Brain Development: The dangers of rubella virus
Some pathogens can be extremely harmfull during pregnancy as they can cross the placenta, infect the fetus, and go on to cause congenital birth defects, miscarriages and stillbirths (Pereira, 2018). The rubella virus, for example, can cause a range of congenital brain defects, and it is also associated with a higher risk of babies developing congenital rubella syndrome, a complex condition associated with developmental delays, cardiac anomalies, hearing impairment and eye abnormalities (Bardeletti et al., 1975; Lazar et al., 2016; Toizumi et al., 2017). These viruses and other pathogens are collectively referred to as TORCH pathogens, which is short for toxoplasmosis (which is caused by a parasite), other pathogens (such as syphilis, varicella, mumps, parvovirus B19 and HIV), rubella, cytomegalovirus, and herpes simplex virus.
Despite the threat they pose to public health, the mechanisms by which TORCH pathogens affect brain development remain poorly understood. Now, in eLife, Tomasz Nowakowski, Joseph DeRisi and colleagues at the University of California San Francisco – including Galina Popova and Hanna Retallack as joint first authors – report new insights into the infection of brain cells by the rubella virus (Popova et al., 2023).
The researchers combined analyses of live human fetal brain slices that were maintained in the laboratory and two-dimensional cell cultures to study how the rubella virus affects brain cells. This revealed that the virus predominantly infects immune cells called microglia, which patrol and scavenge the central nervous system for damaged cells and pathogens. Microglia also have an important role in protecting the brain during development.
The experiments revealed that the rubella virus can only infect microglia when a variety of other brain cells are present. This is likely due to some yet-to-be-identified diffusible factors released by the other brain cells, which could render microglia susceptible to infection. However, the microglia do not need to make direct contact with these other cells in order to get infected.
Microglia play a crucial role in the antiviral immune response by releasing inflammatory cytokines, such as interferons (Sala and Kuka, 2020). Popova et al. found that infection with the rubella virus leads to an excessive interferon response by neighbouring neuronal cells, and this could have a deleterious effect on brain development. This is consistent with previous research, which showed that prenatal infection with rubella and HIV can trigger the overproduction of interferons, leading to prolonged inflammation that may contribute to the atypical development of the fetus (Crow et al., 2003). Certain inflammatory disorders, such as systemic lupus erythematosus and Aicardi-Goutières syndrome, are also characterized by an increased interferon response, and it is possible that some TORCH infections (in particular HIV and Rubella) share certain phenotypic similarities with these conditions.
As with many other TORCH pathogens, the fetus is most vulnerable to the rubella virus during the first trimester of pregnancy, due to the lack of immune defense in the developing fetus. Microglia populate the brain about a month into pregnancy, and the blood-brain barrier in the fetal brain only starts to be functional about two months into pregnancy (Menassa and Gomez-Nicola, 2018; Goasdoué et al., 2017). The brain is therefore extremely vulnerable to viruses during the first trimester of pregnancy, which coincides with a higher risk of developing severe developmental disorders.
The study of Popova et al. highlights the importance of using human cell-based models to better understand the pathophysiological mechanisms of congenital rubella syndrome. It remains to be seen why and how the rubella virus specifically attacks microglia, and what its molecular targets are. Identifying the molecular cues released by other brain cells, which potentially increase infection, will be necessary to eventually develop therapies against congenital rubella syndrome.
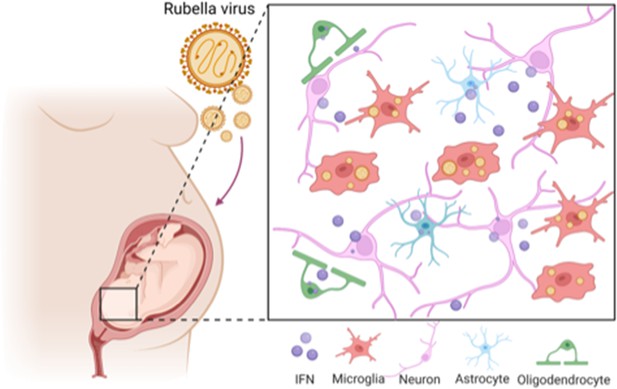
The effect of rubella virus on the brain development.
