Abstract
Blood-based biomarkers reflect systemic inflammation status and have prognostic and predictive value in solid malignancies. As a recently defined biomarker, Pan-Immune-Inflammation-Value (PIV) integrates different peripheral blood cell subpopulations. This retrospective study of collected data aimed to assess whether PIV may predict the pathological complete response (pCR) to neoadjuvant chemotherapy (NAC) in Turkish women with breast cancer. The study consisted of 743 patients with breast cancer who were scheduled to undergo NAC before attempting cytoreductive surgery. A pre-treatment complete blood count was obtained in the two weeks preceding NAC, and blood-based biomarkers were calculated from absolute counts of relevant cell populations. The pCR was defined as the absence of tumor cells in both the mastectomy specimen and lymph nodes. Secondary outcome measures included disease-free survival (DFS) and overall survival (OS). One hundred seven patients (14.4%) had pCR. In receiver operating characteristic analysis, optimal cut-off values for the neutrophile-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), platelet-to-lymphocyte (PLR), PIV, and Ki-67 index were determined as ≥ 2.34, ≥ 0.22, ≥ 131.8, ≥ 306.4, and ≥ 27, respectively. The clinical tumor (T) stage, NLR, MLR, PLR, PIV, estrogen receptor (ER) status, human epidermal growth factor receptor-2 (HER-2) status, and Ki-67 index were significantly associated with NAC response in univariate analyses. However, multivariate analysis revealed that the clinical T stage, PIV, ER status, HER-2 status, and Ki-67 index were independent predictors for pCR. Moreover, the low PIV group patients had significantly better DFS and OS than those in the high PIV group (p = 0.034, p = 0.028, respectively). Based on our results, pre-treatment PIV seems as a predictor for pCR and survival, outperforming NLR, MLR, PLR in predicting pCR in Turkish women with breast cancer who received NAC. However, further studies are needed to confirm our findings.
Similar content being viewed by others
Introduction
Malignancies arising in the mammary gland are the most common type of cancer in women1. The lifetime risk of breast cancer for a woman has been calculated at around 1-in-7 to 1-in-102, indicating that approximately 10% of the female population will be diagnosed with breast cancer during their lifetime1,2.
Neoadjuvant chemotherapy (NAC)—consisting of chemotherapy delivered before local treatment (surgery)—has become the standard of care in patients with locally advanced breast cancer3. In addition, downstaging of the primary tumor in patients with earlier stages of breast cancer may facilitate breast-conserving therapy and offer the opportunity to downstage the axilla—ultimately obviating the need for axillary treatment in some patients4,5. The overarching goal of NAC is to achieve pathological complete response (pCR)—which is, in turn, associated with a lower recurrence rate and more favorable survival outcomes6. Unfortunately, there is considerable interindividual variation in response to NAC amongst women with breast cancer, and several variables have been investigated in relation to this variability7.
In the last two decades, the relationship between chronic inflammation and cancer has become very popular, and both the diagnostic and therapeutic value of inflammatory markers have been studied extensively. Inflammation has been shown to promote tumor initiation and progression, whereas escape from immune surveillance may favor cancer invasiveness8. In the tumor microenvironment, neutrophils, monocytes-derived macrophages, and platelets have adverse prognostic significance by promoting tumoral angiogenesis and tumor growth, whereas tumor-infiltrating lymphocytes portend favorable outcomes9,10,11. Based on the assumption that peripheral blood cell populations can provide information about the intratumoral immune system status, peripheral blood-derived inflammation markers such as neutrophile-lymphocyte ratio (NLR), monocyte-lymphocyte ratio (MLR), and platelet-lymphocyte ratio (PLR) was shown to have prognostic value in many solid organ malignancies12,13,14. In addition to their prognostic value, these markers were reported to predict the neoadjuvant chemotherapy response in breast cancer15,16,17.
In 2020, Fuca et al. reported that a novel systemic immune score called Pan-Immune-Inflammation-Value (PIV) performed better in predicting survival outcomes than other immune-inflammatory biomarkers such as NLR in advanced colorectal cancer patients18. However, PIV's predictive and prognostic value in breast cancer patients receiving NAC has not been studied. We, therefore, designed the current study to address these issues specifically.
