Abstract
DNA molecules have excellent molecular recognition abilities through the complementary hydrogen-bonded base pairing. Since the hybridization of oligonucleotides can be programmed based on the sequences of the nucleobases, a great number of DNA supramolecular architectures have been constructed via self-assembly processes. The development of stimuli-responsive DNA supramolecules has attracted increasing interests because it will contribute to the construction of dynamic molecular systems such as molecular machines. Metal ions are considered as useful chemical stimuli, but the construction of metal-responsive DNA systems is still in the early stage. This review article describes current progress on the development of DNA supramolecules whose structure and function can be regulated in response to metal ions, with mainly focusing on our recent studies. The basic strategy is the introduction of unnatural metal ligands that form interstrand metal complexes in DNA structures. For example, artificial metal-mediated base pairs, formed through complexation between ligand-type nucleobase analogs and a bridging metal ion, were incorporated into known DNAzymes (catalytic DNA) to allosterically regulate their activity in a metal-responsive manner. Novel ligand-type nucleobases that form both metal-mediated and hydrogen-bonded base pairs have been recently devised as metal-responsive building blocks, and were used to construct a simple prototype of DNA molecular machines. Branched DNA structures bearing metal ligands at the junction core were also synthesized as novel structural motifs, with which metal-mediated structure transformation was demonstrated. These metal-responsive DNA supramolecules are expected to expand the toolbox of DNA-based supramolecular chemistry and nanotechnology.
Similar content being viewed by others
Introduction
DNA molecules serve as the bearer of genetic information of living organisms, thus being recognized as one of the most important biomolecules. The DNA molecules form well-defined double helical structures through the formation of complementary Watson–Crick base pairs, A–T and G–C, with two and three internucleobase hydrogen bonds, respectively (Fig. 1a). The complementary base pairing endows DNA with exquisite molecular recognition abilities, allowing for replication and transfer of genetic information in biological systems. The molecular recognition of DNA molecules has also inspired synthetic chemists including researchers in the field of supramolecular chemistry [1]. Since the hybridization of oligonucleotides can be programmed based on the base sequences, a great number of DNA supramolecular architectures have been made from DNA molecules via self-assembly processes (Fig. 1b) [2,3,4]. Particularly, various two- and three-dimensional nano-sized structures can be constructed by sophisticated strategies such as DNA origami, and the computer-aided design is now available (Fig. 1c and d) [5,6,7]. In addition, the development of dynamic DNA molecular devices whose configuration and function can be controlled by external stimuli has attracted increasing interests. By programming the hybridization of DNA components, excellent prototypes of molecular machines as well as computational systems have been developed thus far (Fig. 1e) [8,9,10,11].
a Schematic representation of a natural DNA duplex and the chemical structures of hydrogen-bonded Watson–Crick base pairs. b An example of DNA self-assembly. A DNA four-way junction structure self-assembles into a quadrilateral shape through the hybridization of the single-stranded regions called sticky ends. c Two-dimensional DNA origami structures [5]. Left panel: folding of a long genomic DNA strand into a designed structure with the aid of short staple strands. Right panels: atomic force microscopy images of DNA origami shapes. d Three-dimensional wireframe DNA origami structures imaged by negative-stain dry-state transmission electron microscopy [6]. Scale bars are 50 nm. e A nanoscale rotary device formed from 3D DNA components [8]. Panel b is adapted with permission from ref. 3. Copyright 2017 Springer Nature. Panel c is adapted with permission from ref. 5. Copyright 2006 Springer Nature. Panel d is adapted with permission from ref. 6. Copyright 2015 Springer Nature. Panel e is adapted from ref. 8, CC BY 4.0
Chemical modification of DNA molecules is a powerful way to embed stimuli-responsiveness into DNA molecules [12]. A variety of unnatural components have been incorporated into DNA oligomers based on a well-established phosphoramidite chemistry, by which nucleoside monomers are oligomerized in a stepwise manner. Post-synthetic modifications [13, 14] and enzymatic synthesis [15,16,17] are also useful techniques to introduce artificial functionalities into DNA. One of the successful examples is the incorporation of photo-responsive molecules, through which numerous DNA nanoarchitectures that change their shape and function in response to light irradiation were developed [18,19,20]. Metal ions are also considered as useful chemical stimuli by virtue of the kinetic reversibility of metal coordination bonding [21,22,23,24]. In the field of supramolecular chemistry, metal ions have been widely exploited to operate non-biological molecular switches and machines. However, the construction of metal-responsive DNA systems is still in the early stage due to the limited types of interactions between metal ions and natural DNA molecules.
One of the promising approaches to develop metal-responsive DNA supramolecules is the introduction of unnatural metal ligands into DNA [25]. The formation of interstrand metal complexes is expected to enhance the stability of the whole DNA structures, thus allowing for metal-triggered hybridization and transformation of DNAs [26]. The simplest example is the complexation between two opposing nucleobases (or nucleobase analogs) and a bridging metal ion in DNA duplexes, termed as metal-mediated base pairing (Fig. 2) [27,28,29,30,31]. While the natural pyrimidine nucleobases, T and C, are known to form metal-mediated-base pairs with HgII and AgI ions [32], a variety of unnatural ligand-type nucleobases that can form metal-mediated base pairs have been synthesized so far (Fig. 2a) [33,34,35,36,37]. Monodentate to tridentate nucleobase analogs were designed to form a 2:1 complex, i.e., a metal-mediated base pair, within DNA duplexes. In most cases, metal ions that adopt square-planar (such as CuII and NiII) or linear coordination geometry (such as AgI) are utilized so that the resulting metal-mediated base pairs are fit in very well with the stacked natural base pairs. For example, the 3-hydroxy-4-pyridone-type nucleobase (H) was found to form a stable CuII-mediated base pair (H–CuII–H) inside DNA duplexes [38]. The metal-mediated base pairs have originally been applied to the construction of one-dimensional metal arrays (Fig. 2b) [39,40,41,42], and the physical properties of metallo-DNA structures were then investigated (Fig. 2c) [40, 43]. The T–HgII–T and C–AgI–C base pairs have been also used to create HgII- or AgI-responsive DNA molecular devices [32], but unintended binding of these metal ions to other T and C bases causes difficulties in the molecular design. Therefore, it was highly desired to use other metal-mediated base pairs consisting of ligand-type nucleobase analogs and metal ions other than HgII or AgI for the rational design of metal-responsive DNA systems.
a Schematic representation of a DNA duplex with an artificial metal-mediated base pair. Chemical structures of representative metal-mediated base pairs are shown. b DNA-templated heterologous metal assembly (CuII–CuII–HgII–CuII–CuII) by using two kinds of metal-mediated base pairs [41]. c CuII-dependent regulation of the conductivity of metallo-DNA devices filling gaps in carbon nanotubes [43]. Panel c is reproduced with permission form ref. 43. Copyright 2011 Wiley-VCH
This review describes current progress on the development of DNA supramolecules whose structure and function can be regulated in response to metal ions, with mainly focusing on our recent studies. The basic strategy is the introduction of unnatural metal ligands that form interstrand metal complexes in DNA structures. For example, the incorporation of artificial metal-mediated base pairs into known DNAzymes (catalytic DNA) allows for allosteric regulation of their catalytic activity in a metal-responsive manner. Novel ligand-type nucleobases that form both metal-mediated and hydrogen-bonded base pairs have been devised as metal-responsive building blocks, and were used to construct a simple prototype of DNA molecular machines. Branched DNA structures bearing metal ligands at the junction core were also synthesized as novel structural motifs, with which metal-mediated structure transformation was demonstrated. These metal-responsive DNA supramolecules are expected to expand the toolbox of DNA-based supramolecular chemistry and nanotechnology. The design strategy of each metal-responsive DNA supramolecule will be discussed in detail in the following sections.
Rational deign of metal-responsive split DNAzymes
Among various functional nucleic acids, DNAzymes (deoxyribozymes) have attracted much attention because of their unique property to catalyze chemical reactions [44]. DNAzymes are normally obtained through a process called in vitro selection or SELEX (Systematic Evolution of Ligands by EXponential enrichment), during which catalytically active DNA oligomers are selected from DNA strands with randomized base sequences. Especially, DNAzymes that catalyze the cleavage of nucleic acid substrates have been widely utilized to develop DNA-based molecular sensors, molecular machines, and molecular computing systems [45,46,47]. In such applications, the rational design of stimuli-responsive allosteric DNAzymes is thought to be an important challenge [48]. So far, DNAzymes that are activated by the addition of target DNA/RNA [49] and small molecules [50] and by photoirradiation [51] were developed using unmodified or chemically-modified oligonucleotides. Metal-responsive DNAzymes consisting of only natural nucleotides were previously developed based on the T–HgII–T and C–AgI–C base pairing by Willner, Lu, and others (Fig. 3) [52, 53]. However, the metal ions used as stimuli were limited to only HgII and AgI ions, which strongly interact with natural nucleobases.
HgII-responsive DNAzymes based on T–HgII–T base pairing. a HgII-responsive split DNAzyme reported by Willner et al. [52]. b HgII-responsive single-stranded DNAzyme reported by Lu et al. [53]. Panel a is adapted with permission from ref. 52. Copyright 2010 Royal Society of Chemistry. Panel b is adapted with permission form ref. 53. Copyright 2007 Wiley-VCH
We have developed a series of metal-responsive DNAzymes whose catalytic activity can be regulated in response to metal ions other than HgII and AgI, by using artificial metal-mediated base pairs (Fig. 4). The base sequences were strategically designed based on previously-identified DNAzyme sequences so that the modified DNAzymes are activated through the formation of metal-mediated base pairs. One of the simplest strategies is to split the parent DNAzymes into two fragments and introduce a metal-mediated base pair(s) into the stem duplex (Fig. 4b). The formation of the metal-mediated base pair was expected to induce hybridization of the split strands, resulting in metal-dependent reconstruction of the active DNAzyme structure.
Development of metal-responsive split DNAzymes by using metal-mediated base pairing. a Base sequences of RNA-cleaving E5 DNAzyme consisting of natural nucleotides. “rA” in the substrate strand represents an adenine ribonucleotide at the cleavage site. b Design principle of metal-responsive split DNAzymes. c Base sequences of CuII-responsive split DNAzymes with metal-mediated base pairs. d The RNA-cleaving activity of H-Dz1 in the presence of various transition metal ions. [DNAzyme] = 1.0 µM, [substrate] = 1.0 µM, [metal ions] = 1.0 µM (1.0 equiv) in 10 mM HEPES (pH 7.0) containing 1 M NaCl and 10 mM MgCl2, 25 °C, 3 h. N = 3. For the experiments with HgII and AgI ions, NaCl and MgCl2 in the buffer were replaced with NaNO3 and Mg(NO3)2, respectively. Error bars indicate standard errors. e Iterative switching of the activity of ImC-Dz1. CuII (1 equiv), GHK (2 equiv), CuII (2 equiv), and GHK (4 equiv) were alternately added. [DNAzyme] = 1.0 µM, [substrate] = 10 µM (10 equiv), 25 °C. N = 3. The DNAzyme activity in the presence (red solid lines) and the absence of CuII ions (red dotted lines) are also shown. Error bars indicate standard errors. Panels b and d are reproduced with permission form ref. 55. Copyright 2019 American Chemical Society. Panel e is reproduced with permission form ref. 58. Copyright 2020 Wiley-VCH
This design strategy was initially used to reshape a well-studied RNA-cleaving DNAzyme called E5 [54] into a CuII-responsive DNAzyme [55]. The E5 DNAzyme (Fig. 4a), composed of a catalytic core, a substrate-binding domain, and a variable stem, was segmented at the loop region, and a pair of H nucleotides [38] were incorporated into the stem duplex. The H-modified split DNAzyme was expected to lose its activity and to restore its catalytically active structure in the presence of CuII ions via H–CuII–H base pairing. The length of the stem duplex and the position of the H nucleotides were varied to determine the optimal molecular design. When an H–H pair was introduced near the catalytic core, the modified DNAzyme exhibited a very low activity even in the presence of CuII ions. This is presumably due to the structural distortion of the catalytic core arising from the larger size of the H–CuII–H pair (whose C1′–C1′ distance was estimated to be 12.7 Å in a DNA analog [56]) compared to the natural Watson–Crick base pairs (10.7 Å). When an H–H mismatch was located at the terminal of the stem duplex, the activity of the DNAzyme was little changed by the addition of CuII ions. This is likely because the duplex with an H–H pair at the end is stable enough to be associated even without CuII ions. Among the sequences tested, a split DNAzyme with an 8-base-pair stem possessing an H–H pair at the third position from the catalytic core was found to exhibit the best response to CuII ions. The base sequence of the optimized H-modified DNAzyme (named as H-Dz1) is shown in Fig. 4c.
