Change in newly diagnosed Graves’ disease phenotype between the twentieth and the twenty-first centuries: meta-analysis and meta-regression. J Endocrinol Invest. https://doi.org/10.1007/s40618-020-01479-z
Teng W, Shan Z, Teng X et al (2006) Effect of iodine intake on thyroid diseases in China. N Engl J Med 354:2783–2793
Li Y, Teng D, Shan Z et al (2008) Antithyroperoxidase and antithyroglobulin antibodies in a five- year follow-up survey of populations with different iodine intakes. J ClinEndocrinolMetab 93:1751–1757
Shan Z, Chen L, Lian X, Liu C, Shi B, Shi L, Tong N, Wang S, Weng J, Zhao J, Teng X, Yu X, Lai Y, Wang W, Li C, Mao J, Li Y, Fan C, Teng W (2016) Iodine status and prevalence of thyroid disorders after introduction of mandatory universal salt iodization for 16 years in china: a cross-sectional study in 10 Cities. Thyroid 26:1125–1130. https://doi.org/10.1089/thy.2015.0613
Li Y, Teng D, Ba J et al (2020) Efficacy and safety of long-term universal salt iodization on thyroid disorders: epidemiological evidence from 31 provinces of Mainland China. Thyroid 30:568–579. https://doi.org/10.1089/thy.2019.0067
Teng D, Yang W, Shi X et al (2020) An inverse relationship between iodine intake and thyroid antibodies: a national cross-sectional survey in Mainland China. Thyroid 30:1656–1665. https://doi.org/10.1089/thy.2020.0037
Delange F (1995) Correction of iodine deficiency: benefits and possible side effects. Eur J Endocrinol 132(542):543
Kahaly GJ, Dienes HP, Beyer J, Hommel, (1998) IodG ide induces thyroid autoimmunity in patients with endemic goitre: a randomised, double-blind, placebo-controlled trial. Eur J Endocrinol 139:290–297
Zimmermann MB, Moretti D, Chaouki N, Torresani T (2003) Introduction of iodized salt to severely iodine-deficient children does not provoke thyroid autoimmunity: a one-year prospective trial in northern Morocco. Thyroid 13:199–203
Pedersen IB, Knudsen N, Carlé A, Vejbjerg P, Jørgensen T, Perrild H, Ovesen L, Rasmussen LB, Laurberg P (2011) A cautious iodization program bringing iodine intake to a low recommended level is associated with an increase in the prevalence of thyroid autoantibodies in the population. ClinEndocrinol 75:120–126
Bliddal S, Borresen SW, Feldt-Rasmussen U (2017) Increase in thyroglobulin antibody and thyroid peroxidase antibody levels, but not preterm birth-rate, in pregnant Danish women upon iodine fortification. Eur J Endocrinol 176:603–612
Latrofa F, Fiore E, Rago T, Antonangeli L, Montanelli L, Ricci D, Provenzale MA, Scutari MA, Frigeri M, Tonacchera M, Vitti P (2013) Iodine contributes to thyroid autoimmunity in humans by unmasking a cryptic epitope on thyroglobulin. J ClinEndocrinolMetab 98:E1768–E2177
Premawardhana LD, Parkes AB, Smyth PP, Wijeyaratne CN, Jayasinghe A, de Silva DG, Lazarus JH (2000) Increased prevalence of thyroglobulin antibodies in Sri Lankan schoolgirls-is iodine the cause? Eur J Endocrinol 143:185–188
Mazziotti G, Premawardhana LD, Parkes AB, Adams H, Smyth PP, Smith DF, Kaluarachi WN, Wijeyaratne CN, Jayasinghe A, de Silva DG, Lazarus JH (2003) Evolution of thyroid autoimmunity during iodine prophylaxis. The Sri Lankan experience. Eur J Endocrinol 149:103–110
Premawardhana LDKE, Parker AR, Mazziotti G, Lazarus JG (2003) Autoimmune thyroiditis after elimination of iodine deficiency in Sri Lanka. Thyroid 13:1187–1188
Jayatissa R, Okosieme O, Ranasinghe S, Carter JL, Gunatunga I, Lazarus JH, Premawardhana L (2021) Thyroid autoimmunity and dysfunction in Sri Lankan children and adolescents after twenty-two years of sustained universal salt iodisation. Thyroid. https://doi.org/10.1089/thy.2020.0798
Teng X, Shan Z, Chen Y, Lai Y, Yu J, Shan L, Bai X, Li Y, Li N, Li Z, Wang S, Xing Q, Xue H, Zhu L, Hou X, Fan C, Teng W (2011) More than adequate iodine intake may increase subclinical hypothyroidism and autoimmune thyroiditis: a cross-sectional study based on two Chinese communities with different iodine intake levels. Eur J Endocrinol 164:943–950. https://doi.org/10.1530/EJE-10-104
Chen C, Xu H, Chen Y, Chen Y, Li Q, Hu J, Liang W, Cheng J, Xia F, Wang C, Han B, Zheng Y, Jiang B, Wang N, Lu Y (2017) Iodized salt intake and its association with urinary iodine, thyroid peroxidase antibodies, and thyroglobulin antibodies among urban Chinese. Thyroid 27:1566–1573. https://doi.org/10.1089/thy.2017.0385