Results
The general characteristics of the entire study sample (n = 743) are shown in Table 1. The median age was 48.0 years (range: 22.0–83.5 years). One hundred ninety-seven patients (26.5%) had T3/T4, and 37.5% had node-positive disease. More than two-thirds received chemotherapy regimens containing both anthracycline and taxane. Of patients, 14.4% had pCR.
Table 2 presents the values of the area under the curve, sensitivity, and specificity in receiver operating characteristic (ROC) analysis. The cut-off values for the NLR, MLR, PLR, PIV, and Ki-67 index were determined as ≥ 2.34, ≥ 0.22, ≥ 131.8, ≥ 306.4, and ≥ 27, respectively.
Outcomes
Table 3 depicts the analyses of the association between the patients' characteristics and pCR. The clinical tumor (T) stage, NLR, MLR, PLR, PIV, estrogen receptor (ER) status, human epidermal growth factor receptor-2 (HER-2) status, and Ki-67 index were significantly associated with response to NAC. However, multivariate logistic regression analysis revealed that the clinical T stage (odds ratio [OR], 2.16; 95% confidence interval [CI], 1.10–4.23; p = 0.025) , PIV (OR, 3.32; 95% CI, 1.53–7.21, p = 0.002), ER status (OR, 3.24; %95 CI, 1.84–5.71; p < 0.001), HER-2 status (OR, 3.84; 95% CI, 2.17–6.80; ip < 0.001), and Ki-67 index (OR, 3.30; 95% CI, 1.72–6.32; p < 0.001) were independent predictors for pCR (Table 4). The blood-derived inflammation markers other than PIV lost statistical significance in multivariate analysis.
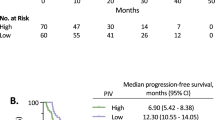
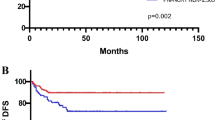
The median follow-up time was 67.5 months (range: 10.5–194.4 months). Median DFS and OS were not reached. 12-, 36-, and 60-months disease-free survival rates were 94.6%, 84.6%, 77.5%, respectively. 12-, 36-, and 60-months overall survival rates were 99.6%, 95.4%, 88.7%, respectively. As a secondary outcome, the disease-free survival (DFS) of patients in the low PIV group was significantly longer than the DFS of the high PIV group patients (hazard ratio [HR], 0.69; 95% CI 0.49–0.97; p = 0.034; Fig. 1A). Similarly, the low PIV group patients had significantly better overall survival (OS) than those in the high PIV group (HR, 0.61; 95% CI 0.39–0.95; p = 0.028; Fig. 1B).
Discussion
In the present study, we demonstrated for the first time that a new inflammatory score, PIV, was one of the independent predictors for pCR to NAC like the well-studied other clinicopathological factors such as T stage, ER status, HER-2 status, and Ki-67 index in breast cancer. Additionally, PIV outperformed other blood-derived inflammation markers in predicting pCR, and it also had a prognostic value for DFS and OS.
In general, white blood cell count reflects an individual's systemic and/or local inflammatory status11. Neutrophils are known to regulate the tumor microenvironment and produce cytokines, chemokines, and growth factors that may promote angiogenesis as well as tumor cell proliferation and migration19. The M2 phenotype tumor-associated macrophages (TAMs) deriving from circulating monocytes exist within the tumor microenvironment and promote metastasis and immunosuppression20,21. It was reported that peripheral monocyte count was associated with the density of the TAMs, and high absolute monocyte count predicted poor survival in cancer patients22,23. Platelets are other cells contributing to cancer-favored inflammation by various mechanisms. For example, the activated platelets inhibit the interaction between tumor cells and cytolytic immune cells by forming a layer around tumor cells and support tumor growth via the secretion of several factors such as TGF-β24,25. Hence, high platelet counts were reported to be associated with adverse outcomes in breast cancer26. In contrast, lymphocytes are responsible for antitumor-specific immune response—including T-lymphocyte tumor infiltration and cytotoxic T-lymphocyte-mediated antitumor activity11,27. Starting from these findings, NLR, MLR, and PLR, indexes reflecting the balance between inflammation and immunoreaction in cancer, were reported to have predictive value in NAC response in many breast cancer studies, supporting our findings of univariate analysis15,16,17,28.