The activity of the resulting H-Dz1 can be estimated by analyzing the time-course of the RNA-cleaving reactions in the absence and the presence of CuII ions. Under the CuII-free conditions, the RNA-cleaving activity of H-Dz1 was significantly lower (apparent first-order rate constant kobs = 0.029 h–1) than that of the original E5 DNAzyme (0.23 h–1). When one equivalent of CuII ions was added, the DNAzyme activity was enhanced 5.5-fold (kobs = 0.16 h–1). In contrast, the activity of the unmodified E5 DNAzyme was not increased upon CuII addition. Under the same reaction conditions, CuII ions cannot cleave the RNA substate without the DNAzyme strands. Therefore, it was concluded that the addition of CuII ions increased the catalytic activity of H-Dz1. Additionally, titration experiments confirmed that one equivalent of CuII ions is sufficient to activate H-Dz1. All of these results agree well with the action mechanism that the formation of an H–CuII–H base pair induces the hybridization of the split strands to restore the active DNAzyme structure. Important to note is that the addition of other transition metal ions such as MnII, FeII, FeIII, CoII, NiII, ZnII, PdII, PtII, HgII, and AgI did not activate H-Dz1, showing a good metal selectivity (Fig. 4d). This result coincides with the metal specificity of the H–CuII–H base pairing. It was also demonstrated that the activity of H-Dz1 can be reversibly regulated by the addition and the removal of CuII ions. Sequential addition of equimolar amounts of CuII ions and a CuII-binding peptide (GHK) [57] resulted in the iterative switching of the DNAzyme activity. Furthermore, H-Dz1 was found to be inactivated and re-activated in responce to the redox of CuII ions.
More efficient on-off switching was demonstrated with a split DNAzyme containing a different CuII-mediated base pair ImC–CuII–ImC (Fig. 4c) [58], which shows a greater duplex stabilization effect (ΔTm = + 35.2 °C) than the H–CuII–H base pair (+ 13.1 °C) [58, 59]. The ImC-modified split DNAzyme (ImC-Dz1) was designed by formally replacing the H nucleotides in H-Dz1 with ImC nucleotides. In the absence of CuII ions, the RNA-cleaving activity of ImC-Dz1 was greatly suppressed. This is because the two fragments of the split DNAzyme was separated by the electric repulsion between the negatively charged ImC bases in the stem duplex. The addition of equimolar CuII ions enhanced the DNAzyme activity ca. 12-fold through the re-association of the split DNAzyme by ImC–CuII–ImC base pairing. These results confirmed that ImC-Dz1 was activated in a CuII-responsive manner as is the case for H-Dz1, and the on-off ratio was improved by changing the ligand-type nucleobases. Additionally, the activity of ImC-Dz1 was shown to be reversibly regulated by addition, removal, and reduction of CuII ions during the reaction. As shown in Fig. 4e, the alternate addition of CuII and GHK peptide resulted in repetitive switching of the DNAzyme activity.
Metal-responsive DNAzymes were further developed by using damaged nucleobases as metal-binding sites (Fig. 4c) [60]. We employed a 1,N6-ethenoadenine (εA), an etheno adduct of a natural adenine base, because it was reported to form CuII-mediated εA–CuII–εA base pairs in DNA duplexes [61]. In a manner similar to the abovementioned split DNAzymes, an RNA-cleaving DNAzyme named NaA43 [62] was split into two fragments. Three εA–εA mismatch pairs were consecutively introduced into the stem duplex because our investigations revealed that the formation of at least three εA–CuII–εA base pairs is needed for substantial stabilization of DNA duplexes. As a result, the activity of the εA-modified split DNAzyme (εA-Dz1) was shown to be enhanced 5.3-fold by the addition of three equivalents of CuII ions (i.e., [CuII]/[εA–εA] = 1.0).
Rational deign of metal-responsive single-stranded allosteric DNAzymes
Metal-responsive allosteric DNAzymes can be rationally designed without splitting the original DNAzymes (Fig. 5) [63]. This strategy involves intrastrand structural transformation caused by metal-mediated base pairing (Fig. 5a). A metal-mediated base pair(s) is incorporated into the stem duplex of the parent DNAzyme in a manner similar to the split DNAzymes discussed above. Additionally, the loop sequence is altered to hybridize with the catalytic core to decrease the DNAzyme activity in the absence of the target metal ions. The addition of the metal ions is expected to trigger structural changes from a catalytically inactive structure to an active DNAzyme structure with metal-mediated base pair(s). For example, the RNA-cleaving E5 DNAzyme was reshaped to a CuII-responsive allosteric DNAzyme by the introduction of an H–CuII–H base pair (Fig. 5b) [63]. An H–H mismatch pair was incorporated into the stem region and a sequence complementary to the catalytic domain was inserted into the loop (shown in orange) to form the inactive structure. The RNA-cleaving activity of the resulting DNAzyme (H-Dz2) was efficiently suppressed (kobs = 0.011 h–1) in the absence of CuII ions as expected. The addition of CuII ions increased the DNAzyme activity by 6.8-fold (kobs = 0.073 h–1), showing better on-off performance than the split DNAzyme H-Dz1. A fluorescence assay using a DNAzyme modified with a fluorogenic nucleobase analog (pyrrolocytosine) [64] confirmed the intrastrand structure conversion induced by the addition of CuII. Titration of CuII ions established that one equivalent of CuII ions is sufficient to activate H-Dz2. These results showed that the non-split H-Dz2 was allosterically regulated through the quantitative formation of an H–CuII–H base pair. Furthermore, reversible control of the DNAzyme activity was demonstrated by the addition and the removal of CuII ions under isothermal conditions. Alternate addition of CuII ions and a CuII-binding peptide (GHK) resulted in a repetitive switching of the DNAzyme activity.
Development of metal-responsive single-stranded allosteric DNAzymes by using metal-mediated base pairs. a Schematic representation of a CuII-responsive single-stranded DNAzyme. b Base sequences of a CuII-responsive DNAzyme with an H–CuII–H base pair (H-Dz2). Both catalytically inactive and active secondary structures are shown. “rA” in the substrate strand represents an adenine ribonucleotide at the cleavage site. Panel a is reproduced with permission form ref. 63. Copyright 2020 American Chemical Society
We subsequently developed a CuII-responsive allosteric DNAzyme containing multiple metal-mediated base pairs [65]. Three H–CuII–H base pairs were consecutively introduced into the parent E5 DNAzyme and the loop sequence was modified as is the case for H-Dz2. The activity of the resulting DNAzyme (H-Dz3) was found to increase 2.2-fold by the addition of three equivalents of CuII ions (i.e., [CuII]/[H–H mismatch pair] = 1.0). Contrary to our expectations, the on-off ratio was lower than that of H-Dz2 containing only a single H–CuII–H pair. It is likely that the structural distortion caused by multiple H–CuII–H base pairs adversely influenced on the DNAzyme activity.
The design strategy of the metal-responsive non-split DNAzymes was further applied to other types of DNAzymes such as an RNA-cleaving NaA43 DNAzyme [63]. A pair of H nucleotides were incorporated into the stem as a CuII-binding site and the stem duplex was shortened to form a catalytically inactive structure in the absence of CuII ions. The activity of the modified DNAzyme (H-Dz4) was enhanced 5.9-fold upon addition of equimolar CuII ions, demonstrating the allosteric regulation of the DNAzyme activity in response to CuII. Recently, we have remade an RNA-cleaving 8–17 DNAzyme to a CuII-responsive allosteric DNAzyme (Fig. 6) [66]. The 8–17 DNAzyme is one of the smallest DNAzymes and does not have any long stem region (Fig. 6a) [67]. Owing to its compactly-folded structure, it has been challenging to modify 8–17 DNAzyme without losing its activity. Based on the reported crystal structure [68], an ImC–CuII–ImC base pair was introduced in the middle of the 3-base-pair duplex region inside the pseudoknot structure (Fig. 6c). It was expected that the modified DNAzyme (ImC-Dz2) is folded into the catalytically active form through the formation of the ImC–CuII–ImC base pair. As a result, the activity of ImC-Dz2 was enhanced 5.1-fold upon addition of one equivalent of CuII ions, showing good metal responsiveness. The development of the compactly-packed CuII-responsive DNAzyme (ImC-Dz2) demonstrates the utility of metal-mediated artificial base pairs for creating stimuli-responsive functional DNAs.
Development of CuII-responsive 8–17 DNAzyme. a Crystal structure of a compact-sized 8–17 DNAzyme reported previously (PDB: 5XM8). The DNAzyme and the substrate are depicted in blue and grey, respectively. The 3-base-pair stem is colored in orange. b Base sequence of the unmodified 8–17 DNAzyme. The broken lines indicate hydrogen-bonded base pairs (including a nonstandard A–G base pair) to form the pseudoknot structure. c Molecular design of CuII-responsive 8–17 DNAzyme containing an ImC–CuII–ImC base pair (ImC-Dz2). “rA” represents an adenine ribonucleotide at the cleavage site. Reprinted with permission from ref. 66. Copyright 2024 Royal Society of Chemistry
The same approach was applied to the development of a DNAzyme that exhibits an AND logic-gate response to two different metal ions, CuII and AgI (Fig. 7) [63]. An AgI-dependent RNA-cleaving DNAzyme Ag10c, previously obtained by SELEX [69], was modified to be activated in the presence of CuII ions. An H–H mismatch pair was incorporated in the stem region and the loop sequence was changed to form inactive structure under CuII-free conditions (Fig. 7a). The catalytic activity of the modified DNAzyme (H-Dz5) was tested in the absence and the presence of CuII (1 equiv) and AgI ions (10 equiv) (Fig. 7b). In the absence of AgI ions, the DNAzyme showed no catalytic activity at all. In the presence of only AgI ions but without CuII, the DNAzyme showed only faint activity. When both CuII and AgI ions were added, the DNAzyme efficiently cleaved the RNA substrate. These results confirmed that H-Dz5 functions as an AND logic-gate system that responds to CuII and AgI ions as inputs.
