INTRODUCTION
Diabetes mellitus is a chronic disease afflicting 425 million individuals worldwide, and it is expected to rise inevitably in the coming decades (IDF Diabetes Atlas 9th edition 2019). Despite significant advances in monitoring and treatment, diabetic complications continue to be a key source of morbidity and mortality (Halban et al., 2010). The defects that result in diabetes are diverse, but the failure of pancreatic β-cell is a large determinant of this disorder (Kahn, 2001). The progressive deterioration of glucose control in both type 1 diabetes (T1D) and type 2 diabetes (T2D) is mainly caused by the insufficient mass of β-cells and gradual dysfunction, which leads to diminished insulin secretion (Stumvoll et al., 2005). In T1D, immunological factors, as well as T-cells activated by immune-mediated responses damage insulin-producing β-cells (Wållberg and Cooke, 2013). T2D, on the other hand, is a degenerative condition caused by both genetic and environmental factors, resulting in insulin resistance in peripheral tissues, often culminating in β-cell death, which cannot compensate for insulin resistance (Benthuysen et al., 2016).
In T2D pancreatic β-cells are compromised or become non-functional as a result of persistently high levels of glucose and lipid, a phenomenon known as glucolipotoxicity (Weir, 2020). Endoplasmic reticulum stress, Reactive oxygen species (ROS) production, islet inflammation, mitochondrial dysfunction, increased synthesis of ceramides, and increased islet amyloid polypeptide (Fig. 1a–c) have all been proposed as mechanisms for glucolipotoxicity-induced β-cell dysfunction and death (Chang-Chen et al., 2008). Furthermore, chronic hyperglycemia can induce β-cell death by increasing the expression of proapoptotic genes, while antiapoptotic gene expression B-cell lymphoma 2 (Bcl-2) remains unaffected (Tomita, 2016). The mechanisms activated by glucolipotoxicity are strongly linked, resulting in a destructive cycle that inevitably leads to the failure of β-cells. The molecular basis of the parameters regulating β-cell mass and function, on the other hand, is a key problem for understanding diabetes, which is characterized by a near total (in T1D) or relative (in T2D) insufficiency in pancreatic β-cell number (Chen et al., 2017). Thus, the discovery of new therapeutics targeting the apoptotic process to delay or reverse β-cell death/dysfunction serves as an attractive strategy for the control of the disease (Venkatesan et al., 2011).
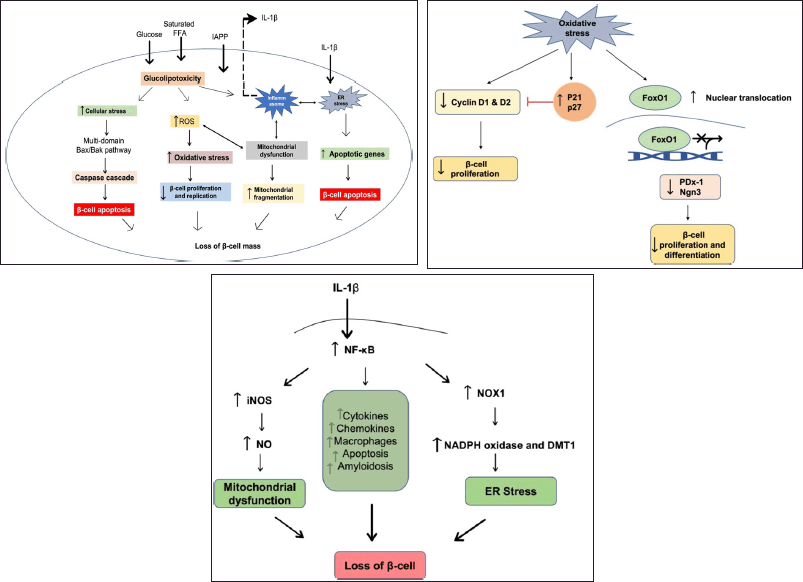 | Figure 1. (a) Glucolipotoxicity leading to decreased β-cell mass and dysfunction: β-cells become dysfunctional as a result of glucolipotoxicity several mechanisms for glucolipotoxicity-induced β-cell dysfunction have been proposed, including endoplasmic reticulum stress, mitochondrial dysfunction, ROS production, islet inflammation, and increased islet amyloid polypeptide. Chronically elevated glucose levels stimulate glucose metabolism via oxidative phosphorylation. As a result, dysfunctional mitochondria and there is an increased production of ROS. ER stress has also been linked to increased biosynthetic demand, elevated FFA levels, and chronic-cell overnutrition. (b) Role of oxidative stress in beta cell cycle: ROS inhibits cyclin D1 and D2 expression while increasing cell cycle inhibitors such as p21 and p27, resulting in decreased beta cell proliferation. Furthermore, ROS promotes nuclear translocation of FoxO1, which inhibits the expression of Pdx-1, Ngn3, and other beta-cell related gene transcription, all of which are required for beta cell proliferation and differentiation. (c) Inflammatory response causing β-cell dysfunction: inflammatory mediators increases ER stress by activating Nicotinamide adenine dinucleotide phosphate oxidase and DMT-1. Also, these mediators stimulate the transcription of various cytokines, chemokines, and other inflammatory markers, exacerbating the inflammatory response. The signaling molecules involved in mitochondrial dysfunction, ER stress, oxidative stress, and inflammation all interact extensively, leading to beta cell dysfunction. [Click here to view] |
There are numerous anti-diabetic medications available today to treat hyperglycemia. Except for insulin, liraglutide, exenatide, and pramlintide, all other anti-diabetic drugs are administered orally. These drugs work through various mechanisms, including enhanced insulin release, improved insulin sensitivity, increased glucose uptake, and decreased carbohydrate absorption. The major downside of existing drugs is that they must be administered throughout one’s life and cause undesirable side effects (Hossain and Pervin, 2018). Therefore, herbal medicines that can manage diabetes and associated complications more efficiently and safely have become increasingly popular among people and scientists working in the field both in developed and emerging nations (Kumar et al., 2021). Plants have proven to be an excellent source of drugs, with many currently available drugs derived explicitly or implicitly from them. The active principles derived from plants work through a wide range of pathways, including the inhibition of several key enzymes involved in glucose metabolism. The world health organization has identified close to 21,000 plants used for a variety of medicinal purposes around the world. Nearly 800 of them have been known to possess anti-diabetic potential (Rizvi and Mishra, 2013). In a review, Salehi et al. (2019a, 2019b) described several medicinal plants, including Aloe vera, Gymnema sylvestre, Ficus benghalenesis, Hibiscus rosa-sinesis, Mangifera indica, Tinospora cordifolia, Trigonella foenum-graecum, Allium sativum, Momordica charantia, Panax ginseng, Azadirachta indica Aegle marmelose with antihyperglycemic, insulin-mimetic, anti-lipidemic, and anti-diabetic properties, with a focus on compounds of high interest from various classes such as flavonoids, terpenoids, coumarins, carbohydrates, polypeptides, and small molecules (Salehi et al., 2019a). Some of these medicinal plants and plant-derived compounds are discussed further in this systematic review.
METHODS USED FOR LITERATURE COLLECTION
Online databases such as PubMed, Science Direct, Google Scholar, Web of Science, Scopus, Clinical Trials.gov, and Wiley online library were used for the literature search for this systematic review. The strategy of search is based on the PRISMA guidelines and the major keywords used in various combinations included: Diabetes mellitus, the anti-diabetic activity of plant extracts and plant-derived compounds, β-cell function, proliferation, and differentiation. Based on the search 625 articles were identified. The full-length articles from peer-reviewed journals related to the subject of interest, published between (2000 and 2021) and written in English were included in the review process. Studies on the beneficial effects of crude extracts/isolated compounds on glucose-lowering effects via restoration of islet cells using in vivo and/or in vitro studies were selected for this review. Commercial oral-hypoglycemic agents that lacked definitive evidence on pancreatic β-cell function and mass but obliquely suggested improvement of those activities were excluded from the review. Plants that have been reported for their beneficial effects on β-cells and have undergone clinical trials were included in this review. The review excluded studies that were unrelated to the research question, duplicated, had unavailable full texts or were abstract-only. Further, only plants with reported antidiabetic phytochemicals and mechanisms of action were considered for the review work. There were no restrictions on the sample size, study design, or method of exposure and outcome measurement. Of the 625 articles, 195 articles were included in this review following the criteria for inclusion. Figure 2 summarizes the strategy for the search.
IS REGENERATION POSSIBLE?
The endocrine pancreas is known to be tissue with slow turnover and reduced renewal capacity than tissues with well-defined adult stem cell niches, such as blood, skin, and gut (Aguayo-Mazzucato and Bonner-Weir, 2018) Despite this, the low frequency of both apoptosis and proliferation allows for sustained expansion of β-cell mass in rats for the initial 7 months (Montanya et al., 2000). For about a decade, the notion of regeneration of islets by implies apart from replication of pre-existing cells was unpopular, but neogenesis/trans differentiation has regained favor. Proliferation and the formation of newer β- cells are both likely islet regeneration mechanisms, with different emphases depending on the injury and species (Aguayo-Mazzucato and Bonner-Weir, 2018). Most of the studies on the regeneration of β-cells have been carried out using various rodent models (Kodama et al., 2003), and there is significant evidence of impressive proliferation capacity of post-natal rodent β-cells in situations of higher metabolic demand (Desgraz et al., 2011). Furthermore, it has been shown in transgenic mice that the mature pancreas can recover fully from almost complete ablation of all existing β-cells (Cano et al., 2008), but it is unspecified whether a similar approach applies to humans. Although it has been hypothesized for decades that the human pancreas contains progenitor cells with the potential to regenerate islets, the regenerative capacity of the human pancreas has not been conclusively demonstrated (Ku, 2008). Diabetes Research Institute reports have shown that there are progenitor cells in the human pancreas that can be induced to develop into glucose-responsive β-cells in the presence of bone morphogenic protein-7 (Qadir et al., 2018). These cells were also distinguished by the presence of Pancreatic and duodenal homeobox-1 (Pdx-1), a protein required for β-cell development. As a result, these findings open up the possibility for the development of regenerative therapies to combat the disease (Qadir et al., 2018).
