Abstract
Background
Immune-inflammatory biomarkers (IIBs) showed a prognostic relevance in patients with metastatic CRC (mCRC). We aimed at evaluating the prognostic power of a new comprehensive biomarker, the Pan-Immune-Inflammation Value (PIV), in patients with mCRC receiving first-line therapy.
Methods
In the present pooled-analysis, we included patients enrolled in the Valentino and TRIBE trials. PIV was calculated as: (neutrophil count × platelet count × monocyte count)/lymphocyte count. A cut-off was determined using the maximally selected rank statistics method. Generalised boosted regression (GBR), the Kaplan–Meier method and Cox hazards regression models were used for survival analyses.
Results
A total of 438 patients were included. Overall, 208 patients (47%) had a low-baseline PIV and 230 (53%) had a high-baseline PIV. Patients with high PIV experienced a worse PFS (HR, 1.66; 95% CI, 1.36–2.03, P < 0.001) and worse OS (HR, 2.01; 95% CI, 1.57–2.57; P < 0.001) compared to patients with low PIV. PIV outperformed the other IIBs in the GBR model and in the multivariable models.
Conclusion
PIV is a strong predictor of survival outcomes with better performance than other well-known IIBs in patients with mCRC treated with first-line therapy. PIV should be prospectively validated to better stratify mCRC patients undergoing first-line therapy.
Similar content being viewed by others
Background
Even in the era of molecular selection,1 a non-negligible fraction of patients with metastatic colorectal cancer (mCRC) receiving first-line treatment has poor outcomes.2 Thus, the identification of new biomarkers for a better prognostic stratification and prediction of treatment outcomes is mandatory. Most of the biomarkers investigated so far are tumour-related, with less focus on host-related factors. Inflammation and immunity play a fundamental role in colorectal cancer initiation and progression,3,4 and several blood-based, easy-to-obtain, immune-inflammatory biomarkers (IIBs) have been investigated in cancer patients.5 Among others, neutrophil-to-lymphocyte ratio (NLR), platelets and monocytes showed a prognostic relevance in the advanced setting,6,7,8 but the clinical usefulness of such single biomarkers is limited by their low discriminative ability. Since the interplay between immunity, inflammation and cancer relies on complex networks, the use of a composite biomarker encompassing different immune-inflammatory populations and reflecting the global inflammation status could achieve a more robust prognostic power. Of note, the systemic immune-inflammation index (SII) based on lymphocyte, neutrophil and platelet counts, but not monocytes, was first developed for prognostic stratification in patients with hepatocellular carcinoma9 and demonstrated a certain relevance also in mCRC.10
In patients with mCRC, the use of such IIBs should be assessed in large datasets of patients enrolled in modern trials. In the present pooled-analysis of patients with mCRC receiving first-line therapy in the frame of two randomised academic trials, Valentino and TRIBE, we aimed to evaluate the prognostic power of a new biomarker, the Pan-Immune-Inflammation Value (PIV), including all the immune-inflammatory populations from peripheral blood with a proved prognostic relevance in mCRC.
Methods
Patients population
The Valentino study (NCT02476045) was a multicentre, randomised, open-label Phase 2 trial that enrolled 229 patients and showed that, in patients with RAS wild-type mCRC, panitumumab plus FOLFOX-4 induction followed by maintenance therapy with single-agent panitumumab (arm B) achieved inferior PFS compared to the same induction regimen followed by panitumumab plus 5-FU/LV (arm A).11 The TRIBE study (NCT00719797) was a multicentre, randomised, open-label Phase 3 trial by Gruppo Oncologico del Nord Ovest (GONO) that enrolled 508 patients and showed that, in patients with molecularly unselected mCRC, first-line FOLFOXIRI plus bevacizumab achieved superior PFS and OS compared with FOLFIRI plus bevacizumab.12
For the present study, we selected patients enrolled in the two trials with available RAS and BRAF mutational status, complete baseline blood-cell count at cycle 1, day 1 (prior to first treatment cycle administration) and clinicopathological data including but not limited to prior exposure to adjuvant chemotherapy, primary tumour resection and primary tumour sidedness.