Development of an AND-gate DNAzyme that responds to CuII and AgI ions. a Base sequence of the H-modified AND-gate DNAzyme (H-Dz5). Only the active structure is shown. The sequence shown in orange is complementary to each other, allowing for the formation of inactive structure in the absence of CuII. “rA” in the substrate represents adenine ribonucleotide at the cleavage site. b RNA-cleaving activity of H-Dz5 in the presence of CuII and/or AgI ions. [DNAzyme] = 1.0 µM, [substrate] = 10 µM, [CuSO4] = 0 or 1.0 µM, [AgNO3] = 0 or 10 µM in 10 mM HEPES buffer (pH 7.5) containing 200 mM NaNO3, 25 °C, 1 h, N = 3. Error bars indicate standard errors. ** p < 0.01 (two-tailed unpaired Student’s t test). Panel b is reproduced with permission form ref. 63. Copyright 2020 American Chemical Society
Collectively, it was demonstrated that various known DNAzymes can be reshaped into metal-responsive allosteric DNAzymes by introducing metal-mediated artificial base pairs. Although many metal-dependent DNAzymes have been obtained via SELEX method [70], chemical modification of existing DNAzymes would be a more versatile strategy to develop metal-responsive DNAzymes. In our approaches, one or a few pairs of ligand-type nucleobase analogs are introduced as the allosteric site. Since only minimal chemical modification is required, the design strategies described above are expected to be applied to the allosteric regulation of other functional DNA molecules. Recently, Müller et al. have developed metal-responsive DNA aptamers whose binding affinity to the target molecules can be regulated by the addition of metal ions [71, 72]. Ligand-type nucleotides, such as Im and ImC, were incorporated into known aptamer sequences so that the active structure can be reconstituted through the formation of metal-meditated base pairs, such as Im–AgI–Im [73] and ImC–CuII–ImC [59]. It is also expected that allosteric DNA molecules responsive to different effector metal ions can be developed by using appropriate metal-mediated base pairs. Designing allosteric DNAs that are regulated by biologically important metal ions and by redox of metal ions would be of significant interest in terms of future applications in biological systems [74, 75]. Furthermore, the use of metal ions that little interfere with natural nucleobases would allow for the applications of the metal-responsive DNAs in more complex DNA systems including DNA nanomachines and DNA computing systems.
Development of metal-responsive bifacial nucleobases
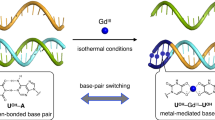
As discussed above, artificial metal-mediated base pairing is a versatile tool to create metal-responsive DNA supramolecules. In order to realize more efficient switching of DNA structures and functions in response to metal ions, we have developed bifacial nucleobases that form both hydrogen-bonded and metal-mediated base pairs (Fig. 8). The bifacial nucleobases were designed so that their base-pairing partner can be altered by the addition of specific metal ions. In particular, the bifacial nucleobases have a Watson–Crick face and an additional metal-coordination site on the other side (Fig. 8a). We first examined a 5-hydroxyuracil (UOH) base, whose 4-carbonyl and 5-hydroxy groups were considered to serve as a bidentate ligand (Fig. 8b) [76]. It was confirmed that the UOH bases form Watson–Crick-type hydrogen-bonded base pair (UOH–A) with natural adenine (A) bases as is the case for natural thymine (T) and uracil (U) bases. In addition, the UOH bases were found to form metal-mediated homo base pairs (UOH–M–UOH) in the presence of most lanthanoid ions (M = GdIII etc.). The formation of metal-mediated base pairs was evidenced by DNA duplex melting analysis, UV titration experiments, and mass spectrometry. It should be noted that the stable formation of UOH–M–UOH base pairs was observed when DNA duplexes contain at least three consecutive UOH–UOH pairs. Since lanthanoid ions generally adopt high coordination numbers, such as 8 or 9, it is most likely that the metal centers are coordinated not only by the two opposing UOH nucleobases but also by additional ligands such as water molecules and adjacent nucleobases. Although the detailed coordination structure is still unrevealed, both terminal regions of the metallo-DNA duplex were shown to adopt a typical right-handed B-form helix as indicated by circular dichroism (CD) spectroscopy and NMR structure analysis. Furthermore, UOH bases were found to form ZnII-mediated UOH–ZnII–UOH base pairs within DNA duplexes under basic conditions [77]. The pH-dependency can be explained by the need for the deprotonation of the 5-hydroxy group of UOH (pKa = 7.7).
Hydrogen-bonded and metal-mediated base pairing of bifacial 5-modified pyrimidine nucleobases. a Concept of the metal-mediated base-pair switching of the bifacial nucleobases. “L” represents a metal coordinating functionality. b 5-Hydroxyuracil (UOH) nucleobase. “X” represents additional coordinating ligands such as water molecules and adjacent nucleobases. c 5-Carboxyuracil (caU) nucleobase. dN,N- Dicarboxymethyl-5-aminouracil (dcaU) nucleobase. e Melting temperatures (Tm) of 15-base-pair DNA duplexes containing three UOH–A or UOH–UOH base pairs in the absence and the presence of GdIII ions (3 equiv). fTm values of 15-base-pair DNA duplexes containing three caU–A or caU–caU base pairs in the absence and the presence of CuII ions (6 equiv). gTm values of 15-base-pair DNA duplexes containing a dcaU–A or dcaU–dcaU base pair in the absence and the presence of GdIII ions (1 equiv). [Duplex] = 2.0 µM in 10 mM HEPES buffer (pH 8.0 for e, pH 7.0 for g and f) containing 100 mM NaCl
DNA duplexes containing bifacial nucleobases such as UOH showed unique thermal denaturing behaviors in the presence of the corresponding metal ions (Fig. 8e). The thermal stability of a 15-base-pair DNA duplex containing three UOH–UOH pairs, (5′-CAC ATT UOHUOHUOH GTT GTA-3′)·(3′-GTG TAA UOHUOHUOH CAA CAT-5′), was greatly increased upon addition of 3 equiv of GdIII ions (ΔTm = + 18.3 °C). The duplex stabilization is ascribed to the additional cross-linkage by the formation of three UOH–GdIII–UOH base pairs. Interestingly, a DNA duplex containing three hydrogen-bonded UOH–A pairs, (5′-CAC ATT UOHUOHUOH GTT GTA-3′)·(3′-GTG TAA AAA CAA CAT-5′) was destabilized by the addition of 3 equiv of GdIII (ΔTm = − 14.3 °C). The GdIII-dependent destabilization was caused by the binding of GdIII ions to the UOH bases, which could weaken the hydrogen-bonding between UOH and A. Taken together, the duplex containing UOH–A pairs (Tm = 45.2 °C) is more stable than the duplex with UOH–UOH pairs (22.8 °C) without GdIII ions, and the duplex with UOH–GdIII–UOH pairs (41.1 °C) becomes more stable than the duplex with UOH–A pairs (30.9 °C) upon GdIII addition (Fig. 8e). These results suggest that the hybridization preference of the UOH-containing strand, 5′-CAC ATT UOHUOHUOH GTT GTA-3′, can be altered by the addition of GdIII ions.
The second example of the metal-responsive bifacial nucleobases is 5-carboxyuracil (caU), which has a 4-carbonyl and a 5-carboxy groups as a bidentate metal binding site (Fig. 8c) [78]. The caU bases form not only hydrogen-bonded caU–A base pairs but also CuII-mediated caU–CuII–caU base pairs inside DNA duplexes. The base-pairing property of caU was elucidated by duplex melting analysis, CD spectroscopy, and mass spectrometry. It is interesting to note that caU forms metal-mediated base pair with other natural nucleobases, T, C, and G. For example, caU–HgII–T and caU–AgI–C base pairs were found to form in the presence of HgII and AgI ions, respectively, through N3 coordination in a manner similar to well-studied T–HgII–T and C–AgI–C base pairs [32]. Furthermore, the caU bases were suggested to pair with G bases in the presence of CuII ions to afford caU–CuII–G base pairs. Control experiments using 7-deazaguanines instead of G bases indicated its [N,O,O,O]-type coordination geometry. Overall, caU bases were shown to form a homo base pair with itself (caU–CuII–caU) and hetero base pairs with all four canonical nucleobases (caU–A, caU–HgII–T, caU–AgI–C, and caU–CuII–G) depending on the coexisting metal ions.
As is the case with UOH bases, metal-dependent stabilization and destabilization were observed with DNA duplexes containing caU nucleobases (Fig. 8f). A duplex containing three consecutive caU–caU pairs, (5′-CAC ATT caUcaUcaU GTT GTA-3′)·(3′-GTG TAA caUcaUcaU CAA CAT-5′), was significantly stabilized by adding 3 equiv of CuII ions (ΔTm = + 30.7 °C). The CuII-mediated duplex stabilization was ascribable to the quantitative formation of three caU–CuII–caU base pairs. Importantly, the degree of the stabilization caused by caU–CuII–caU base pairing is higher than that observed with UOH–GdIII–UOH base pairing. In contrast, a duplex containing three hydrogen-bonded caU–A pairs, (5′-CAC ATT caUcaUcaU GTT GTA-3′)·(3′-GTG TAA AAA CAA CAT-5′), was destabilized when excess CuII ions (6 equiv) were added (ΔTm = − 7.3 °C). In other words, the duplex containing three caU–A pairs (Tm = 41.2 °C) is more stable than the duplex with three caU–caU mismatch pairs (17.7 °C) in the absence of CuII ions (Fig. 8f). In contrast, the duplex with caU–caU (47.0 °C) shows higher stability than the duplex with caU–A (33.9 °C) in the presence of CuII ions (6 equiv). As a result, the addition of CuII ions reversed the order of the stability of the duplexes, indicating that the hybridization preference of caU-containing DNA strands can be changed in response to CuII ions.
The metal-mediated duplex stabilization was observed only when multiple UOH–UOH or caU–caU pairs are incorporated, thus limiting the flexibility of the sequence design. We have recently synthesized a novel metal-responsive bifacial nucleobase, N,N-dicarboxymethyl-5-aminouracil (dcaU), which possesses a tridentate iminodiacetic acid (IDA) ligand at the 5-position of the uracil base (Fig. 8d) [79]. Since the IDA ligand is a partial structure of classical chelating reagents such as EDTA, the dcaU base was expected to form stable complexes with various metal ions. The thermal stability of a 15-base-pair DNA duplex with a dcaU–dcaU pair in the middle, (5′-CAC ATT AdcaUT GTT GTA-3′)·(3′-GTG TAA TdcaUA CAA CAT-5′), was significantly enhanced when one equivalent of GdIII ions was added (ΔTm = + 16.1 °C). Titration of GdIII ions and control experiments suggested that the duplex stabilization results from the quantitative formation of a single dcaU–GdIII–dcaU base pair. In contrast to the case with UOH and caU base, only a single dcaU–GdIII–dcaU base pairing stabilized the DNA duplex. CD spectroscopic analysis showed that the dcaU–GdIII–dcaU base pair was accommodated within the duplex without severe structural distortion, although the GdIII complex is likely to adopt a nonplanar bulky conformation. Since dcaU functions as a tetradentate ligand, GdIII with high coordination number is appropriate for metal-mediated base pairing with dcaU.