RESTORATION OF PANCREATIC Β-CELLS MASS AND ITS FUNCTION
The β-cells play a crucial role in human physiology since they are the only cells able to produce insulin, which can maintain glucose homeostasis by enhancing the uptake of glucose and its utilization in peripheral tissues. Therefore, dysfunctional β-cells or reduced β-cell mass, disrupt glucose homeostasis, leading to diabetes mellitus (Basile et al., 2019). Thus, the restoration of insulin-producing functional β-cells or transplantation of islets has been one of the major themes of treating diabetes mellitus (Tokui et al., 2006). Since β-cell dysfunction has been identified as a key factor in the development and progression of T1D and T2D, scientists are working on strategies to replace the destroyed β-cell. One way to do that is through β-cell replacement therapy (Butler et al., 2003; Rahier et al., 2008). However, the scarcity of donors, the need for immunosuppressive drugs, which are often toxic, and graft failure, which typically occurs within a few years (Halban et al., 2010) have prompted several studies on alternative means, including the use of β-cells deduced from human embryonic/induced pluripotent stem cells and/or the initiation of endogenous regeneration (Aguayo-Mazzucato and Bonner-Weir, 2018). Embryonic stem cells (ESCs) have tremendous potential for producing insulin-secreting cells because of their significant proliferation, self-renewal, and controlled differentiation capacity (D’Amour et al., 2006). Nevertheless, the clinical application of ESCs is limited due to their undesirable teratoma formation property (Wakitani et al., 2003) and the stringent ethical standards followed in their procurement (Fischbach and Fischbach, 2004). As a result, there is ongoing research into the intracellular expansion of β-cells to regenerate their mass (Bonner-Weir et al., 2004). Thus, (a) enhanced replication of existing β-cells (Dor et al., 2004) and (b) generation of new β-cells from cells not expressing insulin, either through converting from a differentiated cell type (trans differentiation) or differentiation from progenitors (neogenesis) found in regenerating pancreatic ducts, which are viewed as a possible source of β-cell regeneration, can result in increased β-cell mass (Fig. 3) (Bonner-Weir et al., 2004).
 | Figure 2. Search strategy. [Click here to view] |
Transdifferentiation is the process through which one specialized cell type is transformed into some other mature cell type via a dedifferentiation intermediate. It has emerged as the most alluring method of generating β-cell sources for cell replacement therapies (Kim and Lee, 2016). This process is contingent on cellular reprogramming, such as the neogenesis of β-cells and alternative progenitor cells as the source for regeneration of new β-cells from the adult pancreas. Acino-ductal trans differentiation is one such example. Given their abundance, proximity, and shared developmental origin with endocrine cells, acinar cells appear to be a viable source for producing large numbers of β-cells. Acinar cell subpopulations have increased expression of the transcription factor sex-determining region Y-Box 9, a progenitor cell marker for the pancreas, indicating the existence of differentiated acinar cells (Furuyama et al., 2011). Furthermore, during the initial stages of pancreatic development in mice, the trans differentiation process was initiated by ductal cells towards the endocrine lineage, acting as a progenitor for an islet cell (Solar et al., 2009). Interestingly, ductal cells positive for immature β-cell indicators were observed in pregnant women and T2D, indicating this as a reparative mechanism to enhance β-cells mass in physiological and pathophysiological conditions with increased metabolic demand (Dirice et al., 2019).
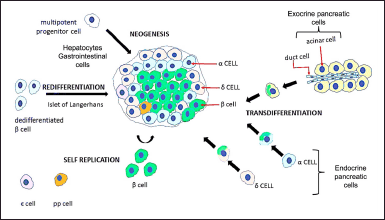 | Figure 3. Expansion of functional beta-cell mass: expansion of the population of functional β-cells represents one of the difficult strategies for compensating for insulin deficiency and improve glucose homeostasis. Cells producing insulin can be derived from either multipotent progenitor cells (hepatocytes or gastrointestinal cells), pancreatic exocrine cells, or endocrine pancreatic islet cells, by inducing cell identity switches termed as trans differentiation. Also, via enhanced replication of existing β-cells (self-replication). [Click here to view] |
The potential of α-cells to differentiate into β-cells is yet another example of pancreatic trans differentiation. Some of the benefits of considering α-to-β trans differentiation as a potential intervention are the close lineage associations and extensive overlap of transcriptomes between α and β-cells, as well as the evident better resistance of α-cells to metabolic stressors (Hancock et al., 2010). It was also found that pancreatic insulin-glucagon-positive cell populations increased in response to an increase in Glucagon like peptide-1 (GLP-1) exposure via an increase in the Pdx-1 expression (Zhang et al., 2019).
Because, hepatocytes and gastrointestinal cells are obtained from the primitive foregut endoderm and share initial developmental stages, they are viewed as a plausible endogenous source of insulin-producing cells. Multiple studies have developed reliable methods for obtaining cells like β-cells from hepatocytes by increasing the expression of transcription factors that are important for β-cell development, resulting in improved expression of biologically active insulin and restoration of glycemic control in various diabetic models (Ber et al., 2003; Kojima et al., 2003). Similarly, transient transgenic expression of genes such as Pdx-1, MafA, and nuclear factor E2-related factor (Nrf2) can induce insulin expression in gastrointestinal cells in vivo (Chen et al., 2014). Another study found that demonstrated that increasing HNF-6-induced Ngn-3 expression with GLP-1 improved insulin-producing cell conversion in rodent and human intestinal epithelial cells (Suzuki et al., 2003). A large number of studies in this research area, however, are based on findings made in rodents and proof-of-concept studies on human cells in vitro. As a result, it remains a significant challenge to determine whether reprogramming of β-cells from extra-pancreatic cells can be stimulated in human patients with the ultimate goal of increasing their mass and treating diabetes.
PHARMACOLOGICAL AGENTS THAT PROMOTE Β-CELL MASS AND IMPROVE THEIR FUNCTION
The search for strategies to enhance β-cell function and its regeneration has resulted in the discovery of several small molecules that have been demonstrated to protect and stimulate human β-cell proliferation. Table 1 lists some of the molecules involved in increased islet cell mass, the proliferation of beta cells, and glucose-stimulated insulin secretion (GSIS). Once all safety concerns have been addressed, these pharmacological agents could be used as a strategic approach to reducing the amelioration of this disorder. However, the shortcomings of presently available oral hypoglycemic agents in terms of their efficacy and/or safety, short-term hypoglycemia, and the emergence of diabetes as a global epidemic have prompted the development of alternative therapies that can manage diabetes more efficiently and safely. Dietary composition is considered to be one of the stimuli for pre-existing cell multiplication; different components may trigger this differentiation individually or synergistically. As a result, it is worthwhile to research natural products that can assist in overcoming some of these limitations and alleviate this disorder.
 | Table 1. Pharmacological agents that aid in the regeneration and function of β –cells. [Click here to view] |
TRADITIONAL MEDICINE CONTRIBUTING TO THE RESTORATION OF Β-CELL MASS AND ITS FUNCTION
Complementary or herbal medicine draws the attention of many diabetics to manage and/or prevent diabetes and its ramifications. Numerous popular herbs are asserted to lower blood glucose levels, and therefore the possibility of improving glucose control or reducing the dependency on insulin therapy via the use of traditional medicine is undeniably appealing (Dey et al., 2002). Herbal medicine is based on the comprehensive and integrated theory, that emphasizes the whole body. In contrast to conventional treatments, which typically contain a single bioactive molecule that targets a particular mechanism, herbal concoctions may include a variety of bioactive components that act synergistically through different mechanisms (Ceylan-Isik et al., 2008).
These medications can have a diverse range of beneficial effects, including increased insulin responsiveness, insulin release, β-cell regeneration, reduced carbohydrate absorption, and so on (Choudhury et al., 2018). However, caution should be exercised before administering any herbal medicine treatment (Watson and Preedy, 2012), as the selection of herbs may be dependent on a variety of factors, such as the stage of diabetes advancement, the types of comorbidities that the patients have, cost and availability, and the health impact of the herbs (Surya et al., 2014). As a result, researchers must increase their efforts in investigating alternative preventive and therapeutic measures, such as the utilization of locally accessible functional plants with novel action mechanisms. Such measures may be useful in a variety of communities where their use is accepted and considered a pivotal customary practice (Njume et al., 2019). Some of the commonly used plants and plant-derived compounds that have been researched extensively for their anti-diabetic potential, with a focus on β-cell function and β-cell preservation, and have undergone clinical trials are discussed further in the review. Table 2 summarizes the role of some of these medicinal plants in restoring pancreatic β-cells functionality in terms of T1D and T2D. In this table, animals induced with chemicals such as streptozotocin (STZ) and alloxan, which can damage pancreatic beta cells, are classified as T1D models, while diet-induced ones are classified as T2D models. Figure 4 depicts the multiple pathways by which medicinal plants and compounds derived from plants may regenerate pancreatic β-cells.