Rubella viruses (orange circles) target immune cells, called microglia (red), in the brain of the fetus. Popova et al. show that other neighbouring brain cells (pink, green and blue) must be present for infection to take place: it is thought that infection relies on diffusible factors released by these cells. Infection causes the release of large amounts of a signaling protein, called interferon (IFN; purple), which can damage the developing brain.
References
-
Morphology, biochemical analysis and neuraminidase activity of rubella virusArchives of Virology 49:175–186.https://doi.org/10.1007/BF01317536
-
Microglial dynamics during human brain developmentFrontiers in Immunology 9:1014.https://doi.org/10.3389/fimmu.2018.01014
-
Congenital viral infection: traversing the uterine-placental interfaceAnnual Review of Virology 5:273–299.https://doi.org/10.1146/annurev-virology-092917-043236
Article and author information
Author details
Publication history
- Version of Record published: June 16, 2023 (version 1)
Copyright
© 2023, Epifanova and Nguyen
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 997
- views
-
- 65
- downloads
-
- 1
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Neuroscience
The fragile X syndrome (FXS) represents the most prevalent form of inherited intellectual disability and is the first monogenic cause of autism spectrum disorder. FXS results from the absence of the RNA-binding protein FMRP (fragile X messenger ribonucleoprotein). Neuronal migration is an essential step of brain development allowing displacement of neurons from their germinal niches to their final integration site. The precise role of FMRP in neuronal migration remains largely unexplored. Using live imaging of postnatal rostral migratory stream (RMS) neurons in Fmr1-null mice, we observed that the absence of FMRP leads to delayed neuronal migration and altered trajectory, associated with defects of centrosomal movement. RNA-interference-induced knockdown of Fmr1 shows that these migratory defects are cell-autonomous. Notably, the primary Fmrp mRNA target implicated in these migratory defects is microtubule-associated protein 1B (MAP1B). Knocking down MAP1B expression effectively rescued most of the observed migratory defects. Finally, we elucidate the molecular mechanisms at play by demonstrating that the absence of FMRP induces defects in the cage of microtubules surrounding the nucleus of migrating neurons, which is rescued by MAP1B knockdown. Our findings reveal a novel neurodevelopmental role for FMRP in collaboration with MAP1B, jointly orchestrating neuronal migration by influencing the microtubular cytoskeleton.
-
- Biochemistry and Chemical Biology
- Neuroscience
In most mammals, conspecific chemosensory communication relies on semiochemical release within complex bodily secretions and subsequent stimulus detection by the vomeronasal organ (VNO). Urine, a rich source of ethologically relevant chemosignals, conveys detailed information about sex, social hierarchy, health, and reproductive state, which becomes accessible to a conspecific via vomeronasal sampling. So far, however, numerous aspects of social chemosignaling along the vomeronasal pathway remain unclear. Moreover, since virtually all research on vomeronasal physiology is based on secretions derived from inbred laboratory mice, it remains uncertain whether such stimuli provide a true representation of potentially more relevant cues found in the wild. Here, we combine a robust low-noise VNO activity assay with comparative molecular profiling of sex- and strain-specific mouse urine samples from two inbred laboratory strains as well as from wild mice. With comprehensive molecular portraits of these secretions, VNO activity analysis now enables us to (i) assess whether and, if so, how much sex/strain-selective ‘raw’ chemical information in urine is accessible via vomeronasal sampling; (ii) identify which chemicals exhibit sufficient discriminatory power to signal an animal’s sex, strain, or both; (iii) determine the extent to which wild mouse secretions are unique; and (iv) analyze whether vomeronasal response profiles differ between strains. We report both sex- and, in particular, strain-selective VNO representations of chemical information. Within the urinary ‘secretome’, both volatile compounds and proteins exhibit sufficient discriminative power to provide sex- and strain-specific molecular fingerprints. While total protein amount is substantially enriched in male urine, females secrete a larger variety at overall comparatively low concentrations. Surprisingly, the molecular spectrum of wild mouse urine does not dramatically exceed that of inbred strains. Finally, vomeronasal response profiles differ between C57BL/6 and BALB/c animals, with particularly disparate representations of female semiochemicals.