The dcaU base also forms a hydrogen-bonded dcaU–A base pair with a natural A base. In a manner similar to UOH–A and caU–A base pairs, the dcaU–A base pair was destabilized by adding GdIII ions. The thermal stability of a duplex (5′-CAC ATT AdcaUT GTT GTA-3′)·(3′- GTG TAA TAA CAA CAT-5′), containing a dcaU − A pair, was decreased (Tm = − 3.5 °C) in the presence of equimolar GdIII ions. As summarized in Fig. 8g, the duplex containing a dcaU–A pair (Tm = 38.0 °C) is more stable than the duplex with a dcaU–dcaU mismatch pair (22.5 °C) in the absence of GdIII ions. When GdIII ions are present, the duplex with a dcaU–GdIII–dcaU pair (38.6 °C) becomes more stable than the duplex with a dcaU–A (34.5 °C). The metal-mediated changes in stability of the two duplexes are accompanied by the switching between the hydrogen-bonded and the metal-mediated base pairs. This characteristic property of the bifacial nucleobases would be applicable to the construction of a wide range of metal-responsive DNA supramolecules.
Development of metal-responsive DNA supramolecules with bifacial nucleobases
The metal-responsive bifacial nucleobases, UOH, caU, and dcaU, form both hydrogen-bonded and metal-mediated base pairs in DNA duplexes. Since the base-pairing partner of the bifacial bases can be switched depending on the coexisting metal ions, these bifacial bases can be utilized to endow DNA structures with metal-responsiveness. We first applied the bifacial UOH bases to induce DNA strand displacement reactions (SDRs) by the addition of GdIII ions [80]. SDRs are reactions in which one of the strands in a pre-hybridized duplex (termed as an incumbent strand) is replaced with an input single-stranded DNA (termed as an invader strand) [81, 82]. Typical SDRs start at a single-stranded segment called a toehold where the invading strand binds. It is safe to say that SDR is one of the most fundamental processes in the operation of DNA-based molecular machines and molecular computing circuits. The development of SDRs triggered by external stimuli other than DNA/RNA molecules has been considered as an important step toward practical applications of DNA molecular systems in various chemical environments.
We investigated metal-triggered SDRs based on GdIII-mediated switching between UOH–A and UOH–GdIII–UOH base pairs (Fig. 9a). To this end, a DNA strand containing four consecutive UOH bases at the 5′ end (1) was used as an invader strand. At the biggening, a DNA strand with the same base sequence as 1 except for four A bases at the 5′ end (2) was hybridized to a complementary strand containing UOH bases at the 3′ end (3) through UOH–A base pairing. The strand 3 and the invader strand 1 were labeled with a fluorophore and a quencher, respectively. Thus, the progression of the SDR can be monitored by fluorescence quenching (Fig. 9b). The fluorescence intensity decreased immediately after the addition of 4 equiv of GdIII ions, showing that the incumbent strand 2 was displaced with the invader strand 1. The fluorescence intensity was hardly changed when DNA strands containing T bases in place of the bifacial UOH bases were utilized. It was revealed that the SDR was triggered by the GdIII-mediated destabilization of the UOH–A pairs and the formation of the UOH–GdIII–UOH base pairs at the terminal of the strands. In addition, the reverse reaction was found to proceed by removing GdIII ions with an equimolar amount of chelating reagent EDTA. Although the GdIII-triggered SDR is slower than conventional toehold-mediated SDRs, the use of bifacial UOH bases enabled reversible DNA strand displacement reactions in response to GdIII ions.
a Schematic representation of metal-mediated DNA strand displacement reactions (SDRs) induced by base-pair switching of UOH nucleobases. UOH nucleotides in the DNA strands are highlighted. b Time-course analysis of GdIII-triggered SDRs. The reaction was started by the addition of GdIII ions (4 equiv). [DNA strand] = 2.0 µM each in 10 mM HEPES buffer (pH 8.0) containing 100 mM NaCl, 25 °C. The strand 3 and the invader strand 1 were labeled with a fluorophore (FAM) and a quencher (Dabcyl), respectively. λex = 495 nm, λem = 519 nm. c GdIII-dependent switching of the hybridization partner of dcaU-containing strand. “A*” represents a fluorogenic 2-aminopurine nucleobase. d Time-course analysis of GdIII-dependent hybridization behaviors of the dcaU-containing strand. After annealing the mixture of the three strands, GdIII (1 equiv) and EDTA (5 equiv) were sequentially added. λex = 303 nm, λem = 371 nm, 20 °C. [DNA strand] = 0.5 µM each in 10 mM HEPES buffer (pH 7.0) containing 100 mM NaCl. Panels a and b are adapted from ref. 80, CC BY 4.0. Panels c and d are adapted with permission from ref. 79. Copyright 2023 Royal Society of Chemistry
Similarly, the hybridization partner of a dcaU-containing strand was exchanged by the addition and the removal of GdIII ions (Fig. 9c) [79]. A 15-mer DNA strand containing a dcaU base in the middle (6) was initially hybridized with its complementary 13-mer strand (5) through dcaU–A base pairing, and a 15-mer strand with a dcaU base (4) was used as an invader. Native polyacrylamide gel electrophoresis (PAGE) and fluorescence measurements using the strand 5 containing fluorogenic 2-aminopurine base [83] (Fig. 9d) revealed that the addition of GdIII ions (1 equiv) induced the release of strand 5 and the formation of duplex 4·6 containing a dcaU–GdIII–dcaU pair. Furthermore, the removal of GdIII ions with EDTA resulted in a strand exchange in the reverse direction. The strand exchange was repeated three times by alternate addition and removal of GdIII ions although the efficiency was gradually decreased.
Metal-meditated dissociation of oligonucleotides with the bifacial nucleobases was also applied to the regulation of the DNA triplex formation [84]. The triple-stranded structures are formed by sequence-specific binding of a triplex-forming oligonucleotide (TFO) to a target DNA duplex via Hoogsteen base pairing between a pyrimidine and a purine bases. Since the triplex formation is applicable to gene regulation and dynamic control of DNA structures [85], the development of stimuli-responsive TFOs has been of great interest. We designed TFOs containing UOH bases to reversibly regulate the triplex formation in a GdIII-dependent manner (Fig. 10). The UOH-modified TFOs such as 5′-TCmCm TTUOH TCmT UOHTCm TUOHT TTCm CmTT-3′ (Cm = 5-methylcytosine) were found to bind to target duplexes by forming UOH·A–T base triads, similar to the natural T·A–T base triads. Melting analysis revealed that the binding affinity of the UOH-modified TFO decreases upon addition of GdIII ions. This is probably because the Hoogsteen UOH·A base pairs were disrupted by the site-selective binding of GdIII to the UOH bases. The GdIII-responsive triplex formation was then studied by native PAGE. Under the GdIII-free conditions, the mixture of the target duplex and the UOH-containing TFO gave the triplex structure in 73% yield. After the incubation with GdIII ions (6 equiv), the amount of the triplex was dropped to 25% in 2 h, suggesting the release of the TFOs. Subsequent addition of EDTA (6 equiv), which removes GdIII ions, resulted in re-association of the TFO to form the triplex. The binding of the TFOs was cycled at least twice albeit with gradually decreasing the efficiency.
Schematic representation of GdIII-responsive reversible binding of triplex-forming oligonucleotides (TFOs) with 5-hydroxyuracil (UOH) nucleobases. The chemical structure of a UOH·A–T base triad is shown. UOH·A: Hoogsteen base pair, A–T: Watson–Crick base pair. Reprinted with permission from ref. 84. Copyright 2021 Royal Society of Chemistry
We have recently utilized UOH bases to construct one of the simplest prototypes of DNA molecular machines [80], so-called DNA tweezers, which take closed and open forms depending on the external stimuli [86]. The UOH-modified DNA tweezers were designed to open and close in response to GdIII ions (Fig. 11a) [80]. The tweezer structure consists of three DNA strands forming two arms (a, b, and c) and a strand that functions as a stopper (d). Four UOH bases were incorporated at both termini of the stopper strand d to close the tweezers via hydrogen-bonded UOH–A base pairing. The addition of GdIII was expected to induce switching from UOH–A to UOH–GdIII–UOH base pairs, resulting in the release of strand d and the opening of the DNA tweezers. Native PAGE analysis confirmed that the closed structure with the stopper d was predominantly formed in the absence of GdIII ions while the open state was formed by the addition of GdIII ions. The yield of the open form increased as the amount of GdIII ions increased, reaching ca. 90% with 12 equiv of GdIII. FRET analysis with the hinge strand b labeled with a fluorophore and a quencher at the both termini demonstrated GdIII-mediated operation of the DNA tweezers under isothermal conditions (Fig. 11b). The fluorescence intensity increased immediately after the addition of GdIII ions (12 equiv), showing the GdIII-triggered opening of the tweezers. Subsequent addition of EGTA (12 equiv), a chelating agent to remove GdIII ions, led to the decrease in the fluorescence, confirming that the DNA tweezers were closed again. Sequential addition and removal of GdIII ions successfully repeated the opening and closing of the DNA molecular tweezers. Although GdIII–EGTA complexes accumulated in the solution, the metal-mediated base-pair switching between UOH–A and UOH–GdIII–UOH was proven to be versatile way to operate DNA-based molecular machines.
a Schematic representation of GdIII-responsive DNA tweezers. U represents UOH nucleotides. b Repeated opening and closing of the UOH-modified DNA tweezers observed by fluorescence analysis. Strand b was labeled with a fluorophore (FAM) and a quencher (Dabcyl) at both termini. λex = 495 nm, λem = 519 nm, 25 °C. [DNA] = 2.0 µM each in 10 mM HEPES buffer (pH 8.0) containing 100 mM NaCl and 3 mM MgCl2, [GdCl3] = [EGTA] = 24 µM. c Molecular design of the UOH-modified DNAzyme (UOH-Dz1). “rA” in the substrate represents an adenosine ribonucleotide at the cleavage site. Both the catalytically inactive form (without GdIII) and the active form (with GdIII) are shown. d Activation of UOH-Dz1 by the addition of GdIII ions (3 equiv). The activity of UOH-Dz1 in the presence and the absence of GdIII ions is also shown. N = 3. Error bars indicate the standard errors. Adapted from ref. 80, CC BY 4.0
The bifacial nucleobases can be also utilized to develop metal-responsive allosteric DNAzymes. A GdIII-responsive DNAzyme was logically designed by applying the switching between UOH–A and UOH–GdIII–UOH base pairs (Fig. 11c) [80]. Three consecutive UOH–UOH mismatches were introduced into the stem region of a known RNA-cleaving NaA43 DNAzyme [62]. The surrounding bases were redesigned to form a catalytically inert structure in the absence of GdIII ions through UOH–A base pairing. The UOH-modified DNAzyme (UOH-Dz1) was expected to undergo an intramolecular structural change upon GdIII addition, from the inactive state containing UOH–A base pairs to the active state containing UOH–GdIII–UOH pairs. The detailed base sequence was rationally designed with the help of the NUPACK software [87], which predicts possible secondary structures from the base sequences. In the simulation, the UOH–A base pairs in the inactive form were replaced with natural T–A base pairs, and the potential UOH–GdIII–UOH base pairs in the active form were replaced with G–C base pairs. The RNA-cleaving activity of UOH-Dz1 was enhanced 14.4-fold by the addition of 3 equiv of GdIII ions (Fig. 11d) while that of the unmodified NaA43 DNAzyme was increased only 1.9-fold. A control DNAzyme in which all UOH bases were replaced with thymine (T) bases did not cleave the RNA substrate. UV spectroscopic analysis of UOH-Dz1 confirmed the metal complexation of the UOH bases. These results suggest that the activity of UOH-Dz1 was allosterically enhanced by the formation of UOH–GdIII–UOH base pairs. Furthermore, the DNAzyme activity was reversibly regulated by adding GdIII or EDTA during the reactions. It was revealed that UOH-Dz1 showed more efficient switching capability (kGd+/kGd− = 14.4) than the CuII-responsive cognate DNAzyme H-Dz4 (kCu+/kCu− = 5.9). This is owing to the unique design strategy based on the bifacial nature of the UOH bases, whereby both the inactive and the active states are stably formed through the formation of UOH–A and UOH–GdIII–UOH base pairs, respectively.