PLANTS FOR RESTORING PANCREATIC Β-CELL MASS AND ITS FUNCTION
Aloe vera
Aloe vera, a succulent plant has a long history of use by many cultures because of its medicinal properties (Sahu et al., 2013). The anti-diabetic potential of A. vera have been studied by various researchers (Rajasekaran et al., 2004; Tanaka et al., 2006), wherein, administration of different preparations results in a significant decrease in plasma glucose levels in different models (Beppu et al., 2006). The antihyperglycemic potential of A. vera was suggested to be mediated via increased insulin synthesis and its secretion, attenuation of oxidative damage, and the associated pancreatic β-cell destruction (Boudreau and Beland, 2006). Furthermore, administration of the ethanolic extract (300 mg/kg body weight) to STZ-induced rats resulted in a 50% reduction in fasting blood glucose (FBG) levels, as well as the prevention of the pancreatic β-cell damage or the recovery of partially destroyed β-cells (Noor et al., 2017, 2008). Many of its medicinal properties are believed to be due to polysaccharides observed in the inner leaf gel (Ni et al., 2004; Yin et al., 2008) anthrachinonic derivatives, phenolic compounds that are metabolized in the intestine and enter the circulatory system to show physiological effects, including protection against β-cell damage (Beppu et al., 2006).
Dyslipidemia is well known to play a crucial role in impaired β–cell function which contributes to the onset of T2D (von Eckardstein and Sibler, 2011). Oral administration of phytosterols-rich fraction of A. vera to Zucker diabetic fatty rats has significantly improved random blood glucose, glycated hemoglobin (HbA1c) levels, serum free fatty acids (FFA) and triglycerides (TG) levels, indicating increased insulin sensitivity and secretion, but no effect was observed on total cholesterol (TC) levels (Misawa et al., 2008). The lack of effect of the fraction on TC levels is thought to be attributable to the low dosage of phytosterols administered by (Misawa et al., 2008). For the treatment of diabetic patients, A. vera in conjunction with metformin could prevent diabetes-associated dyslipidemia, improve cellular integrity, and increase high-density lipoprotein (HDL) levels, reducing cardiovascular disease risk and renal failure. The histopathology studies on the pancreas and kidney demonstrated signs of recovery, in contrast to the diabetic group, which had necrotic pancreatic cells and total glomeruli erosion in the kidney (Atanu et al., 2018).
GLP-1/DPP-IV (Dipeptidyl peptidase- I V) pathway is considered important in restoring the deranged islet-cell balance and various studies have demonstrated beneficial effects on functional β–cell mass and pancreatic insulin content (Gilbert and Pratley, 2020). A dipyrrole derivative from A. vera demonstrated in vitro inhibition of the DPP-IV enzyme in a non-competitive manner suggesting one of the mechanisms by which A. vera extract restores β–cell mass in a diabetic model (Prasannaraja et al., 2020). Furthermore, the polypeptide-rich fraction from A. vera has shown protective effects on β–cells via the GLP-1/DPP-IV pathway, as evident from histopathological studies (Babu et al., 2021). Another study demonstrated that ethanolic A. vera extract alleviated diabetes symptoms by improving β–cell function and lipid metabolism. Scanning electron microscope analysis demonstrated decreased surface irregularities on islet cells in the treated group (Deora et al., 2021). However, it is continued to believe that all these therapeutic effects must be attributed to the synergism of compounds rather than a single chemical entity.
Clinical studies
A 5-year clinical study involving 5,000 patients with coronary artery disease confirmed that including A. vera gel in the diet resulted in a significant reduction in fasting and post-prandial blood glucose levels in diabetics, as well as total serum cholesterol, TG, and an increase in HDL levels was observed (Agarwal, 1985). Surprisingly, the majority of those who benefited were diabetics who were not taking any anti-diabetic medication, and no side effects were observed during the study (Agarwal, 1985). Another study found A. vera to have anti-diabetic activity in new cases of diabetes mellitus, with improved triglyceride levels (Yongchaiyudha et al., 1996). As previously stated, dyslipidemia is known to play an important role in impaired β–cell function, which contributes to the T2D onset. Improving triglyceride, cholesterol, and total lipid levels must therefore be one of the mechanisms for improved β–cell function. Furthermore, in diabetics, oral administration of a high-molecular-weight fraction (AHM-0.05 g) containing acemannan and verectin daily thrice for 12 weeks resulted in a decrease in FBG levels (Yagi et al., 2009). Acemannan, which is digested by intestinal microbiota into oligosaccharides and is responsible for obstructing glucose absorption, was discovered to be responsible for the biological effect of AHM (Boban et al., 2006; Jain et al., 2007; Yagi et al., 2001). In addition, T2Dwho received acemannan (600 mg capsule/day) for 60 days demonstrated a decrease in blood glucose, TC, and Low-density lipoprotein (LDL) levels (Huseini et al., 2012). In a randomized trial, A. vera was considered to be superior to the placebo in reducing FBG levels HbA1c, and TC levels, and also improvement in HDL levels was observed (Alinejad-Mofrad et al., 2015; Devaraj et al., 2013). Another study observed that Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) was found to be lower in an intervention group (Choi et al., 2013).
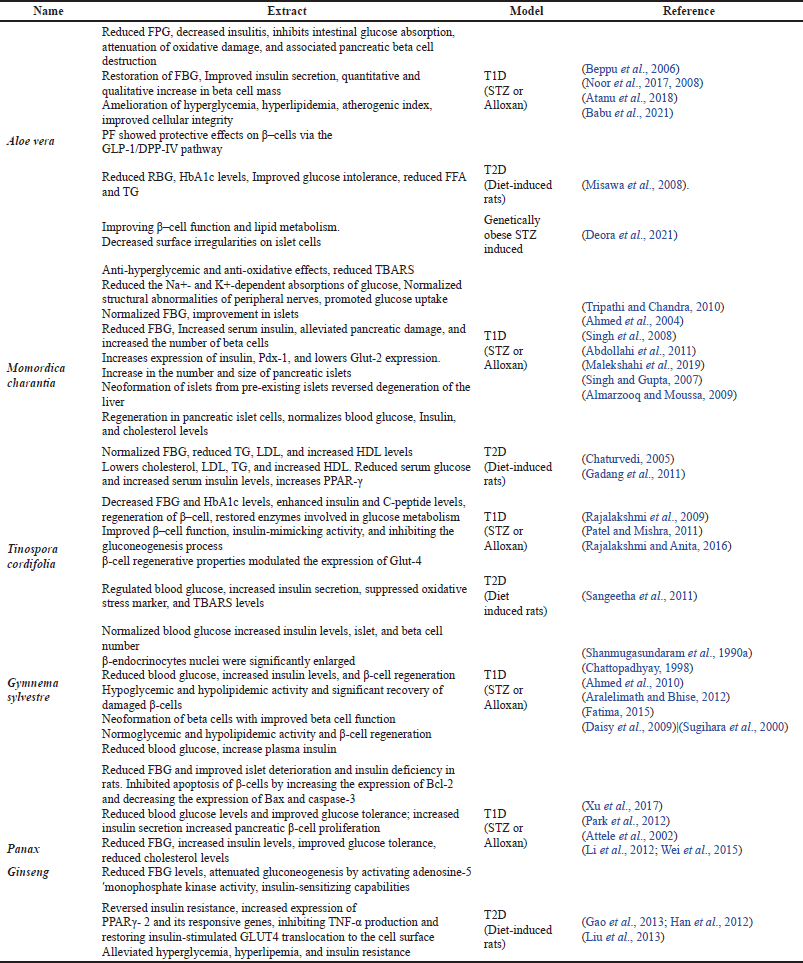 | Table 2. Effects of medicinal plants on diabetes mellitus through pancreatic β–cell regeneration. [Click here to view] |
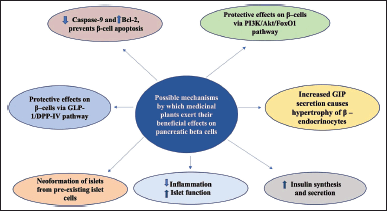 | Figure 4. Possible mechanism for β-cell regeneration: medicinal plants and plant-derived compounds can regenerate pancreatic beta cells through multiple pathways; Inhibition of DPP-IV and increasing shelf life of GLP-1, reduced islet inflammation potentiates GSIS; Prevents beta cell apoptosis by inhibiting Caspase-9 and increasing Bcl-2; Preventing nuclear translocation of Fox-1 increases expression of genes involved in beta cell regeneration and have protective effects on beta cells; reduced cytokine, chemokine production increases insulin synthesis and secretion. [Click here to view] |
Although the antidiabetic activity of A. vera has been reported in various studies, not all of this included preparation are identical. Furthermore, the variability in daily dose makes it difficult to determine the minimum optimal dose of A. vera that can bring glucose homeostasis. Large-scale, multicenter, randomized-controlled long-term trials must be robustly designed to corroborate the recent findings and investigate the long-term impact of A. vera supplementation on managing prediabetes and T2DM with a focus on improved β–cell function (Zhang et al., 2016).
Momordica charantia
Momordica charantia (MC), also known as bitter gourd (karela), is a flowering vine that has been used to treat diabetes since antiquity. (Lucas et al., 2010). Numerous in vitro, and in vivo studies, and clinical trials validated its anti-hyperglycemic potential by significantly lowering blood glucose levels, glycosylated hemoglobin, and insulinotropic activity (Chaturvedi, 2005; Tripathi and Chandra, 2010). MC extract has been shown to restore plasma glucose levels in various animal models and a significant change in pancreatic islet morphology (increase in islet size, total β-cells area, number of cells) was observed, which may be due to the renewal of β-cells or the recovery of partially destroyed β-cells (Ahmed et al., 2004; Almarzooq and Moussa, 2009). The alcoholic extract of whole fruit improved islets significantly and blood glucose levels remained static even after discontinuation of the drug for 15 days (Singh et al., 2008). The regeneration/renewal property of the extract was also demonstrated in alloxan-induced diabetic rabbits (Tahira and Hussain, 2014) and Sprague-Dawley neonatal rats (Abdollahi et al., 2011) where oral administration alleviated pancreatic damage and increased the number of β-cells. In STZ-induced diabetic rats, MC extract upregulated the expression of Insulin and Pdx-1 genes while decreasing the expression of glucose transporter-2 (GLUT2) and the number and size of pancreatic islets increased significantly (Malekshahi et al., 2019). The presence of small scattered islets among the acinar tissue after administering acetone MC extract in alloxan-induced diabetic rats was the most significant finding, which may reflect the neoformation of islets from pre-existing islet cells. Because dietary composition is one of the stimuli for pre-existing cell multiplication, various components present in the acetone fraction may trigger this differentiation (Singh and Gupta, 2007). The reparative effects of the MC extract were also observed in HIT-T15 hamster pancreatic β-cells (Xiang et al., 2007) and MIN6N8 cells via the downregulation of MAPKs and NF-κB pathway (Kim and Kim, 2011) and modulation of Peroxisome proliferator- activated receptor gamma (PPAR-γ) gene expression (Gadang et al., 2011). It was recently reported that saponins from MC improved β-cell morphology and its function in a concentration-dependent manner via the PI3K/Akt/FoxO1 signaling pathway (Liu et al., 2021). These findings imply that this plant has the potential to either repair or prevent the death of damaged pancreatic β-cells.