Although the scientific evidence supporting the predictive value of the blood-derived inflammation indexes has been expanding, there are conflicting reports about which index provides the best prediction for the efficacy of NAC in breast cancer29,30,31,32,33. For example, Eren et al. reported that NLR was the only independent predictive factor of pCR among blood-derived inflammation markers in multivariate analysis29. In another study conducted by Peng et al., multivariate analysis of 808 breast cancer patients showed that the lymphocyte-monocyte ratio was the only independent predictive factor for the efficacy of NAC among these inflammatory markers32. In addition, Hu et al. stated that PLR had superior efficacy to NLR in predicting NAC response33. When these studies are evaluated together with scholars presenting negative results34, it has emerged offering a combination of these markers35,36. The combination of NLR and PLR was reported to predict NAC response more precisely than NLR and PLR alone, claiming that the combination of different biomarkers could better define the patients' inflammatory status35,36.
PIV is a new blood-based biomarker integrating different peripheral blood immune cell subpopulations-neutrophil, platelet, monocyte, and lymphocyte. Due to its potential to represent comprehensively patient's immunity and systemic inflammation, PIV was proposed as a stronger predictor of outcomes in advanced cancer patients receiving cytotoxic chemotherapy, immunotherapy, and targeted therapy18,37,38,39. Recently, Ligorio reported that PIV was firmly associated with survival and outperformed NLR, PLR, and MLR in predicting survival in patients with HER-2 positive advanced breast cancer39. In line with the studies mentioned above, we showed that patients with low PIV scores had better survival outcomes. Furthermore, to our knowledge, this is the first study reporting that PIV was a more reliable predictor of pCR after NAC than other blood-based markers in breast cancer patients.
Meta-analyses and systematic reviews have shown that numerous factors—including age, genetic polymorphisms, tumor-infiltrating lymphocytes, programmed death-ligand 1, ER, progesterone receptor, and HER2 expression status—may be predictive of response to NAC in women with breast cancer7,40,41,42. However, most of these variables become available only following detailed pathological and genetic investigations. There is, therefore, an urgent need for reliable prognostic tools grounded on simple pre-treatment variables. In this scenario, PIV—an easy-to-drive biomarker originating from routine complete blood count—may help clinicians to predict treatment responses after prospective validation and confirmation of our results by further studies.
Some limitations of our study merit comment, including the retrospective design, the presentation of single-center experience, and the inclusion of women of Turkish descent only. Furthermore, although we excluded the patients with hematological disorders and those receiving immunomodulatory treatment, various other conditions may influence the blood-based biomarkers.
Based on our results, pre-treatment PIV seems to have a significant predictive value and outperform NLR, MLR, PLR in predicting pCR in Turkish women with breast cancer who received NAC. In addition, PIV has a prognostic impact on survival. However, further studies are needed to confirm our findings.
Materials and methods
Study population
Figure 2 shows the profile of our study. The electronic records of patients admitted to the Department of Oncology or the Department of General Surgery, Uludag University Medical Center (Bursa, Turkey) between January 2008 and December 2019 due to breast cancer were reviewed. Among patients who underwent NAC before attempting cytoreductive surgery, patients who were aged < 18 years, received immunomodulatory treatment or neoadjuvant endocrine therapy, had a history of malignancies at other sites, hematological disease, incomplete data were excluded. The study consisted of 743 Turkish women.