As described above, switching between hydrogen-bonded and metal-mediated base pairs allows for metal-dependent control of DNA hybridization as well as structure conversion. Thus, these bifacial bases would be versatile building blocks for developing metal-responsive DNA supramolecules and nanodevices. The DNA sequences can be designed by modifying existing functional DNAs, more specifically, by replacing natural T bases with UOH, caU, or dcaU. The unique base-pairing properties of the bifacial nucleobases stem from the metal coordination site on the opposite side of the Watson–Crick face. Therefore, bifacial nucleobases with a different metal affinity could be developed by introducing other coordination functionalities at the 5-position of uracil (U) or cytosine (C) bases. We believe that the concept of the metal-responsive bifacial nucleobases would offer new strtegies in the field of DNA supramolecular chemistry and nanotechnology.
Metal-triggered transformation of DNA three-way junction structures
In order to construct DNA-based functional nanoarchitectures, it is important to control the self-assembly of not only duplexes but also branched structures [88]. A DNA three-way junction (3WJ) is a branching structural motif in which three DNA duplexes emanate from its branching point (Fig. 12a). In a manner similar to DNA duplexes stabilized by metal-mediated base pairing, 3WJ structures can be thermally stabilized by interstrand metal complexation (Fig. 12c) [89, 90]. Toward this end, a nucleoside modified with a metal ligand is incorporated into each strand (Fig. 12d). The ligand-modified 3WJs are stabilized through 3:1 ligand–metal complexation in the presence of specific metal ions.
a An ideal structure of an unmodified DNA three-way junction (3WJ). Dipicted based on a crystal structure reported by Baldwin et al. (PDB ID: 1DRG) [95]. b Molecular structure of an unmodified DNA 3WJ motif. Drawn based on a crystal structure reported by Hannon et al. (PDB ID: 2ET0) [94]. c Schematic representation of metal-dependent stabilization of ligand-modified DNA 3WJ structures. d Molecular design of ligand-modified nucleotides. e Base sequences of the ligand-modified DNA strands forming 3WJs. U represents the ligand-modified nucleotides, Ubpy-1, Ubpy-2, Ubpy-3, or Uphen. f Melting temperatures (Tm) of the unmodified 3WJ and the ligand-modified 3WJs in the absence and the presence of NiII ions. [NiII]/[3WJ] = 1.0 in 10 mM MOPS buffer (pH 7.0) containing 100 mM NaCl. Panel b is reproduced with permission from ref. 93. Copyright 2021 Wiley-VCH
We synthesized bipyridine (bpy)-modified 3WJ structures and investigated metal-dependent stabilization [91,92,93]. The reported crystal structures of natural 3WJs [94, 95] showed that the 2′-hydrogen of the deoxyribose moiety points to the center of the 3WJ (Fig. 12b). Therefore, the metal ligand was attached to the 2′-position to form the 3:1 metal complex at the junction core. We designed a series of bpy-modified nucleosides, Ubpy-1 [91], Ubpy-2 [92], and Ubpy-3 [93], with different linker moieties. The DNA strands containing a Ubpy-1 nucleotide were synthesized by post-synthetic azide–alkyne Huisgen cycloaddition between oligonucleotides containing a 2′-propargyl uridine and an azide-modified bpy ligand [91]. A DNA 3WJ structure with three bpy ligands at the branching point was constructed by hybridizing three bpy-modified strands (Fig. 12e). Melting experiments showed that the thermal stability of the bpy-modified 3WJs was increased by the addition of an equimolar amount of NiII ions (ΔTm = + 8.9 °C) (Fig. 12f). The NiII-dependent stabilization of the 3WJ was ascribed to the formation of an interstrand tris-bipyridine complex, NiII(bpy)3, which was confirmed by UV spectroscopic analysis and control experiments. The addition of transition metal ions other than NiII was found to stabilize the bpy-modified 3WJ as well (ΔTm = + 5.0 °C for FeII; +3.3 °C for CoII). It is interesting to note that the degree of the metal-dependent stabilization (NiII > FeII > CoII) followed the order of the overall stability constants of MII(bpy)3 complexes (log β3 = 20.2, 17.2, and 15.9 for NiII, FeII, and CoII, respectively [96]). Afterward, Wagenknecht et al. reported a 3WJ structure with an analogous bpy-modified cytidines, which was also stabilized by the formation of NiII(bpy)3 [97].
Larger stabilization was observed for 3WJ structures containing the improved version of bpy-modified nucleosides, Ubpy-2 [92] and Ubpy-3 [93]. We hypothesized that the triazole group of Ubpy-1 could interfere with the metal coordination process and the relatively rigid linker may disturb the local structure of the junction core, thus adversely affecting the 3WJ stability. Accordingly, Ubpy-2 and Ubpy-3 were designed to have the bpy ligand with a carbamate or an amide linker, respectively. Ubpy-2 and Ubpy-3 have opposite stereochemistry of the modification sites (i.e., the 2′-position), but the linkers have the same length. The thermal stability of the 3WJ containing Ubpy-2 was significantly enhanced upon addition of one equivalent of NiII ions (ΔTm = + 18.8 °C) (Fig. 12f). Likewise, the 3WJ containing Ubpy-3 was greatly stabilized by adding NiII ions (ΔTm = + 17.7 °C). These results demonstrated that the stereochemistry of the modification site has little influence on the thermal stability of the bpy-modified 3WJs.
Recently, DNA 3WJ structures modified with phenanthroline (phen) ligands were synthesized by using a newly designed phen-modified nucleosides Uphen [98]. The 3WJ containing three phen ligands at the center was found to be stabilized through the formation of an interstrand NiII(phen)3 complex (ΔTm = + 16.9 °C) (Fig. 12f). The phen ligands are known to form 3:1 metal complexes with higher stability constants than bpy (e.g., log β3 = 24.3 for NiII(phen)3 [96]). Nonetheless, the degree of the NiII-dependent stabilization of the phen-modified 3WJ was comparable to that of the bpy-modified 3WJs. The Uphen nucleoside has the same succinate linker as Ubpy-3, but the positions of the coordinating nitrogen atoms are different. This slight structural difference might affect the degree of the metal-mediated 3WJ stabilization.
The NiII-mediated stabilization of the ligand-modified DNA 3WJs was further applied to metal-triggered structural transformation between duplexes and 3WJs (Fig. 13) [92, 93, 96]. For example, a mixture of three DNA strands containing a Ubpy-2 nucleoside (L1, L2, and L3) that can form the bpy-modified 3WJ and their complementary natural strands (S4, S5, and S6) were annealed in the absence and the presence of NiII ions [92]. Native polyacrylamide gel electrophoresis (PAGE) analysis showed that the six DNA strands self-assembled into three DNA duplexes (L1S4, L2S5, and L3S6) under NiII-free conditions. When one equivalent of NiII ions was added, the formation of two kinds of 3WJ structures, namely, a metal-bridged 3WJ (L1L2L3·NiII) and an unmodified 3WJ (S4S5S6) was observed. The yields of the 3WJs were estimated to be about 60%. These results demonstrated that structure conversion from duplexes to 3WJs occurred via the formation of an interstrand NiII(bpy)3 complex. More efficient duplex-to-3WJ conversion was realized by redesigning the base sequences of the counter natural strands (M4, M5, and M6) (Fig. 13a). Two nucleobases at the junction core were altered to introduce mismatches, which decreased the stability of the duplexes. As a result, the efficiency of the NiII-mediated 3WJ formation was improved to ca. 90% (Fig. 13b). Furthermore, the addition of EDTA to remove NiII ions resulted in the regeneration of the duplexes, showing reversible metal-responsive conversion between the duplexes and the 3WJs.
a Schematic representation of NiII-mediated structural transformation between DNA duplexes and 3WJs. L1, L2, and L3 contain a Ubpy-2 nucleotide in the middle. M4, M5, and M6 have the complementary base sequences except for two bases in the middle. b Native polyacrylamide gel electrophoresis (PAGE) of a mixture of L1, L2, L3, M4, M5, and M6 in the absence and the presence of NiII ions (1.0 equiv). M4 was labeled with a fluorophore (FAM). [DNA strand] = 1.0 µM each in 10 mM MOPS buffer (pH 7.0) containing 100 mM NaCl, [NiII] = 1.0 µM or [EDTA] = 10 µM. Reproduced with permission from ref. 92. Copyright 2016 Royal Society of Chemistry
NiII-triggered structure conversion was also demonstrated with 3WJ containing Ubpy-3 [93]. With optimized base sequences, the duplexes were formed exclusively in the absence of NiII ions, and the 3WJs were formed in 83% yield by the addition of NiII ions. In the case of the phen-modified DNA strands containing Uphen [98], the addition of NiII ions did not induce the formation of 3WJs when unmutated complementary strands (i.e., S4, S5, and S6) were used. This is probably due to the high stability of the duplexes, in which the large aromatic moiety of the phen ligand intercalates into the base pairs. When the mutated strands (M4, M5, and M6) were utilized, the 3WJs were formed in 88% yield in the presence of NiII ions while duplexes were mainly formed without NiII ions.
The 3WJ stabilization via NiII(bpy)3 complexation was also applied to metal-mediated self-sorting of 3WJ structures (Fig. 14) [93]. Self-sorting is a process wherein molecules in a complex mixture discriminate self from non-self to selectively self-assemble into specific structures [99]. We have demonstrated NiII-mediated self-sorting of 3WJs by using DNA strands containing a Ubpy-3 nucleotide in the middle (L1, L2, and L3). Their unmodified counterparts (S1′, S2′, and S3′) were tailed with oligo-thymidine so that the different 3WJs exhibit different mobilities in native PAGE analysis (Fig. 14a). When all the six strands were annealed in the absence of NiII ions, at least three bands were observed on the gel, indicating that 3WJs with zero to three bpy ligands (S1′S2′S3′, S1′S2′L3, S1′L2L3, etc.) were randomly formed (Fig. 14b). In the presence of one equivalent of NiII ions, only two bands appeared, confirming the selective formation of the metal-bridged 3WJ L1L2L3·NiII and the unmodified 3WJ S1′S2′S3′. Taken together, the self-sorting of the 3WJs was successfully demonstrated by NiII(bpy)3 complexation at the junction core.