Clinical studies
Even though the multiple mechanisms underlying bitter melon’s beneficial effects, as well as the results reported in previous in vitro and in vivo studies, provide a strong base for well-designed randomized controlled trials to evaluate bitter melon as a treatment option for diabetes mellitus, only a few randomized, double-blind controlled studies of bitter melon have been conducted. The majority of bitter melon clinical studies have focused on blood glucose management (Fuangchan and Ingkaninan, 2009), a substantial reduction in HbA1c levels after four months of intervention (Zanker et al. 2012), fructosamine reduction following a 4-week regimen of 2,000 mg/day (Fuangchan et al., 2011), reduced glucose area under the curve (AUC), weight, BMI, fat percentage, and waist circumference, with an increment of insulin AUC, first phase and total insulin secretion (Cortez-Navarrete et al., 2018). The clinical potential of MC extract and its components shows convincing results in normalizing blood glucose levels, however, studies based on regenerating effects are needed in a larger population to use this dietary supplement for the management of diabetes. The clinical potential of MC extract and its components demonstrates convincing results in normalizing blood glucose levels; however, more research on the regeneration and stimulation of β-cells is needed before using this dietary supplement for diabetes management.
Tinospora cordifolia
Tinospora cordifolia, also known as Guduchi (Giloy), is an extremely potent herb that is extensively used in Ayurveda to combat diabetes and keep the function of various organs in balance (Sharma et al., 2015). The anti-diabetic potential of this herb is mediated by a slew of biologically active phytocompounds isolated from different parts of the plant (Sudha et al., 2011).
Various studies demonstrate amelioration of experimental diabetes in various animal models (Upadhyay et al., 2010) and this effect is mediated by reducing oxidative stress, increasing insulin secretion, and inhibiting gluconeogenesis and glycogenolysis, all of which help to regulate blood glucose levels (Sangeetha et al., 2011). Tinospora cordifolia extract supplementation significantly lowers glycated hemoglobin while increasing insulin and C-peptide levels. This increase in C-peptide levels suggests that pancreatic β–cell regeneration could be one of the possible mechanisms for this effect (Rajalakshmi et al., 2009). When compared with diabetic controls, histological examination of the pancreas in TCS methanol extract-treated groups support the regenerative capacity of the extract (Rajalakshmi et al., 2009). Sharma et al. (2019) suggested that T. cordifolia stem water extract had beneficial effects on pancreatic β–cells and increased glucose uptake in 3T3-L1 adipocytes, which may regulate glucose metabolism in diabetic rats. The signaling mechanism of isoquinoline alkaloids isolated from the plant (Palmatine, jatrorrhizine, and magnoflorine) was studied in vitro and in vivo for their insulinotropic effect using the RINm5F and rat pancreatic-cell line (Sudha et al., 2011). Further, an alkaloid-rich fraction from T. cordifolia extract had antihyperglycemic activity by improving β–cell function, insulin-mimicking activity, and inhibiting the gluconeogenesis process (Patel and Mishra, 2011). Purified polysaccharide from TC methanolic extract showed β-cell regenerative properties in STZ-induced Wistar rats, and the compound treatment positively modulated the Glut-4 expression in the gastrocnemius muscle, pointing to the development of anti-diabetic drugs with few side effects (Rajalakshmi and Anita, 2016).
Clinical studies
The studies mentioned above confirm the hypoglycemic effect of T. cordifolia and the isolated compound (s) by either promoting glucose uptake, inhibiting hepatic gluconeogenesis, or absorption of glucose into muscle and adipose tissues via stimulation of regeneration and rejuvenation of β-cells. All of this active component(s) with different physiological roles demonstrate the versatility of the plant.
Despite the anti-diabetic activities observed in pre-clinical studies, there have been very few clinical trials reported. The decrease in lipoproteins was accompanied by stimulation of the plasma lecithin cholesterol acyltransferase levels (Kumar, 2015). Importantly, no herb-drug interaction has been reported (Sharma et al., 2015). However, more research on the β-cell regenerating capacity of the plant is needed before this plant could lead its way to clinical use.
Gymnema sylvestre
Gymnema sylvestre, also known as Gurmar, is an indigenous herb that is officially mentioned in the Indian Pharmacopoeia for the treatment and/or prevention of diabetes (Tiwari et al., 2014). Gymnema leaves’ hypoglycemic action was first documented in the late 1920s (Mhaskar and Caius, 1930). Numerous in vivo studies have since confirmed the hypoglycemic activity of G. sylvestre root and leaf extracts (Sugihara et al., 2000). The antidiabetic array of molecules has been identified as a family of closely related gymnemic acids and gymnemasaponins, which are classified as oleanane saponins (Kaur et al., 2011). Furthermore, the leaves of this plant contain anthraquinones, tannins, flavonoids, phenols, cardiac glycosides, and other compounds (Senthilkumar, 2015) exhibiting therapeutic potential.
The insulinotropic activity of G. sylvestre leaves has been demonstrated in numerous diabetic models, with a concurrent reduction in fasting and postprandial glucose levels (Shanmugasundaram et al., 1990a), increasing endogenous insulin levels, presumably due to the pancreas β-cells regeneration (Murakami et al., 1996). Microscopy examination on GS extract-treated animals revealed that the nuclei of β-endocrinocytes nuclei were significantly increased in size in all sections of the organ with the same volume fraction and area of pancreatic islets (Chattopadhyay, 1998). This means that the effect of GS extract on gastric inhibitory polypeptide secretion is most likely responsible for β-endocrinocytes hypertrophy (Flier, 2001).
In vitro, GS extract induced insulinogenesis in HIT-T15, MIN6, and RINm5F β-cell lines as well as isolated islets of Langerhans, and these effects were mediated by increased cell permeability (Persaud et al., 1999a). In wistar rats, G. sylvestre leaf and callus extract showed a beneficial effect on β-cell regeneration, and gymnemic acid was found to be the active ingredient responsible for pancreatic β-cell regeneration and restoration of function (Ahmed et al., 2010). Similarly, long-term treatment with standardized dry extract of G. sylvestre leaves resulted in regenerative effects in diabetic rats (Aralelimath and Bhise, 2012). The number of β-cells increased significantly following GS treatment in STZ-induced diabetic rats. The immunohistochemical evidence revealed pancreatic islet regeneration and/or neoformation (Fatima, 2015). A cluster of β-cells without any α-cells was observed in GS-treated diabetic rats, implying β-cells regeneration rather than stem-cell-induced growth (Fatima, 2015). According to a recent study, G. sylvestre extract (Om Santal Adivasi) protects islets from cytokine-induced apoptosis in vitro and in vivo by upregulating cell survival pathways and the free radical scavenger system (Al-Romaiyan et al., 2020). Several compounds isolated from GS extract have been shown to have beneficial effects on blood glucose levels, TC levels, and TG levels suggesting that this plant has the potential to regenerate and/or neo-form pancreatic islets (Daisy et al., 2009).
Clinical studies
Gymnema sylvestre has been tested in humans as an anti-hyperglycemic agent in combination with other drugs with promising results (Shanmugasundaram et al., 1990b). Fasting plasma glucose, glycated hemoglobin, and glycosylated plasma proteins were reduced significantly in T2D patients after administering GS extract or Beta Fast GXR (supplement), and some of them were able to decrease the dosage or even discontinue the use of available hypoglycemic agents (Baskaran et al., 1990). Endogenous insulin appears to be improved by GS therapy, possibly through β-cell regeneration. In T2D patients, oral administration of GS extract (Om Santal Adivasi) revealed an insulinotropic effect, as well as a significant improvement in glycemic control. In vitro measurements using patient-derived islets derived revealed a stimulatory effect on insulin secretion, which corresponded to the in vivo mode of action (Al-Romaiyan et al., 2010). In addition to the above action, a decrease in the HOMA-IR, and an increase in the Homeostatic model of beta cell function Homeostasis Model Assessment of β-cell function (HOMA-B) were observed upon administration of GS extract, suggesting its beneficial effect in the management of diabetes (Kumar et al., 2010). Further research should be conducted to determine the drug’s long-term effect in larger populations, as well as to understand the mechanism of action of purified compounds so that their therapeutic applications can be widely explored.
Panax ginseng
For thousands of years, P. ginseng has been used as a tonic and reparative remedy in traditional Chinese medicine (Hou, 1977). Ginsenosides, widely recognized as ginseng saponins, are a type of natural triterpene saponin that is considered to be accountable for the antihyperglycemic potential of ginseng (Yang and Wu, 2016). Near about 200 ginsenosides are identified in ginseng and heat-processed ginseng products so far (Attele et al., 1999).