Statistical analyses
In order to represent a weight of the interaction between inflammatory pro-tumour populations (i.e. neutrophils, platelets and monocytes) and anti-cancer immune populations (i.e. lymphocytes), PIV was calculated as: [neutrophil count (103/mmc) × platelet count (103/mmc) × monocyte count (103/mmc)]/lymphocyte count (103/mmc). Maximally selected rank statistics method for PFS was used to find an optimal cut-off value13 to stratify patients in low PIV vs high PIV. NLR was calculated as: neutrophil count (103/mmc)/lymphocyte count (103/mmc). NLR was defined high if >3, platelet count was defined high if >310 × 103/mmc and monocyte count was defined high if >0.5 × 103/mmc based on literature data.6,7,8 SII was calculated as [neutrophil count (103/mmc) × platelet count(103/mmc)/lymphocyte count (103/mmc) and defined high if >730 based on literature data.10
Fisher exact test, Chi-square test, Mann–Whitney U test or Kruskal-Wallis test, as appropriate, were used to analyse the association between baseline PIV and the other clinicopathological characteristics. PFS was defined as the time from randomisation to disease progression or death from any cause. OS was defined as the time from randomisation to death from any cause. Generalised boosted regression was used to screen the association of PIV and the other IIBs with PFS and OS.14,15 Further survival analyses were performed using the Kaplan–Meier method and the Cox proportional hazards regression models. All the variables showing a P below the significance threshold in the univariate models were included in a multivariable model. The variables showing a P below the significance threshold in the multivariable models were considered to be independent prognostic factors. All tests were 2-sided with a significance threshold of 0.05. Statistical analyses were performed using the R (version 3.5.0) and R Studio (version 1.1.447).
Results
Patients characteristics according to Pan-Immune-Inflammation Value
A total of 438 patients were included in the present analysis: 207 from the Valentino study and 231 from the TRIBE study. The process of patients’ selection is illustrated in Supplementary Fig. S1. In terms of patients’ characteristics, the subsets of patients included in the present study was representative of the overall trial populations (Supplementary Table S1). Median PIV in the entire study population was 417 (IQR, 239–780). The distribution of median PIV according to patients’ and disease characteristics is shown in Supplementary Table S2.
The optimal cut-off value for PIV using a maximally selected rank statistics method for PFS was 390 (Supplementary Fig. S2). Overall, 208 patients (47%) had a low PIV and 230 (53%) had a high PIV. The distribution of high vs low PIV patients in the two studies was well balanced (Table 1). Compared to patients with low PIV, a higher proportion of patients with high PIV had ECOG PS1 (P < 0.001), no primary tumour resection (P < 0.001), presence of synchronous metastases (P = 0.003) and more than 1 site of metastases (P = 0.032) (Table 1). The association between PIV and the classical immune-inflammatory biomarkers is shown in Supplementary Table S3.
Prognostic analyses according to Pan-Immune-Inflammation Value
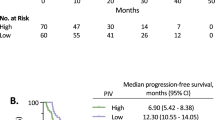
Median follow-up was 38.4 months (IQR, 27.4–50.9). A total of 389 PFS events were recorded with a pooled median PFS of 11.1 months (95% CI, 10.3–11.9). Median PFS was 9.5 months (95% CI, 8.8–10.7) for patients with high PIV and 12.9 months (95% CI, 11.7–14.6) for those with low PIV (HR high vs low, 1.66; 95% CI, 1.36–2.03, P < 0.001) (Fig. 1, panel a). Similar results were observed in the two separate populations in the Valentino and TRIBE studies (Supplementary Fig. S3, panels a and b, respectively). At univariate analysis, also NLR, platelet count, monocyte count and SII were significantly associated with PFS (Table 2). In the generalised boosted regression model, PIV showed the higher relative influence on PFS among the IIBs (Fig. 2, panel a). In the multivariable model including all the variables significantly associated with PFS, PIV was the only IIB that showed an independent prognostic impact on PFS (adjusted HR high vs low, 1.53; 95% CI, 1.09–2.15; P = 0.015) (Table 2).