NiII-mediated self-sorting of 3WJs. a Schematic representation of the self-sorting. Blue: DNA strands containing a Ubpy-3 (L1, L2, and L3), black: unmodified strands with an oligo-T tail (S1′, S2′, and S3′). b Native polyacrylamide gel electrophoresis (PAGE) of a mixture of L1, L2, L3, S1′, S2′, and S3′ in the absence and the presence of NiII ions (1.0 equiv) after staining with SYBR Gold. The 3WJs L1L2L3, L1L2 L3·NiII, L1L2S3′, L1S2′S3′, and S1′S2′S3′ were also analyzed as markers. [DNA strand] = 1.0 µM each in 10 mM MOPS buffer (pH 7.0) containing 100 mM NaCl, [NiII] = 1.0 µM or [EDTA] = 10 µM. Reproduced with permission from ref. 93. Copyright 2021 Wiley-VCH
DNA 3WJ structures are one of the fundamental building blocks for synthesizing DNA nanoarchitectures such as DNA polyhedra and three-dimensional networks [88]. DNA hydrogels [100] and nanodroplets [101] can be made based on the 3WJ formation as well. Thus, metal-mediated stabilization and structure induction of ligand-modified 3WJs are expected to offer novel approaches to develop metal-responsive DNA materials. For example, DNA polyhedra [102, 103] containing ligand-modified 3WJs as their vertices would be formed in a metal-dependent manner. It is expected that metal complexation increases the rigidity of the 3WJ structures. Thus, the physical property of DNA hydrogels and droplets composed of ligand-modified 3WJs could be changed in response to metal ions. In theory, metal selectivity can be altered by careful selection of the ligand moiety. Therefore, the metal-responsive DNA 3WJs will be utilized as novel building blocks in future studies of DNA supramolecular chemistry and nanotechnology.
Conclusion
In this review, the recent progress on the development of metal-responsive DNA supramolecules were described. Metal-mediated base pairs, formed through metal complexation of opposing ligand-type nucleobase analogs, have been employed to develop metal-responsive allosteric DNAzymes (catalytic DNA). Split DNAzymes and single-stranded DNAzymes were rationally designed so that metal-mediated base pairing reorganizes the catalytically active structures. In the case of the non-split single-stranded DNAzymes, the base sequences were strategically designed to block the catalytic domain in the absence of the metal ions. As a result, the DNAzyme activity can be reversibly regulated in response to the specific metal ions. Notably, an AND-gate DNAzyme activated only in the presence of both AgI and CuII ions was created based on the same strategy. This design strategy would be applicable to embedding metal-responsiveness to various types of functional DNAs and DNA nanoarchitectures.
The novel concept of metal-responsive base-pairing system was proposed by using bifacial nucleobases that form both hydrogen-bonded and metal-mediated base pairs. The bifacial nucleobases such as UOH, caU, and dcaU were designed to have a multidentate metal-binding site in addition to the Watson–Crick hydrogen-bonding site. While DNA duplexes are thermally stabilized by the formation of the metal-mediated base pairs (such as UOH-GdIII–UOH), duplexes containing hydrogen-bonded base pairs (such as UOH–A) are destabilized by the addition of the corresponding metal ions. This unique property of the bifacial nucleobases allows for the metal-triggered switching between the hydrogen-bonded and the metal-mediated base pairs. Subsequently, the metal-mediated base-pair switching was applied to metal-triggered DNA strand displacement reactions (SDRs), which would be employed to metal-dependent operation of DNA-based molecular machines and computing systems. As a simplest model, the DNA tweezers that open and close in response to GdIII ions were successfully constructed. The GdIII-responsive allosteric DNAzyme was also developed by the incorporation of UOH bases into a known DNAzyme sequence. Since the duplex stability and the hybridization behavior can be drastically changed by metal complexation, the metal-mediated base-pair switching of the bifacial nucleobases would be useful for dynamic control of DNA nanostructures.
Metal-dependent stabilization of DNA structures other than duplexes is also an important topic. Metal-bridged DNA three-way junction (3WJ) structures were constructed by using DNA strands containing a bipyridine-modified or a phenanthroline-modified nucleotide. The metal ligands were introduced at the 2′-position of the nucleoside so that three ligands are preorganized in the center of the junction. The thermal stability of the ligand-modified 3WJs were found to be significantly enhanced through the formation of an interstrand 3:1 metal complex such as NiII(bpy)3 and NiII(phen)3. Metal-mediated structural transformation between DNA duplexes and 3WJs was demonstrated by using the ligand-modified DNA strands and their natural complementary strands. Likewise, NiII-mediated self-sorting of the 3WJ structures was successfully demonstrated. Since DNA branched structures are essential structural motifs to build up DNA nanoarchitectures, the metal-mediated stabilization and structural induction of the metallo-3WJ motifs would have many potential applications in the field of supramolecular nucleic acid chemistry.
Metal-mediated base pairs consisting of natural nucleobases, T–HgII–T and C–AgI–C, have been broadly utilized to develop HgII- and AgI-responsive DNA materials [32]. However, the sequence design is often troublesome because HgII and AgI ions could bind to T and C bases at unintended positions. In contrast, the incorporation of artificial ligand-bearing nucleotide into DNA structures enables the use of metal ions that rarely interact with natural nucleobases. Our molecular design discussed here would be highly compatible with standard DNA nanotechnologies because only a few modified nucleosides are introduced as additional building blocks. Therefore, we are convinced that the rational design of the metal-responsive DNA systems will largely contribute to a wide range of DNA material sciences, particularly, DNA supramolecular chemistry and DNA nanotechnology. The metal-dependent transformation of DNA nanostructures as well as the metal-mediated operation of DNA molecular machines are now under investigation.
Data availability
No datasets were generated or analysed during the current study.
References
Stulz, E., Clever, G.H. (eds.): DNA in Supramolecular Chemistry and Nanotechnology. Wiley, Chichester (2015)
Seeman, N.C.: Structural DNA Nanotechnology. Cambridge University Press, Cambridge (2016)
Seeman, N.C., Sleiman, H.F.: DNA nanotechnology. Nat. Rev. Mater. 3, 17068 (2017). https://doi.org/10.1038/natrevmats.2017.68
Jonoska, N., Winfree, E. (eds.): Visions of DNA Nanotechnology at 40 for the next 40: A Tribute to Nadrian C. Seeman. Springer, Singapore (2023)
Rothemund, P.W.: Folding DNA to create nanoscale shapes and patterns. Nature. 440, 297–302 (2006). https://doi.org/10.1038/nature04586
Benson, E., Mohammed, A., Gardell, J., Masich, S., Czeizler, E., Orponen, P., Hogberg, B.: DNA rendering of polyhedral meshes at the nanoscale. Nature. 523, 441–444 (2015). https://doi.org/10.1038/nature14586
Endo, M. (ed.): DNA Origami: Structures, Technology, and Applications. Wiley, Hoboken (2022)
Ketterer, P., Willner, E.M., Dietz, H.: Nanoscale rotary apparatus formed from tight-fitting 3D DNA components. Sci. Adv. 2 (2016). https://doi.org/10.1126/sciadv.1501209 e1501209
Ramezani, H., Dietz, H.: Building machines with DNA molecules. Nat. Rev. Genet. 21, 5–26 (2020). https://doi.org/10.1038/s41576-019-0175-6
Del Grosso, E., Franco, E., Prins, L.J., Ricci, F.: Dissipative DNA nanotechnology. Nat. Chem. 14, 600–613 (2022). https://doi.org/10.1038/s41557-022-00957-6
Murata, S., Toyota, T., Nomura, S.M., Nakakuki, T., Kuzuya, A.: Molecular cybernetics: Challenges toward cellular chemical artificial intelligence. Adv. Funct. Mater. 32, 2201866 (2022). https://doi.org/10.1002/adfm.202201866
Madsen, M., Gothelf, K.V.: Chemistries for DNA nanotechnology. Chem. Rev. 119, 6384–6458 (2019). https://doi.org/10.1021/acs.chemrev.8b00570
Ivancová, I., Leone, D., Hocek, M.: Reactive modifications of DNA nucleobases for labelling, bioconjugations and cross-linking. Curr. Opin. Chem. Biol. 52, 136–144 (2019). https://doi.org/10.1016/j.cbpa.2019.07.007
Ito, Y., Hari, Y.: Synthesis of nucleobase-modified oligonucleotides by Post-synthetic Modification in Solution. Chem. Rec. 22, e202100325 (2022). https://doi.org/10.1002/tcr.202100325
Hollenstein, M.: Enzymatic synthesis of base-modified nucleic acids. In: Sugimoto, N. (ed.) Handbook of Chemical Biology of Nucleic Acids, pp. 687–726. Springer, Singapore (2023)
Kobayashi, T., Takezawa, Y., Sakamoto, A., Shionoya, M.: Enzymatic synthesis of ligand-bearing DNAs for metal-mediated base pairing utilising a template-independent polymerase. Chem. Commun. 52, 3762–3765 (2016). https://doi.org/10.1039/C5CC10039A
Takezawa, Y., Kobayashi, T., Shionoya, M.: The effects of Magnesium ions on the enzymatic synthesis of ligand-bearing Artificial DNA by template-independent polymerase. Int. J. Mol. Sci. 17, 906 (2016). https://doi.org/10.3390/ijms17060906
Wang, C., O’Hagan, M.P., Li, Z., Zhang, J., Ma, X., Tian, H., Willner, I.: Photoresponsive DNA materials and their applications. Chem. Soc. Rev. 51, 720–760 (2022). https://doi.org/10.1039/D1CS00688F
Lubbe, A.S., Szymanski, W., Feringa, B.L.: Recent developments in reversible photoregulation of oligonucleotide structure and function. Chem. Soc. Rev. 46, 1052–1079 (2017). https://doi.org/10.1039/C6CS00461J
Kamiya, Y., Asanuma, H.: Light-driven DNA nanomachine with a photo-responsive molecular engine. Acc. Chem. Res. 47, 1663–1672 (2014). https://doi.org/10.1021/ar400308f