Ginseng has gained a great deal of attention for its anti-diabetic efficacy. Oral administration of P. ginseng berry extract and its major constituent, ginsenoside Re, demonstrated a hypoglycemic effect in obese diabetic C57BL/6J ob/ob mice, and that ginsenoside Re plays a crucial role as an anti-hyperglycemic agent (Attele et al., 2002). High glucose and fatty acid levels upregulate uncoupling protein-2 (UCP2) expression, which is relatively high in diabetic models (Reilly and Thompson, 2000; Zhang et al., 2011). Furthermore, superoxide stimulates UCP2 activity (Krauss et al., 2003). According to these findings, UCP2 may have a significant role in beta-cell pathology and the advancement of T2D. Interestingly, American ginseng water extract inhibited UCP-2 expression, which has been linked to insulin synthesis and β-cell survival (Luo and Luo, 2006). Further, the level of pro-apoptotic protein caspase-9 was reduced while the anti-apoptotic protein Bcl-2 level was increased, contributing to ginseng’s ability to protect against β-cell death and improve insulin synthesis (Luo and Luo, 2006). Ginseng extract (GE) and several ginsenosides were reported to inhibit cytokine-induced pancreatic cell death in MIN6N8 cells by reducing the production of nitric oxide and ROS. Also, the expression of p53/p21, caspase, and poly (ADP-ribose) polymerase cleavage was inhibited, which may contribute to antidiabetic influence in T1D (Kim and Kim, 2007). Similar results were reported by another group where GE increased GSIS in MIN6 cells while protecting pancreatic β-cell from apoptosis (Park et al., 2008). Furthermore, in STZ-induced diabetic mice, administration of ginseng berry extract (150 mg/kg) substantially lowered levels of blood sugar and improved glucose tolerance; enhanced insulin secretion was potential because of increased pancreatic β-cell proliferation (Park et al., 2012).
Another study found that rats fed an high fat diet (HFD) reversed insulin resistance in muscle glucose transport quickly. This was attained by increased and improved expression of PPAR?- 2 and its responsive genes, adiponectin, and Insulin receptor substrate-1 (IRS-1), impeding tumor necrosis factor- α (TNF-α) production and restoring insulin-stimulated GLUT4 translocation to the cell surface (Gao et al., 2013; Han et al., 2012). Compound K, a metabolite of protopanaxadiol-type ginsenosides demonstrated the hypoglycemic effect by reducing FBG levels, attenuating gluconeogenesis by activating adenosine-5 ′monophosphate kinase activity (Li et al., 2012; Wei et al., 2015). Malonyl ginsenosides are naturally occurring ginsenosides found both in fresh and air-dried ginseng that can alleviate hyperglycemia, hyperlipemia, and insulin resistance in STZ-induced diabetic rats fed a high-fat diet (Liu et al., 2013). Another study found that feeding GE to C57BL/6 mice significantly reduced HOMA-IR and fasting insulin levels, as well as alleviated pancreatic islet hypertrophy, implying that reduced insulin resistance may contribute to reduced insulin demand (Seo et al., 2015). As per the histopathological and immunohistochemistry studies, ginseng oligopeptides (GOPs) treatment significantly decreased FBG in induced animals and improved islet deterioration and insulin deficiency in rats. GOPs inhibited β-cell apoptosis by decreasing the expression of pro-apoptotic Bax and caspase-3 while increasing the levels of anti-apoptotic Bcl-2 (Xu et al., 2017). As previously stated, an increasing amount of research from in vitro and in vivo studies indicates that the hypoglycemic action of ginseng is complex due to a variety of mechanisms. Furthermore, clinical trials evaluating the safety and efficacy of GE in diabetics are needed.
Clinical studies
Panax ginseng has been used in Asia for a long time. Several clinical studies studying the impact of ginseng on parameters related to diabetes have been published, with widely disparate results. Panax ginseng (6 g/day) consumption for 12 weeks ameliorates hyperglycemia in T2D (Vuksan et al., 2000). Another study by the same group found that Korean red ginseng (KRG) treatment improved glycemic control and insulin levels in T2D patients (Vuksan et al., 2008). Bang et al. (2014) reported similar results, where 12 weeks of KRG supplementation improved blood glucose levels and decreased HOMA-IR in newly diagnosed T2D (Bang et al., 2014). In diabetics, regular supplementation with fermented red ginseng reduced postprandial glucose levels while increasing postprandial insulin levels (Oh et al., 2014). Ginsam, a vinegar extract of P. ginseng, improved blood glucose and HbA1c levels in T2Dwith poor glycemic control (Yoon et al., 2012). Also, the evaluation of hydrolyzed GE (HGE) in Korean participants was effective in treating people with impaired fasting glucose levels (Park et al., 2014). Furthermore, in another study ginseng berry extract reduced fasting glucose levels by 3.7%, postprandial glucose at 60 minutes, and the AUC for glucose by 7.7% in a double-blind placebo-controlled study; however, this study failed to show anti-diabetic potential concerning other blood glucose and lipid metabolism-related parameters. Importantly, no adverse effects from long-term berry extract consumption were reported by (Choi et al., 2018). Reeds et al. (2011) on the other hand, found that oral ginseng or ginsenoside Re therapy has no effect on β-cell function in morbidly obese subjects with poor glucose tolerance or recently diagnosed diabetes. The lack of a therapeutic effect could be attributed to poor systemic bioavailability (Reeds et al., 2011) and differences in ginsenoside concentrations may also have contributed to outcome variability. Standardization of ginsenosides types and ratios is required to achieve consistent efficacy and use it as a prospective drug for the treatment of diabetes mellitus.
COMPOUNDS IMPLICATED IN THE RESTORATION OF PANCREATIC Β-CELL MASS AND ITS FUNCTION
Different natural compounds, such as flavonoids and alkaloids, have been shown in preclinical and clinical studies to have anti-diabetic potential.
Geniposide
Geniposide, a natural dietary pigment obtained from gardenia fruits, has emerged as an exciting candidate due to its ability to significantly increase T-cell factor 7-like 2 (TCF7L2) mRNA levels in cells exposed to high glucose levels. Blood glucose levels were normalized following geniposide treatment in db/db and HFD mice, accompanied by an increase in β-cell mass (Yao et al., 2015). Wnt/β-catenin signaling is an important regulator for β-cell insulin secretion. TCF7L2, a key transcription factor of Wnt signaling, mediates its effects through GLP- 1 receptor. Increased expression of TCF7L2 may enhance GLP-1R expression and activate the AKT pathway (Shu et al., 2009) promoting β-cell regeneration in vivo (Shu et al., 2012). Numerous studies have confirmed the role of TCF7L2 in improving β-cell function, survival, and regeneration (da Silva Xavier et al., 2009; Figeac et al., 2010). Furthermore, geniposide could prevent β-cell apoptosis in lipotoxicity-induced INS-1 cells via GLP-1R (Liu et al., 2012) and protect β-cell mass from pro-inflammatory cytokine-mediated toxicity by upregulating TCF7L2 expression (Yao et al., 2015).
Berberine
Berberine is an isoquinoline alkaloid found in different parts of the plant. Berberis has been used to treat various disorders including diabetes. The effect of berberine on insulin production is controversial. Prolonged treatment with berberine increased insulin secretion in various cell lines and mouse islets of Langerhans in a dose-dependent manner (1–10 μM) (Leng et al., 2004), whereas acute administration with high concentrations (50 M for 1 hours) lowered insulin secretion (Zhou et al., 2008). Even though its effects on insulin release in vitro have been debated, berberine has been shown to reduce hyperglycemia, enhance insulin sensitivity, and stimulate pancreatic β-cell regeneration in T2D animal models. Oral administration of berberine (380 mg/kg) resulted in weight reduction and a substantial improvement in glucose homeostasis in db/db mice (Lee et al., 2006). It has also been shown to have a protective effect against STZ-induced β-cell death (Zhou et al., 2009). Unpublished data from our lab showed that berberine isolated from natural resources inhibits DPP-IV activity in vitro, and the results were confirmed by in silico studies.
Phenylpropenoic acid glucoside
Phenylpropenoic acid glucoside (PPAG) from rooibos (Aspalathus linearis) extract increases in vitro glucose uptake and improves glycemic control in the T2D animal rat model, suggesting that the anti-hyperglycemic effect of rooibos is attributable to PPAG and thus it has the potential to be a new class of antidiabetic therapeutics (Muller et al., 2013). Furthermore, oral administration of PPAG to mice fed a high fat and fructose diet (similar to an unhealthy western diet) for 12 weeks prevented diabetes. PPAG increased β-cell mass in this chronic, long-term treatment by decreasing lipotoxic β-cell apoptosis. PPAG also protected β-cells from palmitate-induced apoptosis in vitro and from ER stress by increasing the expression of the anti-apoptotic BCL2 protein while not affecting proapoptotic signals (Mathijs et al., 2014). PPAG also protects pancreatic β-cells against the harmful effects of STZ, oxidative stress, and glucotoxicity, in addition to having anti-apoptotic and anti-necrotic properties and protecting human beta cells from a diabetogenic insult. The cytoprotective effect was preceded by the normalization of anti-apoptotic protein BCL2 expression in pancreatic islets (Himpe et al., 2016).
Genistein
Genistein is the most extensively researched isoflavone for the management of diabetes (Gilbert and Liu, 2013) and can be found in a wide range of plants, including lupine, fava beans, soybeans, and soybean products. It has been researched in several diabetic models and has consistently demonstrated hypoglycemic potential across experimental models (Elmarakby et al., 2011; Fu et al., 2010). Numerous in vitro studies using isolated islets and insulinoma cell lines have evidenced that genistein has anti-diabetic properties due to its direct effect on β-cells (Elliott et al., 2002; Liu et al., 2006; Ohno et al., 1993). However, genistein’s beneficial effects are limited to a certain concentration. Chronic treatment (100 mol/l) with genistein prevented the cultured islet cell proliferation, whereas acute treatment (5 mol/l) resulted in increased proliferation of β-cells in vitro and in vivo (Sorenson et al., 1994).