A total of 269 OS events were reported during the study period with a pooled median OS of 28.5 months (95% CI, 25.6–31.61). Median OS was 21.6 months (95% CI, 19.9–24.7) for patients with high PIV and 34.4 months (95% CI, 32.1–42.7) for patients with low PIV (HR high vs low, 2.01; 95% CI, 1.57–2.57; P < 0.001) (Fig. 1, panel b). Results were consistent when the two populations were analysed separately (Supplementary Fig. S4). NLR, platelet count, monocyte count and SII were also significantly associated with OS (Table 3). In the generalised boosted regression model, PIV showed the higher relative influence on OS among the immune-inflammatory biomarkers (Fig. 2, panel b). As for PFS, PIV was the only inflammation-based biomarker that showed an independent prognostic impact on OS (adjusted HR high vs low, 1.55; 95% CI, 1.02–2.37, P = 0.042) (Table 3).
Predictive analyses according to Pan-Immune-Inflammation Value
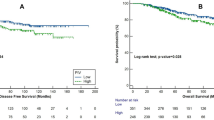
In the Valentino study, PIV was not significantly associated with a differential effect of the two maintenance arms in terms of PFS (interaction P = 0.449) and OS (interaction P = 0.612) (Fig. 3, panels a and b, respectively).
Kaplan–Meier curve for PFS and OS according to PIV and treatment arm in the Valentino study (a and b, respectively) and in the TRIBE study (c and d, respectively). PIV was not significantly associated with a differential effect of the two maintenance arms in the Valentino study nor with a differential effect of triplet-based vs doublet-based therapy in the TRIBE study.
Similar results were observed about the predictive role of PIV in the TRIBE study for patients treated with triplet-based vs doublet-based therapy (interaction P for PFS = 0.924; interaction P for OS = 0.951) (Fig. 3, panels C and D). Supplementary Table S4 summarises the results of the predictive analyses.
Discussion
In the present pooled-analysis of two first-line trials, we investigated PIV as a new inflammation-based biomarker that integrates NLR, platelet count and monocyte count. PIV demonstrated an extensive and powerful prognostic impact on both PFS and OS in patients with mCRC receiving first-line chemotherapy plus a biological agent. We observed that patients with high baseline PIV had significantly worse survival outcomes compared to patients with low baseline PIV. PIV had the highest relative influence on survival outcomes in the generalised boosted regression models including the other canonical IIBs (i.e. NLR, platelet count, monocyte count) and was the only one that retained an independent prognostic role for PFS and OS in the multivariable models.
To avoid a fragmentated information about systemic inflammation, both nomogram systems and scores have been also developed to integrate the various components in the prognostic modelling of CRC,7,16 but there is no consensus about the best approach. Rather than analysing the individual contribution of each cellular components (i.e. lymphocytes, neutrophils, platelets and monocytes) on clinical outcomes and then building a calculator (i.e. nomogram or score) in a statistical-driven approach, we tested the prognostic relevance of a new biomarker incorporating lymphocytes, neutrophils, platelets and monocytes in a way that allowed us to “globally quantify” the cellular compartment of systemic inflammation (i.e. biological-driven approach).
Of note, PIV also outperformed another multicomponent inflammatory index, the SII that does not include information about monocyte count.