Data collection
The following variables were extracted from medical records in all participants: age; menopausal status; pre-treatment T stage; pre-treatment axillary lymph node status; pre-treatment histotype; pre-treatment expression of ER, HER-2, and Ki-67; and chemotherapy regimens. By immunohistochemical staining, ER expression levels ≥ 1% were considered positive. The final pathological reports of all patients were reviewed for pCR. According to the American Joint Committee on Cancer (AJCC) Staging Manual, eighth edition, the staging of the patients was carried out. A pre-treatment complete blood count was obtained in the two weeks preceding NAC. The absolute counts of neutrophils, monocytes, platelets, and lymphocytes were used to estimate NLR, MLR, and PLR. The PIV was calculated by multiplying neutrophil count (103/mL) by platelet count (103/mL) and monocyte count (103/mL) and dividing the result by lymphocyte count (103/mL).
Ethical statement
The study protocol complied with the tenets of the Helsinki declaration, and ethical approval was granted by the Institutional Review Board of Bursa Uludag University (Approval Number: 2020-6/31). The Clinical Research Ethics Committee of the Bursa Uludag University Faculty of Medicine waived the need for informed consent due to the study's retrospective nature.
Outcomes
The primary outcome measure was pCR to NAC. The pCR was defined as the absence of tumor cells in both the mastectomy specimen and the sampled or dissected regional lymph nodes. Secondary outcome measures included DFS and OS. DFS was calculated as the time (in months) from curative surgery until recurrence or death, whichever occurred first. OS was calculated as the time (in months) from breast cancer diagnosis to death.
Statistical analysis
The optimal cut-off points for NLR, MLR, PLR, PIV, and Ki-67 index were determined using ROC curve analysis, taking pCR as the endpoint of interest. The general characteristics of the study patients are presented using descriptive statistics (median, ranges, counts, and percentages). The Pearson's Chi-squared test (categorical variables) or the Mann–Whitney U test (continuous variables) were used to analyze the association between pCR and the variables. Binary logistic regression analysis was employed for multivariate analysis, including the factors having a p-value below 0.25 in univariate analysis. Survival curves were plotted using the Kaplan–Meier method and compared with the log-rank test. All calculations were performed using SPSS, version 22.0 (IBM, Armonk, NY, USA) and MedCalc Statistical Software trial version 20.009 (MedCalc Software bv, Ostend, Belgium; www.medcalc.org; 2021). Two-tailed p values < 0.05 were considered statistically significant.
References
Winters, S., Martin, C., Murphy, D. & Shokar, N. K. Breast cancer epidemiology, prevention, and screening. Prog. Mol. Biol. Transl. Sci. 151, 1–32 (2017).
Rojas, K. & Stuckey, A. Breast cancer epidemiology and risk factors. Clin. Obstet. Gynecol. 59, 651–672 (2016).
Doval, D. C., Dutta, K., Batra, U. & Talwar, V. Neoadjuvant chemotherapy in breast cancer: Review of literature. J. Indian Med. Assoc. 111, 629–631 (2013).
Mathew, J. et al. Neoadjuvant chemotherapy for locally advanced breast cancer: A review of the literature and future directions. Eur. J. Surg. Oncol. 35, 113–122 (2009).
National Comprehensive Cancer Network. Breast Cancer. Version 5.2021. Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
Spring, L. M. et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: A comprehensive meta-analysis. Clin. Cancer Res. 26, 2838–2848 (2020).
Parekh, T., Dodwell, D., Sharma, N. & Shaaban, A. M. Radiological and pathological predictors of response to neoadjuvant chemotherapy in breast cancer: A brief literature review. Pathobiology 82, 124–132 (2015).
Guthrie, G. J. K. et al. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol. Hematol. 88, 218–230 (2013).
Olingy, C. E., Dinh, H. Q. & Hedrick, C. C. Monocyte heterogeneity and functions in cancer. J. Leukoc. Biol. 106, 309–322 (2019).
Huong, P. T., Nguyen, L. T., Nguyen, X. B., Lee, S. K. & Bach, D. H. The role of platelets in the tumor-microenvironment and the drug resistance of cancer cells. Cancers (Basel) 11, 240 (2019).
Dupré, A. & Malik, H. Z. Inflammation and cancer: What a surgical oncologist should know. Eur. J. Surg. Oncol. 44, 566–570 (2018).
Templeton, A. J. et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 106, dju124 (2014).