McConnell, A.J., Wood, C.S., Neelakandan, P.P., Nitschke, J.R.: Stimuli-responsive metal-ligand assemblies. Chem. Rev. 115, 7729–7793 (2015). https://doi.org/10.1021/cr500632f
Champin, B., Mobian, P., Sauvage, J.-P.: Transition metal complexes as molecular machine prototypes. Chem. Soc. Rev. 36, 358–366 (2007). https://doi.org/10.1039/B604484K
Sakata, Y.: Creation of kinetically-controlled supramolecular systems based on coordination chemistry. J. Incl. Phenom. Macrocycl. Chem. 103, 161–188 (2023). https://doi.org/10.1007/s10847-023-01190-5
Miyake, R.: Cooperative systems constructed using crystalline metal complexes of short flexible peptides. J. Incl. Phenom. Macrocycl. Chem. 102, 711–722 (2022). https://doi.org/10.1007/s10847-022-01145-2
Takezawa, Y., Shionoya, M.: Recent advances in the development of metal-responsive functional DNAs based on metal-mediated Artificial Base Pairing. In: Müller, J., Lippert, B. (eds.) Modern Avenues in Metal-Nucleic Acid Chemistry (Metal Ions in Life Science, vol. 25, pp. 257–289. CRC, Boca Raton (2023)
Takezawa, Y., Shionoya, M.: Metal Ion-Induced changes in the Stability of DNA duplexes. In: Sugimoto, N. (ed.) Handbook of Chemical Biology of Nucleic Acids, pp. 2645–2683. Springer, Singapore (2023)
Takezawa, Y., Müller, J., Shionoya, M.: Artificial DNA base pairing mediated by diverse metal ions. Chem. Lett. 46, 622–633 (2017). https://doi.org/10.1246/cl.160985
Takezawa, Y., Shionoya, M.: Metal-mediated DNA base pairing: Alternatives to hydrogen-bonded Watson–Crick base pairs. Acc. Chem. Res. 45, 2066–2076 (2012). https://doi.org/10.1021/ar200313h
Lippert, B.: Metal-modified base pairs vs. metal-mediated pairs of bases: Not just a semantic issue! J. Biol. Inorg. Chem. 27, 215–219 (2022). https://doi.org/10.1007/s00775-022-01926-7
Naskar, S., Guha, R., Müller, J.: Metal-modified nucleic acids: Metal-mediated base pairs, triples and tetrads. Angew Chem. Int. Ed. 59, 1397–1406 (2020). https://doi.org/10.1002/anie.201905913
Müller, J.: Nucleic acid duplexes with metal-mediated base pairs and their structures. Coord. Chem. Rev. 393, 37–47 (2019). https://doi.org/10.1016/j.ccr.2019.05.007
Tanaka, Y., Kondo, J., Sychrovsky, V., Sebera, J., Dairaku, T., Saneyoshi, H., Urata, H., Torigoe, H., Ono, A.: Structures, physicochemical properties, and applications of T-HgII-T, C-AgI-C, and other metallo-base-pairs. Chem. Commun. 51, 17343–17360 (2015). https://doi.org/10.1039/C5CC02693H
Takezawa, Y., Shionoya, M., Müller, J.: Self-assemblies based on metal-mediated Artificial Nucleobase Pairing. In: Atwood, J.L. (ed.) Comprehensive Supramolecular Chemistry II, vol. 4, pp. 259–293. Elsevier, Oxford (2017)
Tanaka, K., Shionoya, M.: Synthesis of a novel nucleoside for alternative DNA base pairing through metal complexation. J. Org. Chem. 64, 5002–5003 (1999). https://doi.org/10.1021/jo990326u
Takezawa, Y., Tanaka, K., Yori, M., Tashiro, S., Shiro, M., Shionoya, M.: Soft metal-mediated base pairing with Novel Synthetic nucleosides possessing an O,S-Donor ligand. J. Org. Chem. 73, 6092–6098 (2008). https://doi.org/10.1021/jo800587d
Hu, L., Takezawa, Y., Shionoya, M.: CuII-mediated DNA base pairing of a triazole-4-carboxylate nucleoside prepared by click chemistry. Chem. Commun. 59, 892–895 (2023). https://doi.org/10.1039/D2CC06205D
Hu, L., Takezawa, Y., Shionoya, M.: Metal-mediated DNA base pairing of easily prepared 2-oxo-imidazole-4-carboxylate nucleotides. Chem. Sci. 13, 3977–3983 (2022). https://doi.org/10.1039/D2SC00926A
Tanaka, K., Tengeiji, A., Kato, T., Toyama, N., Shiro, M., Shionoya, M.: Efficient incorporation of a copper hydroxypyridone base pair in DNA. J. Am. Chem. Soc. 124, 12494–12498 (2002). https://doi.org/10.1021/ja027175o
Takezawa, Y., Shionoya, M.: DNA inspired self-assembled metal arrays. In: Jabbari, E., Kim, D.-H., Lee, L.P., Ghaemmaghami, A., Khademhosseini, A. (eds.) Biomimetics Bioinspired Materials, Mechanics, and Dynamics, Handbook of Biomimetics and Bioinspiration, vol. 1, pp. 217–245. World Scientific Publishing, Singapore (2014)
Tanaka, K., Tengeiji, A., Kato, T., Toyama, N., Shionoya, M.: A Discrete Self-assembled metal array in Artificial DNA. Science. 299, 1212–1213 (2003). https://doi.org/10.1126/science.1080587
Tanaka, K., Clever, G.H., Takezawa, Y., Yamada, Y., Kaul, C., Shionoya, M., Carell, T.: Programmable self-assembly of metal ions inside artificial DNA duplexes. Nat. Nanotechnol. 1, 190–194 (2006). https://doi.org/10.1038/nnano.2006.141
Takezawa, Y., Maeda, W., Tanaka, K., Shionoya, M.: Discrete self-assembly of Iron(III) ions inside Triple-stranded Artificial DNA. Angew Chem. Int. Ed. 48, 1081–1084 (2009). https://doi.org/10.1002/anie.200804654
Liu, S., Clever, G.H., Takezawa, Y., Kaneko, M., Tanaka, K., Guo, X., Shionoya, M.: Direct conductance measurement of individual Metallo-DNA duplexes within single-molecule Break junctions. Angew Chem. Int. Ed. 50, 8886–8890 (2011). https://doi.org/10.1002/anie.201102980
Ma, L., Liu, J.: Catalytic nucleic acids: Biochemistry, chemical biology, biosensors, and nanotechnology. iScience. 23, 100815 (2020). https://doi.org/10.1016/j.isci.2019.100815
Liu, M., Chang, D., Li, Y.: Discovery and biosensing applications of diverse RNA-cleaving DNAzymes. Acc. Chem. Res. 50, 2273–2283 (2017). https://doi.org/10.1021/acs.accounts.7b00262
Orbach, R., Willner, B., Willner, I.: Catalytic nucleic acids (DNAzymes) as functional units for logic gates and computing circuits: From basic principles to practical applications. Chem. Commun. 51, 4144–4160 (2015). https://doi.org/10.1039/C4CC09874A
Huang, Z., Wang, X., Wu, Z., Jiang, J.-H.: Recent advances on DNAzyme-based sensing. Chem. Asian J. 17, e2021014 (2022). https://doi.org/10.1002/asia.202101414
Wang, S., Liu, Y., Shang, J., Wang, H., Yang, C., Liu, X., Wang, F.: Stimuli-responsive RNA-Cleaving DNAzyme for Biomedical Application. Anal. Sens. 3, e20220006 (2023). https://doi.org/10.1002/anse.202200065
Stojanovic, M.N., Mitchell, T.E., Stefanovic, D.: Deoxyribozyme-based Logic Gates. J. Am. Chem. Soc. 124, 3555–3561 (2002). https://doi.org/10.1021/ja016756v
Shen, Y., Chiuman, W., Brennan, J.D., Li, Y.: Catalysis and Rational Engineering of trans-acting pH6DZ1, an RNA-Cleaving and fluorescence-signaling deoxyribozyme with a four-way Junction structure. ChemBioChem. 7, 1343–1348 (2006). https://doi.org/10.1002/cbic.200600195
Asanuma, H., Hayashi, H., Zhao, J., Liang, X., Yamazawa, A., Kuramochi, T., Matsunaga, D., Aiba, Y., Kashida, H., Komiyama, M.: Enhancement of RNA cleavage activity of 10–23 DNAzyme by covalently introduced intercalator. Chem. Commun. 42, 5062–5064 (2006). https://doi.org/10.1039/B611078A
Shimron, S., Elbaz, J., Henning, A., Willner, I.: Ion-induced DNAzyme switches. Chem. Commun. 46, 3250–3252 (2010). https://doi.org/10.1039/B926003J
Liu, J., Lu, Y.: Rational design of Turn-On Allosteric DNAzyme Catalytic beacons for Aqueous Mercury ions with Ultrahigh Sensitivity and Selectivity. Angew Chem. Int. Ed. 46, 7587–7590 (2007). https://doi.org/10.1002/anie.200702006
Breaker, R.R., Joyce, G.F.: A DNA enzyme with Mg2+-dependent RNA phosphoesterase activity. Chem. Biol. 2, 655–660 (1995). https://doi.org/10.1016/1074-5521(95)90028-4
Takezawa, Y., Nakama, T., Shionoya, M.: Enzymatic synthesis of Cu(II)-responsive deoxyribozymes through polymerase incorporation of artificial ligand-type nucleotides. J. Am. Chem. Soc. 141, 19342–19350 (2019). https://doi.org/10.1021/jacs.9b08955
Schlegel, M.K., Zhang, L., Pagano, N., Meggers, E.: Metal-mediated base pairing within the simplified nucleic acid GNA. Org. Biomol. Chem. 7, 476–482 (2009). https://doi.org/10.1039/B816142A
Trapaidze, A., Hureau, C., Bal, W., Winterhalter, M., Faller, P.: Thermodynamic study of Cu2+ binding to the DAHK and GHK peptides by isothermal titration calorimetry (ITC) with the weaker competitor glycine. J. Biol. Inorg. Chem. 17, 37–47 (2012). https://doi.org/10.1007/s00775-011-0824-5
Takezawa, Y., Hu, L., Nakama, T., Shionoya, M.: Sharp switching of DNAzyme activity through the formation of a CuII-mediated carboxyimidazole base pair. Angew Chem. Int. Ed. 59, 21488–21492 (2020). https://doi.org/10.1002/anie.202009579
Sandmann, N., Defayay, D., Hepp, A., Müller, J.: Metal-mediated base pairing in DNA involving the artificial nucleobase imidazole-4-carboxylate. J. Inorg. Biochem. 191, 85–93 (2019). https://doi.org/10.1016/j.jinorgbio.2018.10.013
Rajasree, S.C., Takezawa, Y., Shionoya, M.: CuII-mediated stabilisation of DNA duplexes bearing consecutive ethenoadenine lesions and its application to a metal-responsive DNAzyme. Chem. Commun. 59, 1006–1009 (2023). https://doi.org/10.1039/D2CC06179A
Mandal, S., Wang, C., Prajapati, R.K., Kösters, J., Verma, S., Chi, L., Müller, J.: Metal-mediated assembly of 1,N6-Ethenoadenine: From surfaces to DNA duplexes. Inorg. Chem. 55, 7041–7050 (2016). https://doi.org/10.1021/acs.inorgchem.6b00927
Torabi, S.-F., Wu, P., McGhee, C.E., Chen, L., Hwang, K., Zheng, N., Cheng, J., Lu, Y.: In vitro selection of a sodium-specific DNAzyme and its application in intracellular sensing. Proc. Natl. Acad. Sci. U S A. 112, 5903–5908 (2015). https://doi.org/10.1073/pnas.1420361112
Nakama, T., Takezawa, Y., Sasaki, D., Shionoya, M.: Allosteric regulation of DNAzyme activities through intrastrand transformation induced by Cu(II)-mediated artificial base pairing. J. Am. Chem. Soc. 142, 10153–10162 (2020). https://doi.org/10.1021/jacs.0c03129
Berry, D.A., Jung, K.-Y., Wise, D.S., Sercel, A.D., Pearson, W.H., Mackie, H., Randolph, J.B., Somers, R.L.: Pyrrolo-dC and pyrrolo-C: Fluorescent analogs of cytidine and 2′-deoxycytidine for the study of oligonucleotides. Tetrahedron Lett. 45, 2457–2461 (2004). https://doi.org/10.1016/j.tetlet.2004.01.108
Nakama, T., Takezawa, Y., Shionoya, M.: Site-specific polymerase incorporation of consecutive ligand-containing nucleotides for multiple metal-mediated base pairing. Chem. Commun. 57, 1392–1395 (2021). https://doi.org/10.1039/D0CC07771B