Among circulating white blood cells, monocytes are one of the most important subpopulations with an emerging role in cancer progression17 and potential prognostic impact also in patients with mCRC.7 Indeed, circulating macrophages represent the primary source of tumour-associated macrophages (TAMs) and the peripheral monocyte count was associated with the density of TAMs in colorectal cancer.18 Peripheral monocytes are also the source of monocytic (M-) myeloid-derived suppressor cells (MDSCs) that, together with polymorphonuclear (PMN-) MDSCs, characterise a population of immune cells driving immunosuppression and progression in mCRC.19,20 Of note, PMN-MDSCs are a particular phenotype of circulating neutrophils,21 so using a biomarker like PIV including monocytes and neutrophils rather than neutrophils only might easily summarise the immunosuppressive contribution of the two components of MDSCs without the need of complex cytofluorimetric analyses.
Even if with some limitations consisting in its retrospective nature and lack of prospective validation, our study included patients enrolled in two randomised clinical trials guaranteeing a high quality of data, especially in weighting the prognostic contribution of monocyte count, a parameter usually not included in the case report forms of clinical studies,22 particularly academic ones.
In conclusion, our study identifies PIV as a new IIB strongly associated with overall prognosis of mCRC patients receiving first-line treatment and outperforms the other well-known IIBs, suggesting its possible role as a stratification factor in future first-line clinical trials. Further studies should assess the role of PIV as predictive biomarker, particularly regarding its early modifications during treatment as a potentially dynamic biomarker associated with treatment outcomes, and in different settings (for instance, patients with pre-treated mCRC or early stage) or histologies, and with specific regard to immunotherapy approaches.
References
National Comprehensive Cancer Network. Colon Cancer (Version 4.2019). http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. (2019).
Morano, F., Corallo, S., Lonardi, S., Raimondi, A., Cremolini, C., Rimassa, L. et al. Negative hyperselection of patients with RAS and BRAF wild-type metastatic colorectal cancer who received panitumumab-based maintenance therapy. J. Clin. Oncol. 37, 3099–3110 (2019).
Terzić, J., Grivennikov, S., Karin, E. & Karin, M. Inflammation and colon cancer. Gastroenterology 138, 2101–2114.e5 (2010).
Markman, J. L. & Shiao, S. L. Impact of the immune system and immunotherapy in colorectal cancer. J. Gastrointest. Oncol. 6, 208–223 (2015).
Dolan, R. D., McSorley, S. T., Horgan, P. G., Laird, B. & McMillan, D. C. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 116, 134–146 (2017).
Dell’Aquila, E., Cremolini, C., Zeppola, T., Lonardi, S., Bergamo, F., Masi, G. et al. Prognostic and predictive role of neutrophil/lymphocytes ratio in metastatic colorectal cancer: a retrospective analysis of the TRIBE study by GONO. Ann. Oncol. 29, 924–930 (2018).
Sjoquist, K. M., Renfro, L. A., Simes, R. J., Tebbutt, N. C., Clarke, S., Seymour, M. T. et al. Personalizing survival predictions in advanced colorectal cancer: the ARCAD nomogram project. J. Natl Cancer Inst. 110, 638–648 (2018).
Wen, S., Chen, N., Peng, J., Ling, W., Fang, Q., Yin, S. F. et al. Peripheral monocyte counts predict the clinical outcome for patients with colorectal cancer: a systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 31, 1313–1321 (2019).
Hu, B., Yang, X. R., Xu, Y., Sun, Y. F., Sun, C., Guo, W. et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 20, 6212–6222 (2014).
Passardi, A., Scarpi, E., Cavanna, L., Dall’Agata, M., Tassinari, D., Leo, S. et al. Inflammatory indexes as predictors of prognosis and bevacizumab efficacy in patients with metastatic colorectal cancer. Oncotarget 7, 33210–33219 (2016).
Pietrantonio, F., Morano, F., Corallo, S., Miceli, R., Lonardi, S., Raimondi, A. et al. Maintenance therapy with panitumumab alone vs panitumumab plus fluorouracil–leucovorin in patients with RAS wild-type metastatic colorectal cancer: a phase 2 randomized clinical trial. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2019.1467 (2019).