Gu, L. et al. Prognostic role of lymphocyte to monocyte ratio for patients with cancer: Evidence from a systematic review and meta-analysis. Oncotarget 7, 31926–31942 (2016).
Templeton, A. J. et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: A systematic review and meta-analysis. Cancer Epidemiol. Biomark. Prev. 23, 1204–1212 (2014).
Ni, X. J. et al. An elevated peripheral blood lymphocyte-to-monocyte ratio predicts favorable response and prognosis in locally advanced breast cancer following neoadjuvant chemotherapy. PLoS ONE 9, e111886 (2014).
Asano, Y. et al. Predictive value of neutrophil/lymphocyte ratio for efficacy of preoperative chemotherapy in triple-negative breast cancer. Ann. Surg. Oncol. 23, 1104–1110 (2016).
Cuello-López, J., Fidalgo-Zapata, A., López-Agudelo, L. & Vásquez-Trespalacios, E. Platelet-To-lymphocyte ratio as a predictive factor of complete pathologic response to neoadjuvant chemotherapy in breast cancer. PLoS ONE 13, e0207224 (2018).
Fucà, G. et al. The Pan-Immune-Inflammation Value is a new prognostic biomarker in metastatic colorectal cancer: Results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br. J. Cancer 123, 403–409 (2020).
Wu, L., Saxena, S., Awaji, M. & Singh, R. K. Tumor-associated neutrophils in cancer: Going pro. Cancers (Basel) 11, 564 (2019).
De Palma, M. & Lewis, C. E. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell 23, 277–286 (2013).
Marech, I. et al. Tumour-associated macrophages correlate with microvascular bed extension in colorectal cancer patients. J. Cell. Mol. Med. 20, 1373–1380 (2016).
Shibutani, M. et al. The peripheral monocyte count is associated with the density of tumor-associated macrophages in the tumor microenvironment of colorectal cancer: A retrospective study. BMC Cancer 17, 1–7 (2017).
Shigeta, K. et al. High absolute monocyte count predicts poor clinical outcome in patients with castration-resistant prostate cancer treated with docetaxel chemotherapy. Ann. Surg. Oncol. 23, 4115–4122 (2016).
Amo, L. et al. Involvement of platelet-tumor cell interaction in immune evasion. Potential role of podocalyxin-like protein 1. Front. Oncol. 4, 245 (2014).
Schmied, L., Höglund, P. & Meinke, S. Platelet-mediated protection of cancer cells from ımmune surveillance: Possible ımplications for cancer ımmunotherapy. Front. Immunol. 12, 640578 (2021).
Taucher, S. et al. Impact of pre-treatment thrombocytosis on survival in primary breast cancer. Thromb. Haemost. 89, 1098–1106 (2003).
Chen, S. C., Wu, P. C., Wang, C. Y. & Kuo, P. L. Evaluation of cytotoxic T lymphocyte-mediated anticancer response against tumor interstitium-simulating physical barriers. Sci. Rep. 10, 1–13 (2020).
Eryilmaz, M. K. et al. The neutrophil to lymphocyte ratio has a high negative predictive value for pathologic complete response in locally advanced breast cancer patients receiving neoadjuvant chemotherapy. Asian Pac. J. Cancer Prev. 15, 7737–7740 (2014).
Eren, T. et al. Correlation between peripheral blood inflammatory indicators and pathologic complete response to neoadjuvant chemotherapy in locally advanced breast cancer patients. Medicine (Baltimore) 99, e20346 (2020).
Caziuc, A., Schlanger, D., Amarinei, G. & Dindelegan, G. C. Neutrophils-to-lymphocytes, lymphocytes to-monocytes and platelets-to-lymphocytes ratios: Predictive biomarkers for response to neoadjuvant chemotherapy in breast cancer. J. BU ON 25, 182–187 (2020).
Ma, Y., Zhang, J. & Chen, X. Lymphocyte-to-monocyte ratio is associated with the poor prognosis of breast cancer patients receiving neoadjuvant chemotherapy. Cancer Manag. Res. 13, 1571–1580 (2021).