Another study reported that acute treatment with genistein (5 μM for 30 m) potentiated GSIS in islets of mice and the INS-1 β-cell line (Liu et al., 2006) and that moderate concentration of genistein (50 μM for 1 hour) also produced similar results in isolated islets of the mice (Persaud et al., 1999b). In addition, genistein has been shown to modulate both β-cell proliferation and apoptosis. A low concentration of genistein-induced proliferation was detected using the incorporation of BrdU in INS-1 cells (0.1–10 M for 24 hours) and isolated human islets (1 and 5 M for 24 hours) respectively. (Fu et al., 2010). In contrast, a higher concentration of genistein (100 μM for 24 hours) increased β-cell death in RINm5F cells at 4 hours (Elliott et al., 2001) and 24 hours (Elliott et al., 2002). It is believed that the insulinotropic and proliferative effects of genistein are mediated partly via the accumulation of cAMP and activation of protein kinase A (Fu et al., 2010). Also, the observed increase in cell death associated with higher concentrations of genistein could be due to the inhibition of topoisomerase-II (Elliott et al., 2001). Inhibiting topoisomerase-II can cause fragmentation of DNA, cell death, and G2/M cell cycle arrest (Collier et al., 2006).
Naringenin
Naringenin is a bioactive flavonoid that is found primarily in citrus fruits like grapes, oranges, lemons, and tomatoes (Sumathi et al., 2015). Nrf2 is a leucine zipper transcription element that regulates the expression of intracellular antioxidant enzymes, preventing cell death from oxidative stress (Lacher et al., 2015). It is theorized that targeted Nrf2 activation will protect pancreatic cells from oxidative damage, thereby reducing diabetes complications (Lu et al., 2016). Naringenin showed protective effects against STZ-induced apoptosis in MIN6 cells by increasing Nrf2 expression and its target genes GST and NQO1 and decreasing the expression of caspase-3 (Rajappa et al., 2018). Further, in vivo studies revealed that 45 days of oral naringenin administration significantly decreased blood glucose levels, improved lipid profile, and increased the level of antioxidants in pancreatic tissues (Rajappa et al., 2018). It also improved glucose homeostasis and response to insulin in STZ-treated mice. Immunohistochemical analysis demonstrated that insulin expression was restored in control animals. In addition, naringenin increased glycolysis while inhibiting gluconeogenesis. These in vitro and in vivo results highlight the potential of naringenin to enhance the expression of Nrf2 and protect pancreatic β-cells from STZ-induced oxidative damage (Rajappa et al., 2018).
Baicalein
Baicalein is a flavonoid extracted from the roots of the medicinal plant Scutellariae baicalensis Georgi (SBG), which is generally grown in Asian countries (Bruno et al., 2002). Baicalein, baicalin, wogonin, and wogonoside are supposedly the major biologically active components in SBG, accounting for 5.41%, 10.11%, 1.3%, and 3.55% of the dry material respectively (Li-Weber, 2009). Because of the potential health benefits of baicalein and its glucuronide baicalin, they have received a great deal of attention. In vivo studies demonstrated oral administration of baicalin for 30 days, decreased hyperglycemia-induced mitochondrial damage in β-cells (Waisundara et al., 2009). Baicalein has been shown to have positive effects on diabetes-associated health complications (Pu et al., 2012). Further, it has been shown to improve glycemic control and GSIS in HFD-induced middle-aged obese mice. However, no significant change was observed in the intake of food, body weight, lipid profile, and insulin sensitivity in obese mice. However, in the HFD model with a low dose of STZ, baicalein treatment improved glucose homeostasis and blood insulin levels, which are linked to enhanced β-cell survival and mass. Furthermore, baicalein significantly improved GSIS and increased the effectiveness of insulin-secreting cells and cultured islets either in the basal medium or under prolonged hyperlipidemic conditions. These findings suggest that baicalein may be involved in modulating pancreatic β-cell function (Fu et al., 2014). Baicalein may have anti-diabetic properties by increasing glucose uptake and glycolysis and inhibiting gluconeogenesis in hepatocytes, possibly through regulation of the Ins/IRS-1/PI3K/AKT pathway (Yang et al., 2019). Baicalein has been shown in vivo to improve glucose and fat metabolism in T2D rats by normalizing the MAPK/PI3K/AKT signaling pathway, thereby improving insulin sensitivity (Cui et al., 2018).
Apigenin
Apigenin is a flavonoid derived from plants with innumerable nutritional and organoleptic properties. It is mostly found in the glycosylated form in vegetables, fruits, herbs, and plant-based beverages and oils (Marrano et al., 2021; Salehi et al., 2019b). The antihyperglycemic potential of apigenin may be due to its ability to inhibit the activity of α-glucosidase, enhance insulin secretion (Pamunuwa et al., 2016), and neutralize ROS in the cell (Shay et al., 2015), all of which help to prevent diabetes and its complications. Furthermore, it has been shown to have a protective effect against cytokine-induced β-cell damage via suppression of nuclear factor kappa B activation (Kim et al., 2007) and was able to reverse the cytotoxic effects of STZ and inhibit β-cell apoptosis. The protective effects of apigenin were related to ameliorating the loss of antioxidant enzymes (Wang et al., 2017). Apigenin present in extra virgin olive oil augmented beta-cell proliferation and enhanced GSIS in INS-1E cells and human islet cells (Marrano et al., 2021). Protein kinase B (AKT) activation and cAMP response element-binding protein (CREB) is important for the regulation of β-cell mass and its function (Dalle et al., 2011; Elghazi et al., 2007). Apigenin was found to significantly increase both AKT and CREB phosphorylation, suggesting that it can foster β-cell health and may increase insulin release and improve glycemic control (Marrano et al., 2021).
CONCLUSION
β-cell death contributes to the progression of T1D and T2D by reducing the mass of β-cells and, as a result, endogenous secretion of insulin. Therapeutics to impede or even reverse the apoptosis and dysfunction of β-cells are urgently needed. Several studies have demonstrated that the average contributions of β-cell proliferation, neogenesis, and apoptosis to overall β-cell mass vary with age and in response to stress. We reviewed some medicinal plants and plant-derived compounds, inspired by traditional healthcare systems. Several pieces of scientific evidence suggested that these plant extracts and/or phytochemicals have anti-hyperglycemic properties. Some of these extracts such as A. vera, P. ginseng, M. charantia, and a few compounds, such as Geniposide, Baicalein, and Apigenin, demonstrated highly promising effects. The extracts and the phytochemicals described in this review are believed to ameliorate diabetic symptoms via multiple pathways including increased half-life of GLP-1 and inhibiting DPP-IV, decreasing β-cell apoptosis by inhibiting caspase 9 and increasing the expression of Bcl-2, enhancing the expression of genes involved in β-cell proliferation, decreasing islet inflammation and increasing insulin synthesis and secretion. However, the most significant limitation is the paucity of controlled clinical trials. The short duration of these studies, small sample size, lack of randomization, and dose variation. Thus, further investigation and clinical trials are needed to determine the correct dosage for diabetes prevention and its complications, as well as to better comprehend the mechanisms through which these extracts impart their therapeutic benefits. Importantly, prospective anti-diabetic plants should be evaluated for toxicity to ensure they are therapeutically safe and effective phytomedicines.
ACKNOWLEDGMENT
The lead author (ND) would like to acknowledge the Indian Council of Medical Research for providing a fellowship under grant 45/39/2018/MP/BMS. Also, the authors are thankful to VIT, Vellore for their support in the research activities.There is any other financial support to report.
LIST OF ABBREVIATIONS
AUC, Area under curve; BCL2, B-cell lymphoma 2; cAMP, Cyclic adenosine monophosphate; DPP-IV, Dipeptidyl peptidase-IV; ESCs, Embryonic stem cells; FFA, Free fatty acid; GABA, Gamma-aminobutyric acid; GLP-1, Glucagon-like peptide-1; Glut2, Glucose transported-2; Glut-4, Glucose transporter-4; GOPs, Ginseng oligopeptides; GSIS, Glucose stimulated insulin secretion; Gymnema sylvestre, G. sylvestre; HbA1c, Glycated hemoglobin; HDL, High-density lipoprotein; HFD, High fat diet; HOMA-B, Homeostasis model assessment of β-cell function; HOMA-IR, Homeostatic model assessment of insulin resistance; IRS-1, Insulin receptor substrate-1; KRG, Korean red ginseng; LDL, Low-density lipoprotein; Momordica charantia, M. charantia (MC); Nrf2, Nuclear factor E2-related factor; Panax Ginseng, P. ginseng; Pdx-1, Pancreatic and duodenal homeobox 1; PPAG, Phenylpropenoic acid glucoside; PPAR-γ, Peroxisome proliferator- activated receptor gamma; STZ, Streptozotocin; T1D, Type 1 diabetes; T2D, Type 2 diabetes; TCF7L2, T-cell factor 7-like 2; Tinospora cordifolia, T. cordifolia; TNF-α, Tumor necrosis factor alpha.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abdollahi M, Zuki ABZ, Goh YM, Rezaeizadeh A, Noordin MM. Effects of Momordica charantia on pancreatic histopathological changes associated with streptozotocin-induced diabetes in neonatal rats. Histol Histopathol, 2011; 26(1):13–21.