Cremolini, C., Loupakis, F., Antoniotti, C., Lupi, C., Sensi, E., Lonardi, S. et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 16, 1306–1315 (2015).
Lausen, B. & Schumacher, M. Maximally selected rank statistics. Biometrics 48, 73–85 (1992).
Friedman, J. H. Greedy function approximation: a gradient boosting machine. Ann. Stat. 29, 1189–1232 (2001).
Friedman, J. H. Stochastic gradient boosting. Comput. Stat. Data Anal. 38, 367–378 (2002).
Dolan, R. D., McSorley, S. T., Park, J. H., Watt, D. G., Roxburgh, C. S., Horgan, P. G. et al. The prognostic value of systemic inflammation in patients undergoing surgery for colon cancer: comparison of composite ratios and cumulative scores. Br. J. Cancer 119, 40–51 (2018).
Kitamura, T., Qian, B. Z. & Pollard, J. W. Immune cell promotion of metastasis. Nat. Rev. Immunol. 15, 73–86 (2015).
Shibutani, M., Maeda, K., Nagahara, H., Fukuoka, T., Nakao, S., Matsutani, S. et al. The peripheral monocyte count is associated with the density of tumour-associated macrophages in the tumour microenvironment of colorectal cancer: a retrospective study. BMC Cancer 17, 404 (2017).
Karakasheva, T. A., Dominguez, G. A., Hashimoto, A., Lin, E. W., Chiu, C., Sasser, K. et al. CD38+ M-MDSC expansion characterizes a subset of advanced colorectal cancer patients. JCI Insight 3, 97022 (2018).
Tada, K., Kitano, S., Shoji, H., Nishimura, T., Shimada, Y., Nagashima, K. et al. Pretreatment immune status correlates with progression-free survival in chemotherapy-treated metastatic colorectal cancer patients. Cancer Immunol. Res. 4, 592–599 (2016).
Shaul, M. E. & Fridlender, Z. G. Tumour-associated neutrophils in patients with cancer. Nat. Rev. Clin. Oncol. 16, 601–620 (2019).
Dolan, R. D., Laird, B. J. A., Horgan, P. G. & McMillan, D. C. The prognostic value of the systemic inflammatory response in randomised clinical trials in cancer: a systematic review. Crit. Rev. Oncol. Hematol. 132, 130–137 (2018).
Author information
Authors and Affiliations
Contributions
G.F.: conceptualisation, data curation, formal analysis, investigation, writing of the original draft, review/editing and approval of the final manuscript. V.G.: data curation, investigation, review/editing and approval of the final manuscript. C.A.: data curation, investigation, review editing and approval of the final manuscript. F.Mo.: investigation, review/editing and approval of the final manuscript. R.Mo.: investigation, review/editing and approval of the final manuscript. S.C.: data curation, investigation, review/editing and approval of the final manuscript. F.Ma.: data curation, investigation, review/editing and approval of the final manuscript. S.L.: investigation, review/editing and approval of the final manuscript. L.M.: investigation, review/editing and approval of the final manuscript. A.S.B.: investigation, review/editing and approval of the final manuscript. B.B.: investigation, review/editing and approval of the final manuscript. M.T.: investigation, review/editing and approval of the final manuscript. S.B.: investigation, review/editing and approval of the final manuscript. M.C.: investigation, review/editing and approval of the final manuscript. A.B.: investigation, review/editing and approval of the final manuscript. R.Mu.: investigation, review/editing and approval of the final manuscript. A.Z.: investigation, review/editing and approval of the final manuscript. G.Tom.: investigation, review/editing and approval of the final manuscript. F.L.: investigation, review/editing and approval of the final manuscript. V.A.: investigation, review/editing and approval of the final manuscript. G.Ton.: investigation, review/editing and approval of the final manuscript. E.C.: investigation, review editing and approval of the final manuscript. F.d.B.: investigation, review/editing and approval of the final manuscript. C.C.: conceptualisation, data curation, investigation, review/editing and approval of the final manuscript. F.P.: conceptualisation, data curation, formal analysis, investigation, supervision, writing of the original draft, review/editing and approval of the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Institutional review board and ethics committee approval was obtained from all participating centres. All the patients provided written informed consent before any study-related procedures. The study was conducted in accordance with the Declaration of Helsinki.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