Peng, Y. et al. Low pre-treatment lymphocyte/monocyte ratio is associated with the better efficacy of neoadjuvant chemotherapy in breast cancer patients. Cancer Biol. Ther. 21, 189–196 (2020).
Hu, Y. et al. Platelet/lymphocyte ratio is superior to neutrophil/lymphocyte ratio as a predictor of chemotherapy response and disease-free survival in luminal B-like (HER2−) breast cancer. Clin. Breast Cancer 20, e403–e409 (2020).
Suppan, C. et al. Neutrophil/lymphocyte ratio has no predictive or prognostic value in breast cancer patients undergoing preoperative systemic therapy. BMC Cancer 15, 1–8 (2015).
Graziano, V. et al. Combination of peripheral neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio is predictive of pathological complete response after neoadjuvant chemotherapy in breast cancer patients. Breast 44, 33–38 (2019).
Kim, H. Y., Kim, T. H., Yoon, H. K. & Lee, A. The role of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in predicting neoadjuvant chemotherapy response in breast cancer. J. Breast Cancer 22, 425–438 (2019).
Guven, D. C. et al. PILE: A candidate prognostic score in cancer patients treated with immunotherapy. Clin. Transl. Oncol. https://doi.org/10.1007/s12094-021-02560-6 (2021).
Fucà, G. et al. The pan-immune-inflammation value in patients with metastatic melanoma receiving first-line therapy. Target. Oncol. https://doi.org/10.1007/s11523-021-00819-0 (2021).
Ligorio, F. et al. The pan-immune-inflammation-value predicts the survival of patients with human epidermal growth factor receptor 2 (Her2): Positive advanced breast cancer treated with first-line taxane-trastuzumab-pertuzumab. Cancers (Basel) 13, 1964 (2021).
Du, Q. et al. PD-L1 acts as a promising immune marker to predict the response to neoadjuvant chemotherapy in breast cancer patients. Clin. Breast Cancer 20, e99–e111 (2020).
Gao, Z., Li, C., Liu, M. & Jiang, J. Predictive and prognostic role of tumour-infiltrating lymphocytes in breast cancer patients with different molecular subtypes: A meta-analysis. BMC Cancer 20, 1–14 (2020).
Boland, M. R. et al. Value of a 21-gene expression assay on core biopsy to predict neoadjuvant chemotherapy response in breast cancer: systematic review and meta-analysis. Br. J. Surg. 108, 24–31 (2021).
Author information
Authors and Affiliations
Contributions
A.B.S. conceived the study design, A.B.S, G.Y., R.G., B.O, K.S., Z.B.Y., S.O.O., and U.H. collected the data, A.B.S. performed statistical analyses. A.B.S. and E.C. wrote the manuscript. T.E., A.D., S.T., M.S.G., and S.C. edited the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Şahin, A.B., Cubukcu, E., Ocak, B. et al. Low pan-immune-inflammation-value predicts better chemotherapy response and survival in breast cancer patients treated with neoadjuvant chemotherapy. Sci Rep 11, 14662 (2021). https://doi.org/10.1038/s41598-021-94184-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-94184-7
This article is cited by
-
A Novel Prognostic Model Using Pan-Immune-Inflammation Value and Programmed Death Ligand 1 in Patients with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma Receiving Immune Checkpoint Inhibitors: A Retrospective Multicenter Analysis
Targeted Oncology (2024)
-
Evaluation of multiple biological indicators for the combined diagnosis of metastases from colorectal cancer—a retrospective study based on 1163 patients
World Journal of Surgical Oncology (2023)
-
Pan-immune inflammation value; a novel biomarker reflecting inflammation associated with frailty
Aging Clinical and Experimental Research (2023)
-
Tumor immune microenvironment components and the other markers can predict the efficacy of neoadjuvant chemotherapy for breast cancer
Clinical and Translational Oncology (2023)
-
High pre-chemoradiotherapy pan-immune-inflammation value levels predict worse outcomes in patients with stage IIIB/C non-small-cell lung cancer
Discover Oncology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.