Takezawa, Y., Hu, L., Nakama, T., Shionoya, M.: Metal-dependent activity control of a compact-sized 8–17 DNAzyme based on metal-mediated unnatural base pairing. Chem. Commun. 60, 288 (2024). https://doi.org/10.1039/D3CC05520E
Santoro, S.W., Joyce, G.F.: A general purpose RNA-cleaving DNA enzyme. Proc. Natl. Acad. Sci. U S A. 94, 4262–4266 (1997). https://doi.org/10.1073/pnas.94.9.4262
Liu, H., Yu, X., Chen, Y., Zhang, J., Wu, B., Zheng, L., Haruehanroengra, P., Wang, R., Li, S., Lin, J., Li, J., Sheng, J., Huang, Z., Ma, J., Gan, J.: Crystal structure of an RNA-cleaving DNAzyme. Nat. Commun. 8, 2006 (2017). https://doi.org/10.1038/s41467-017-02203-x
Saran, R., Liu, J.: A silver DNAzyme. Anal. Chem. 88, 4014–4020 (2016). https://doi.org/10.1021/acs.analchem.6b00327
McGhee, C.E., Lake, R.J., Lu, Y.: Preparation of MetalloDNAzymes. In: Diéguez, M., Bäckvall, J.E., Pàmies, O. (eds.) Artificial Metalloenzymes and MetalloDNAzymes in Catalysis, pp. 41–68. Wiley-VCH, Weinheim (2018)
Heddinga, M.H., Müller, J.: Incorporation of a metal-mediated base pair into an ATP aptamer – using silver(I) ions to modulate aptamer function. Beilstein J. Org. Chem. 16, 2870–2879 (2020). https://doi.org/10.3762/bjoc.16.236
Heddinga, M.H., Müller, J.: Modulating aptamer function by copper(II)-mediated base pair formation. Org. Biomol. Chem. 20, 4787–4793 (2022). https://doi.org/10.1039/D2OB00788F
Johannsen, S., Megger, N., Böhme, D., Sigel, R.K.O., Müller, J.: Solution structure of a DNA double helix with consecutive metal-mediated base pairs. Nat. Chem. 2, 229–234 (2010). https://doi.org/10.1038/nchem.512
Zhou, W., Saran, R., Liu, J.: Metal sensing by DNA. Chem. Rev. 117, 8272–8325 (2017). https://doi.org/10.1021/acs.chemrev.7b00063
Hwang, K., Mou, Q., Lake, R.J., Xiong, M., Holland, B., Lu, Y.: Metal-dependent DNAzymes for the Quantitative Detection of Metal Ions in living cells: Recent progress, current challenges, and latest results on FRET ratiometric sensors. Inorg. Chem. 58, 13696–13708 (2019). https://doi.org/10.1021/acs.inorgchem.9b01280
Takezawa, Y., Nishiyama, K., Mashima, T., Katahira, M., Shionoya, M.: Bifacial base-pairing behaviors of 5-hydroxyuracil DNA bases through hydrogen bonding and metal coordination. Chem. Eur. J. 21, 14713–14716 (2015). https://doi.org/10.1002/chem.201502772
Nishiyama, K., Takezawa, Y., Shionoya, M.: pH-dependence of the thermal stability of metallo-DNA duplexes containing ligand-type 5-hydroxyuracil nucleobases. Inorg. Chim. Acta. 452, 176–180 (2016). https://doi.org/10.1016/j.ica.2016.04.040
Takezawa, Y., Suzuki, A., Nakaya, M., Nishiyama, K., Shionoya, M.: Metal-dependent DNA base pairing of 5-carboxyuracil with itself and all four canonical nucleobases. J. Am. Chem. Soc. 142, 21640–21644 (2020). https://doi.org/10.1021/jacs.0c11437
Mori, K., Takezawa, Y., Shionoya, M.: Metal-dependent base pairing of bifacial iminodiacetic acid-modified uracil bases for switching DNA hybridization partner. Chem. Sci. 14, 1082–1088 (2023). https://doi.org/10.1039/D2SC06534G
Takezawa, Y., Mori, K., Huang, W.-E., Nishiyama, K., Xing, T., Nakama, T., Shionoya, M.: Metal-mediated DNA strand displacement and molecular device operations based on base-pair switching of 5-hydroxyuracil nucleobases. Nat. Commun. 14, 4759 (2023). https://doi.org/10.1038/s41467-023-40353-3
Zhang, D.Y., Seelig, G.: Dynamic DNA nanotechnology using strand displacement reactions. Nat. Chem. 3, 103–113 (2011). https://doi.org/10.1038/nchem.957
Simmel, F.C., Yurke, B., Singh, H.R.: Principles and applications of nucleic acid strand displacement reactions. Chem. Rev. 119, 6326–6369 (2019). https://doi.org/10.1021/acs.chemrev.8b00580
Jean, J.M., Hall, K.B.: 2-Aminopurine fluorescence quenching and lifetimes: Role of base stacking. Proc. Natl. Acad. Sci. U S A. 98, 37–41 (2001). https://doi.org/10.1073/pnas.98.1.37
Nishiyama, K., Mori, K., Takezawa, Y., Shionoya, M.: Metal-responsive reversible binding of triplex-forming oligonucleotides with 5-hydroxyuracil nucleobases. Chem. Commun. 57, 2487–2490 (2021). https://doi.org/10.1039/D1CC00553G
Hu, Y., Cecconello, A., Idili, A., Ricci, F., Willner, I.: Triplex DNA nanostructures: From Basic properties to applications. Angew Chem. Int. Ed. 56, 15210–15233 (2017). https://doi.org/10.1002/anie.201701868
Yurke, B., Turberfield, A.J., Mills, A.P., Simmel, F.C., Neumann, J.L.: A DNA-fuelled molecular machine made of DNA. Nature. 406, 605–608 (2000). https://doi.org/10.1038/35020524
Zadeh, J.N., Steenberg, C.D., Bois, J.S., Wolfe, B.R., Pierce, M.B., Khan, A.R., Dirks, R.M., Pierce, N.A.: NUPACK: Analysis and design of nucleic acid systems. J. Comput. Chem. 32, 170–173 (2011). https://doi.org/10.1002/jcc.21596
Dong, Y., Yao, C., Zhu, Y., Yang, L., Luo, D., Yang, D.: DNA functional materials assembled from branched DNA: Design, synthesis, and applications. Chem. Rev. 120, 9420–9481 (2020). https://doi.org/10.1021/acs.chemrev.0c00294
Takezawa, Y., Duprey, J.-L., Shionoya, M.: Metal-aided construction of unusual DNA structural motifs. In: Stulz, E., Clever, G.H. (eds.) DNA in Supramolecular Chemistry and Nanotechnology, pp. 65–77. Wiley, Chichester (2015)
Takezawa, Y., Shionoya, M.: Supramolecular DNA three-way Junction Motifs with a bridging Metal Center. Front. Chem. 7, 925 (2020). https://doi.org/10.3389/fchem.2019.00925
Duprey, J.-L., Takezawa, Y., Shionoya, M.: Metal-locked DNA three-way junction. Angew Chem. Int. Ed. 52, 1212–1216 (2013). https://doi.org/10.1002/anie.201207338
Takezawa, Y., Yoneda, S., Duprey, J.-L., Nakama, T., Shionoya, M.: Metal-responsive structural transformation between artificial DNA duplexes and three-way junctions. Chem. Sci. 7, 3006–3010 (2016). https://doi.org/10.1039/C6SC00383D
Takezawa, Y., Sakakibara, S., Shionoya, M.: Bipyridine-modified DNA three-way junctions with Amide linkers: Metal-dependent structure induction and self-sorting. Chem. Eur. J. 27, 16626–16633 (2021). https://doi.org/10.1002/chem.202102977
Oleksi, A., Blanco, A.G., Boer, R., Usón, I., Aymamí, J., Rodger, A., Hannon, M.J., Coll, M.: Molecular recognition of a three-way DNA junction by a metallosupramolecular helicate. Angew Chem. Int. Ed. 45, 1227–1231 (2006). https://doi.org/10.1002/anie.200503822
Woods, K.C., Martin, S.S., Chu, V.C., Baldwin, E.P.: Quasi-equivalence in site-specific recombinase structure and function: Crystal structure and activity of trimeric cre recombinase bound to a three-way Lox DNA Junction. J. Mol. Biol. 313, 49–69 (2001). https://doi.org/10.1006/jmbi.2001.5012
Smith, R.M., Martell, A.E.: Critical Stability Constants, Vol. 2. Plenum Press, New York (1975)
Stubinitzky, C., Bijeljanin, A., Antusch, L., Ebeling, D., Hölscher, H., Wagenknecht, H.-A.: Bifunctional DNA architectonics: Three-way junctions with sticky perylene bisimide caps and a central metal lock. Chem. Eur. J. 20, 12009–12014 (2014). https://doi.org/10.1002/chem.201402956
Takezawa, Y., Kanemaru, D., Kudo, N., Shionoya, M.: Phenanthroline-modified DNA three-way junction structures stabilized by interstrand 3: 1 metal complexation. Dalton Trans. 52, 11025–11029 (2023). https://doi.org/10.1039/D3DT01508D
Safont-Sempere, M.M., Fernandez, G., Würthner, F.: Self-sorting Phenomena in Complex Supramolecular systems. Chem. Rev. 111, 5784–5814 (2011). https://doi.org/10.1021/cr100357h
Wang, D., Hu, Y., Liu, P., Luo, D.: Bioresponsive DNA hydrogels: Beyond the conventional stimuli responsiveness. Acc. Chem. Res. 50, 733–739 (2017). https://doi.org/10.1021/acs.accounts.6b00581
Sato, Y., Sakamoto, T., Takinoue, M.: Sequence-based engineering of dynamic functions of micrometer-sized DNA droplets. Sci. Adv. 6, eaba3471 (2020). https://doi.org/10.1126/sciadv.aba3471
Chen, J.H., Seeman, N.C.: Synthesis from DNA of a molecule with the connectivity of a cube. Nature. 350, 631–633 (1991). https://doi.org/10.1038/350631a0
Goodman, R.P., Schaap, I.A.T., Tardin, C.F., Erben, C.M., Berry, R.M., Schmidt, C.F., Turberfield, A.J.: Rapid chiral assembly of rigid DNA building blocks for molecular nanofabrication. Science. 310, 1661–1665 (2005). https://doi.org/10.1126/science.1120367
Acknowledgements
The author appreciates the organizing committee of Host-Guest and Supramolecular Chemistry Society, Japan for giving him the SHGSC Japan Award of Excellence 2023 and the opportunity to write this review. The author is most grateful to Prof. Mitsuhiko Shionoya of the University of Tokyo for his valuable and constructive suggestions. The coworkers who contributed to the studies described in this review are also appreciated. This work was supported by JSPS KAKENHI Grant Numbers 16K14029, JP18H02081, JP21H02055, JP22K19100, and MEXT KAKENHI Grant Numbers JP21H00384 (Molecular Engine), JP21H05866 and JP23H04399 (Molecular Cybernetics). The author also acknowledges the Foundation Advanced Technology Institute (ATI), the Kao Foundation for Arts and Sciences, the Foundation for the Promotion of Ion Engineering, Foundation for Interaction in Science and Technology (FIST), the Noguchi Institute, Iketani Science and Technology Foundation, Kanamori Foundation, and Asahi Glass Foundation for financial support.
Funding
Open Access funding provided by The University of Tokyo.
Author information
Authors and Affiliations
Contributions
Y.T. wrote the manuscript and prepared all of the figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This is a paper selected for the “SHGSC Japan Award of Excellence 2023”.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Takezawa, Y. Rational design of metal-responsive functional DNA supramolecules. J Incl Phenom Macrocycl Chem (2024). https://doi.org/10.1007/s10847-024-01224-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10847-024-01224-6