F.M. reported honoraria from: Servier; travel grants from: Sanofi, Servier. S.L. reported consulting/advisory board fees from: Amgen, Merck–Serono, Lilly; lecture fees from: Roche, Lilly, Bristol-Myers Squibb, Servier, Merck–Serono; research Funding: Amgen, Merck–Serono. L.R. reported consulting/advisory board fees from: Amgen, Arqule, Basilea, Baxter, Bayer, Celgene, Eisai, Exelixis, Hengrui, Incyte, Ipsen, Italfarmaco, Eli Lilly, MSD, Roche, Sanofi, and Sirtex Medical; lecture fees from: AbbVie, AstraZeneca, and Gilead; travel grants from: Arqule and Ipsen. A.S.B. reported consulting/advisory board fees from: Amgen, Bayer, Sanofi; lecture fees from: Amgen, Bayer, Sanofi; travel grants from: Amgen, Bayer, Sanofi. M.C. reported lecture fees from: Sanofi, Aventis, Amgen; travel grants from: Roche, Genentech, Sanofi, Aventis. A.Z. reported consulting/advisory board fees from: Amgen, Servier, Bayer, Merck–Serono; lecture fees from: Servier. F.L. reported consulting/advisory board fees from: Amgen, Sanofi, Bayer; lecturer fees from: Roche, Sanofi, Bayer, Amgen; institutional research funding from: Roche, Merck–Serono, Amgen, Bayer; travel grants from: Roche, Amgen, Merck–Serono. F.d.B. reported receiving honoraria for speaker activities and participation in advisory boards from: Amgen, Inc, Roche, and Novartis International AG. C.C. reported receiving honoraria for speaker activities and participation in advisory boards from: Roche, Amgen, Inc, Bayer AG, and Servier Laboratories; research grants from: Merck–Serono. F.P. reported receiving honoraria for speaker activities and participation in advisory boards from: Sanofi SA, Amgen, Inc, Bayer AG, Merck–Serono, Roche, and Servier Laboratories. All remaining authors have declared no conflicts of interest.
Funding information
The present study was funded by institutional funds from the Fondazione IRCCS Istituto Nazionale dei Tumori di Milano.
Additional information
Note This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution 4.0 International (CC BY 4.0).
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fucà, G., Guarini, V., Antoniotti, C. et al. The Pan-Immune-Inflammation Value is a new prognostic biomarker in metastatic colorectal cancer: results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br J Cancer 123, 403–409 (2020). https://doi.org/10.1038/s41416-020-0894-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-020-0894-7
This article is cited by
-
The prognostic importance of the pan-immune-inflammation value in patients with septic shock
BMC Infectious Diseases (2024)
-
Relationship between pan-immune- inflammation value and in major cardiovascular and cerebrovascular events in stable coronary artery disease patients undergoing on-pump coronary artery bypass graft surgery
Journal of Cardiothoracic Surgery (2024)
-
Pan-immune-inflammation value and body mass index to predict survival in diffuse large B-cell lymphoma
The Egyptian Journal of Internal Medicine (2024)
-
A Novel Prognostic Model Using Pan-Immune-Inflammation Value and Programmed Death Ligand 1 in Patients with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma Receiving Immune Checkpoint Inhibitors: A Retrospective Multicenter Analysis
Targeted Oncology (2024)
-
Pan-immune-inflammation and its dynamics: predictors of survival and immune-related adverse events in patients with advanced NSCLC receiving immunotherapy
BMC Cancer (2023)