Agarwal OP. Prevention of atheromatous heart disease. Angiology, 1985; 36(8):485–92. CrossRef
Aguayo-Mazzucato C, Bonner-Weir S. Pancreatic β cell regeneration as a possible therapy for diabetes. Cell Metab, 2018; 27(1):57–67. CrossRef
Ahmed I, Adeghate E, Cummings E, Sharma AK, Singh J. Beneficial effects and mechanism of action of Momordica charantia juice in the treatment of streptozotocin-induced diabetes mellitus in rat. Mol Cell Biochem, 2004; 261(1–2):63–70. CrossRef
Ahmed ABA, Rao AS, Rao MV. In vitro callus and in vivo leaf extract of Gymnema sylvestre stimulate β-cells regeneration and anti-diabetic activity in Wistar rats. Phytomedicine, 2010; 17(13):1033–9. CrossRef
Alinejad-Mofrad S, Foadoddini M, Saadatjoo SA, Shayesteh M. Improvement of glucose and lipid profile status with Aloe vera in pre-diabetic subjects: a randomized controlled-trial. J Diabetes Metab Disord, 2015; 14:22. CrossRef
Almarzooq M, Moussa EA. Hypoglycemic effect of Momordica charantia (karela) on normal and alloxan diabetic albino mice. Egypt J Exp Biol (Zoology), 2009; 5:487–93.
Al-Romaiyan A, Liu B, Asare-Anane H, Maity CR, Chatterjee SK, Koley N, Biswas T, Chatterji AK, Huang GC, Amiel SA, Persaud SJ, Jones PM. A novel Gymnema sylvestre extract stimulates insulin secretion from human islets in vivo and in vitro. Phytother Res, 2010; 24(9):1370–6. CrossRef
Al-Romaiyan A, Liu B, Persaud S, Jones P. A novel Gymnema sylvestre extract protects pancreatic beta-cells from cytokine-induced apoptosis. Phytother Res, 2020; 34(1):161–72. CrossRef
Aralelimath VR, Bhise SB. Anti-diabetic effects of Gymnema sylvester extract on streptozotocin induced diabetic rats and possible b-cell protective and regenerative evaluations. Dig J Nanomater Biostructures, 2012; 7(1):135–42.
Atanu FO, Avwioroko OJ, Momoh S. Anti-diabetic effect of combined treatment with Aloe vera gel and metformin on alloxan-induced diabetic rats. J Ayu Her Med, 2018; 4(1):1–5. CrossRef
Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol, 1999; 58(11):1685–93. CrossRef
Attele AS, Zhou YP, Xie JT, Wu JA, Zhang L, Dey L, Pugh W, Rue PA, Polonsky KS, Yuan CS. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes, 2002; 51(6):1851–8. CrossRef
Babu SN, Govindarajan S, Vijayalakshmi MA, Noor A. Role of zonulin and GLP-1/DPP-IV in alleviation of diabetes mellitus by peptide/polypeptide fraction of Aloe vera in streptozotocin- induced diabetic wistar rats. J Ethnopharmacol, 2021; 272:113949. CrossRef
Bang H, Kwak JH, Ahn HY, Shin DY, Lee JH. Korean red ginseng improves glucose control in subjects with impaired fasting glucose, impaired glucose tolerance, or newly diagnosed type 2 diabetes mellitus. J Med Food, 2014; 17(1):128–34. CrossRef
Basile G, Kulkarni RN, Morgan NG. How, when, and where do human β-cells regenerate? Curr Diab Rep, 2019; 19(8):48. CrossRef
Baskaran K, Kizar Ahamath B, Radha Shanmugasundaram K, Shanmugasundaram ER. Antidiabetic effect of a leaf extract from Gymnema sylvestre in non-insulin-dependent diabetes mellitus patients. J Ethnopharmacol, 1990; 30(3):295–300. CrossRef
Belgardt BF, Lammert E. DYRK1A: a promising drug target for islet transplant-based diabetes therapies diabetes. Diabetes, 2016; 65(6):1496–8. CrossRef
Benthuysen JR, Carrano AC, Sander M. Advances in β cell replacement and regeneration strategies for treating diabetes. J Clin Invest, 2016; 126(10):3651–60. CrossRef
Beppu H, Shimpo K, Chihara T, Kaneko T, Tamai I, Yamaji S, Ozaki S, Kuzuya H, Sonoda S. Antidiabetic effects of dietary administration of Aloe arborescens miller components on multiple low-dose streptozotocin-induced diabetes in mice: investigation on hypoglycemic action and systemic absorption dynamics of aloe components. J Ethnopharmacol, 2006; 103(3):468–77.
Ber I, Shternhall K, Perl S, Ohanuna Z, Goldberg I, Barshack I, Benvenisti-Zarum L, Meivar-Levy I, Ferber S. Functional, persistent, and extended liver to pancreas transdifferentiation. J Biol Chem, 2003; 278(34):31950–7. CrossRef
Boban PT, Nambisan B, Sudhakaran PR. Hypolipidaemic effect of chemically different mucilages in rats: a comparative study. Br J Nutr, 2006; 96(6):1021–9. CrossRef
Bonner-Weir S, Toschi E, Inada A, Reitz P, Fonseca SY, Aye T, Sharma A. The pancreatic ductal epithelium serves as a potential pool of progenitor cells. Pediatr Diabetes, 2004; 5(Suppl 2):16–22. CrossRef
Boudreau MD, Beland FA. An evaluation of the biological and toxicological properties of Aloe barbadensis (miller), Aloe vera. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev, 2006; 24(1):103–54. CrossRef
Bruno M, Piozzi F, Rosselli S. Natural and hemisynthetic neoclerodane diterpenoids from scutellaria and their antifeedant activity. Nat Prod Rep, 2002; 19(3):357–78. CrossRef
Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes, 2003; 52(1):102–10. CrossRef
Cano DA, Rulifson IC, Heiser PW, Swigart LB, Pelengaris S, German M, Evan GI, Bluestone JA, Hebrok M. Regulated beta-cell regeneration in the adult mouse pancreas. Diabetes, 2008; 57(4):958–66. CrossRef
Ceylan-Isik AF, Fliethman RM, Wold LE, Ren J. Herbal and traditional Chinese medicine for the treatment of cardiovascular complications in diabetes mellitus. Curr Diabetes Rev, 2008; 4(4):320–8. CrossRef
Chang-Chen KJ, Mullur R, Bernal-Mizrachi E. Beta-cell failure as a complication of diabetes. Rev Endocr Metab Disord, 2008; 9(4):329–43. CrossRef
Chattopadhyay RR. Possible mechanism of antihyperglycemic effect of Gymnema sylvestre leaf extract, part I. Gen Pharmacol, 1998; 31(3):495–6. CrossRef
Chaturvedi P. Role of Momordica charantia in maintaining the normal levels of lipids and glucose in diabetic rats fed a high-fat and low-carbohydrate diet. Br J Biomed Sci, 2005; 62(3):124–6. CrossRef
Chen C, Cohrs CM, Stertmann J, Bozsak R, Speier S. Human beta cell mass and function in diabetes: recent advances in knowledge and technologies to understand disease pathogenesis. Mol Metab, 2017; 6(9):943–57. CrossRef
Chen YJ, Finkbeiner SR, Weinblatt D, Emmett MJ, Tameire F, Yousefi M, Yang C, Maehr R, Zhou Q, Shemer R, Dor Y, Li C, Spence JR, Stanger BZ. De novo formation of insulin-producing “neo-β cell islets” from intestinal crypts. Cell Rep, 2014; 6(6):1046–58. CrossRef
Choi HS, Kim S, Kim MJ, Kim MS, Kim J, Park CW, Seo D, Shin SS, Oh SW. Efficacy and safety of Panax ginseng berry extract on glycemic control: a 12-wk randomized, double-blind, and placebo-controlled clinical trial. J Ginseng Res, 2018; 42(1):90–7. CrossRef
Choi HC, Kim SJ, Son KY, Oh BJ, Cho BL. Metabolic effects of Aloe vera gel complex in obese prediabetes and early non-treated diabetic patients: randomized controlled trial. Nutrition, 2013; 29(9):1110–4. CrossRef
Choudhury H, Pandey M, Hua CK, Mun CS, Jing JK, Kong L, Ern LY, Ashraf NA, Kit SW, Yee TS, Pichika MR, Gorain B, Kesharwani P. An update on natural compounds in the remedy of diabetes mellitus: a systematic review. J Tradit Complement Med, 2018; 8(3):361–76. CrossRef
Collier JJ, Fueger PT, Hohmeier HE, Newgard CB. Pro- and antiapoptotic proteins regulate apoptosis but do not protect against cytokine-mediated cytotoxicity in rat islets and beta-cell lines. Diabetes, 2006; 55(5):1398–406. CrossRef
Cortez-Navarrete M, Martínez-Abundis E, Pérez-Rubio KG, González-Ortiz M, Méndez-Del Villar M. Momordica charantia administration improves insulin secretion in type 2 diabetes mellitus. J Med Food, 2018; 21(7):672–7. CrossRef
Cui X, Qian DW, Jiang S, Shang EX, Zhu ZH, Duan JA. Scutellariae radix and coptidis rhizoma improve glucose and lipid metabolism in T2DM rats via regulation of the metabolic profiling and mapk/pi3k/akt signaling pathway. Int J Mol Sci, 2018; 19(11):3634. CrossRef
Daisy P, Eliza J, Mohamed Farook KAM. A novel dihydroxy gymnemic triacetate isolated from Gymnema sylvestre possessing normoglycemic and hypolipidemic activity on STZ-induced diabetic rats. J Ethnopharmacol, 2009; 126(2):339–44. CrossRef
Dalle S, Quoyer J, Varin E, Costes S. Roles and regulation of the transcription factor CREB in pancreatic β-cells. Curr Mol Pharmacol, 2011; 4(3):187–95. CrossRef
D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol, 2006; 24(11):1392–401. CrossRef
da Silva Xavier G, Loder MK, McDonald A, Tarasov AI, Carzaniga R, Kronenberger K, Barg S, Rutter GA. TCF7L2 regulates late events in insulin secretion from pancreatic islet beta-cells. Diabetes, 2009; 58(4):894–905. CrossRef
Deora N, Sunitha MM, Satyavani M, Harishankar N, Vijayalakshmi MA, Venkataraman K, Venkateshan V. Alleviation of diabetes mellitus through the restoration of β-cell function and lipid metabolism by Aloe vera (L.) Burm. f. extract in obesogenic WNIN/GR-Ob rats. J Ethnopharmacol, 2021; 272:113921. CrossRef
Desgraz R, Bonal C, Herrera PL. β-cell regeneration: the pancreatic intrinsic faculty. Trends Endocrinol Metab, 2011; 22(1):34–43. CrossRef
Devaraj S, Yimam M, Brownell LA, Jialal I, Singh S, Jia Q. Effects of Aloe vera supplementation in subjects with prediabetes/metabolic syndrome. Metab Syndr Relat Disord, 2013; 11(1):35–40. CrossRef
Dey L, Attele AS, Yuan CS. Alternative therapies for type 2 diabetes. Altern Med Rev, 2002; 7(1):45–58.