Sun J, Teng D, Li C, Peng S, Mao J, Wang W, Xie X, Fan C, Li C, Meng T, Zhang S, Du J, Gao Z, Shan Z, Teng W (2020) Association between iodine intake and thyroid autoantibodies: a cross-sectional study of 7073 early pregnant women in an iodine-adequate region. J Endocrinol Invest 43(1):43–51. https://doi.org/10.1007/s40618-019-01070-1
Allen EM, Appel MC, Braverman LE (1986) The effect of iodide ingestion on the development of spontaneous lymphocytic thyroiditis in the diabetes-prone BB/W rat. Endocrinology 118:1977–1981
Sundick RS, Bagchi N, Brown TR (1996) The obese strain chicken as a model for human Hashimoto’s thyroiditis. ExpClinEndocrinol Diabetes 104:4–6. https://doi.org/10.1055/s-0029-1211668
Rasooly L, Burek CL, Rose NR (1996) Iodine-induced autoimmune thyroiditis in NOD-H-2h4 mice. ClinImmunolImmunopathol 81:287–292. https://doi.org/10.1006/clin.1996.0191
Kolypetri P, King J, Larijani M, Carayanniotis G (2015) Genes and environment as predisposing factors in autoimmunity: acceleration of spontaneous thyroiditis by dietary iodide in NOD.H2(h4) mice. Int Rev Immunol 34:542–556. https://doi.org/10.3109/08830185.2015.1065828
Burek CL, Rose NR (2008) Autoimmune thyroiditis and ROS. Autoimmun Rev 7:530–537. https://doi.org/10.1016/j.autrev.2008.04.006
Barin JG, Talor MV, Sharma RB, Rose NR, Burek CL (2005) Iodination of murine thyroglobulin enhances autoimmune reactivity in the NOD.H2h4 mouse. ClinExpImmunol 142:251–259
Kolypetri P, Carayanniotis G (2014) Apoptosis of NOD.H2h4 thyrocytes by low concentrations of iodide is associated with impaired control of oxidative stress. Thyroid 24:1170–1178. https://doi.org/10.1089/thy.2013.0676
Sharma R, Traore K, Trush MA, Rose NR, Burek CL (2008) Intracellular adhesion molecule-1 up-regulation on thyrocytes by iodine of non-obese diabetic.H2(h4) mice is reactive oxygen species-dependent. ClinExpImmunol 152:13–20. https://doi.org/10.1111/j.1365-2249.2008.03590.x
Ruggeri RM, CampennÌ A, Giuffrida G, Casciaro M, Barbalace MC, Hrelia S, Trimarchi F, Cannavò S, Gangemi S (2020) Oxidative stress as a key feature of autoimmune thyroiditis: an update. Minerva Endocrinol 45:326–344. https://doi.org/10.23736/S0391-1977.20.03268-X
Weetman AP (2021) An update on the pathogenesis of Hashimoto’s thyroiditis. J Endocrinol Invest. https://doi.org/10.1007/s40618-020-01477
Ajjan RA, Weetman AP (2015) The pathogenesis of Hashimoto’s thyroiditis: further developments in our understanding. HormMetab Res 47(10):702–710. https://doi.org/10.1055/s-0035-1548832
Ruggeri RM, Barbalace C, Cristani MT et al (2020) Serum levels of advanced glycation end products (AGEs) are increased and their soluble receptor (sRAGE) reduced in Hashimoto’s thyroiditis. J Endocrinol Invest 43:1337–1342
Weetman AP (2013) The immunopathogenesis of chronic autoimmune thyroiditis one century after Hashimoto. Eur Thyroid J 1:243–250. https://doi.org/10.1159/000343834
Ruggeri RM, Cristani M, Vicchio TM, Alibrandi A, Giovinazzo S, Saija A, Campennì A, Trimarchi F, Gangemi S (2019) Increased serum interleukin-37 (IL-37) levels correlate with oxidative stress parameters in Hashimoto’s thyroiditis. J Endocrinol Invest 42:199–205. https://doi.org/10.1007/s40618-018-0903-3
Funding
This work was not supported by any grant.
Author information
Authors and Affiliations
Contributions
Both authors contributed to the review article. Francesco Trimarchi had the idea for the article. Rosaria M. Ruggeri performed the literature search and data analysis and drafted the work. Francesco Trimarchi critically revised the work. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This paper does not contain any studies with human participants performed by the authors.
Informed consent
No informed consent.
Research involving human participants and/or animals
No animals were used for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ruggeri, R.M., Trimarchi, F. Iodine nutrition optimization: are there risks for thyroid autoimmunity?. J Endocrinol Invest 44, 1827–1835 (2021). https://doi.org/10.1007/s40618-021-01548-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-021-01548-x