Dirice E, De Jesus DF, Kahraman S, Basile G, Ng RW, El Ouaamari A, Teo AKK, Bhatt S, Hu J, Kulkarni RN. Human duct cells contribute to β cell compensation in insulin resistance. JCI Insight, 2019; 4(8):e99576. CrossRef
Dirice E, Walpita D, Vetere A, Meier BC, Kahraman S, Hu J, Dan?ík V, Burns SM, Gilbert TJ, Olson DE, Clemons PA, Kulkarni RN, Wagner BK. Inhibition of DYRK1A stimulates human β-cell proliferation. Diabetes, 2016; 65(6):1660–71. CrossRef
Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature, 2004; 429(6987):41–6. CrossRef
Elghazi L, Rachdi L, Weiss AJ, Cras-Méneur C, Bernal-Mizrachi E. Regulation of beta-cell mass and function by the Akt/protein kinase B signalling pathway. Diabetes Obes Metab, 2007; 9(Suppl 2):147–57. CrossRef
Elliott J, Scarpello JH, Morgan NG. Effects of tyrosine kinase inhibitors on cell death induced by sodium fluoride and pertussis toxin in the pancreatic beta-cell line, RINm5F. Br J Pharmacol, 2001; 132(1):119–26. CrossRef
Elliott J, Scarpello JHB, Morgan NG. Differential effects of genistein on apoptosis induced by fluoride and pertussis toxin in human and rat pancreatic islets and RINm5F cells. J Endocrinol, 2002; 172(1):137–43. CrossRef
Elmarakby AA, Ibrahim AS, Faulkner J, Mozaffari MS, Liou GI, Abdelsayed R. Tyrosine kinase inhibitor, genistein, reduces renal inflammation and injury in streptozotocin-induced diabetic mice. Vascul Pharmacol, 2011; 55(5–6):149–56. CrossRef
Fatima N. Immunohistochemical evidence of pancreatic beta-cell regeneration in streptozotocin-induced type 2 diabetic rats treated with Gymnema sylvestre extract. J Cytol Histol, 2015; 06(04) :1. CrossRef
Figeac F, Uzan B, Faro M, Chelali N, Portha B, Movassat J. Neonatal growth and regeneration of beta-cells are regulated by the Wnt/beta-catenin signaling in normal and diabetic rats. Am J Physiol Endocrinol Metab, 2010; 298(2):E245–56. CrossRef
Fischbach GD, Fischbach RL. Stem cells: science, policy, and ethics. J Clin Invest, 2004; 114(10):1364–70. CrossRef
Flier JS. The missing link with obesity? Nature, 2001; 409(6818):292–3. CrossRef
Fu Y, Luo J, Jia Z, Zhen W, Zhou K, Gilbert E, Liu D. Baicalein protects against type 2 diabetes via promoting islet β-cell function in obese diabetic mice. Int J Endocrinol, 2014; 2014:846742. CrossRef
Fu Z, Zhang W, Zhen W, Lum H, Nadler J, Bassaganya-Riera J, Jia Z, Wang Y, Misra H, Liu D. Genistein induces pancreatic beta-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice. Endocrinology, 2010; 151(7):3026–37.
Fuangchan A, Ingkaninan K. Retrospective study on the use of bitter melon for type 2 diabetes at Dansai Crown Prince Hospital, Thailand. Srinagarind Med J, 2009; 24(4):332–8. CrossRef
Fuangchan A, Sonthisombat P, Seubnukarn T, Chanouan R, Chotchaisuwat P, Sirigulsatien V, Ingkaninan K, Plianbangchang P, Haines ST. Hypoglycemic effect of bitter melon compared with metformin in newly diagnosed type 2 diabetes patients. J Ethnopharmacol, 2011; 134(2):422–8. CrossRef
Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, Koizumi M, Masui T, Kawaguchi M, Takaori K, Doi R, Nishi E, Kakinoki R, Deng JM, Behringer RR, Nakamura T, Uemoto S. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet, 2011; 43(1):34–41. CrossRef
Gadang V, Gilbert W, Hettiararchchy N, Horax R, Katwa L, Devareddy L. Dietary bitter melon seed increases peroxisome proliferator-activated receptor-γ gene expression in adipose tissue, down-regulates the nuclear factor-κB expression, and alleviates the symptoms associated with metabolic syndrome. J Med Food, 2011; 14(1–2):86–93. CrossRef
Gao Y, Yang MF, Su YP, Jiang HM, You XJ, Yang YJ, Zhang HL. Ginsenoside re reduces insulin resistance through activation of PPAR-γ pathway and inhibition of TNF-α production. J Ethnopharmacol, 2013; 147(2):509–16. CrossRef
Gilbert ER, Liu D. Anti-diabetic functions of soy isoflavone genistein: mechanisms underlying its effects on pancreatic β-cell function. Food Funct, 2013; 4(2):200–12. CrossRef
Gilbert MP, Pratley RE. GLP-1 analogs and DPP-4 inhibitors in type 2 diabetes therapy: review of head-to-head clinical trials. Front Endocrinol (Lausanne), 2020; 11:178. CrossRef
Halban PA, German MS, Kahn SE, Weir GC. Current status of islet cell replacement and regeneration therapy. J Clin Endocrinol Metab, 2010; 95(3):1034–43. CrossRef
Han DH, Kim SH, Higashida K, Jung SR, Polonsky KS, Klein S, Holloszy JO. Ginsenoside re rapidly reverses insulin resistance in muscles of high-fat diet fed rats. Metab Clin Exp, 2012; 61(11):1615–21. CrossRef
Hancock AS, Du A, Liu J, Miller M, May CL. Glucagon deficiency reduces hepatic glucose production and improves glucose tolerance in adult mice. Mol Endocrinol, 2010; 24(8):1605–14. CrossRef
Himpe E, Cunha DA, Song I, Bugliani M, Marchetti P, Cnop M, Bouwens L. Phenylpropenoic acid glucoside from rooibos protects pancreatic beta cells against cell death induced by acute injury. PLoS One, 2016; 11(6):e0157604. CrossRef
Hossain MA, Pervin R. Current antidiabetic drugs. Nutritional and therapeutic interventions for diabetes and metabolic syndrome. Elsevier, Amsterdam, The Netherlands, pp 455–73, 2018. CrossRef
Hou JP. The chemical constituents of ginseng plants. Comp Med East West, 1977; 5(2):123–45. CrossRef
Huseini HF, Kianbakht S, Hajiaghaee R, Dabaghian FH. Anti-hyperglycemic and anti-hypercholesterolemic effects of Aloe vera leaf gel in hyperlipidemic type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Planta Med, 2012; 78(4):311–6. CrossRef
Jain A, Gupta Y, Jain SK. Perspectives of biodegradable natural polysaccharides for site-specific drug delivery to the colon. J Pharm Pharm Sci, 2007; 10(1):86–128.
Kahn SE. Clinical review 135: the importance of beta-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab, 2001; 86(9):4047–58. CrossRef
Kaur N, Chaudhary J, Jain A, Kishore L. Stigmasterol: a comprehensive review. Int J Pharm Sci Res, 2011; 2(9):2259–65.
Kim HY, Kim K. Protective effect of ginseng on cytokine-induced apoptosis in pancreatic beta-cells. J Agric Food Chem, 2007; 55(8):2816–23. CrossRef
Kim K, Kim HY. Bitter melon (Momordica charantia) extract suppresses cytokineinduced activation of MAPK and NF-κB in pancreatic β-cells. Food Sci Biotechnol, 2011; 20(2):531–5. CrossRef
Kim EK, Kwon KB, Song MY, Han MJ, Lee JH, Lee YR, Lee JH, Ryu DG, Park BH, Park JW. Flavonoids protect against cyçokine-induced pancreatic beta-cell damage through suppression of nuclear factor kappaB activation. Pancreas, 2007; 35(4):e1–9. CrossRef
Kim HS, Lee MK. β-Cell regeneration through the transdifferentiation of pancreatic cells: pancreatic progenitor cells in the pancreas. J Diabetes Investig, 2016; 7(3):286–96. CrossRef
Kodama S, Kühtreiber W, Fujimura S, Dale EA, Faustman DL. Islet regeneration during the reversal of autoimmune diabetes in NOD mice. Science, 2003; 302(5648):1223–7. CrossRef
Kojima H, Fujimiya M, Matsumura K, Younan P, Imaeda H, Maeda M, Chan L. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat Med, 2003; 9(5):596–603. CrossRef
Krauss S, Zhang CY, Scorrano L, Dalgaard LT, St-Pierre J, Grey ST, Lowell BB. Superoxide-mediated activation of uncoupling protein 2 causes pancreatic beta cell dysfunction. J Clin Invest, 2003; 112(12):1831–42. CrossRef
Ku HT. Minireview: pancreatic progenitor cells--recent studies. Endocrinology, 2008; 149(9):4312–6. CrossRef
Kumar V. Antidyslipidemic and antioxidant activities of Tinospora cordifolia stem extract in alloxan induced diabetic rats. Indian J Clin Biochem, 2015; 30(4):473–8. CrossRef
Kumar SN, Mani UV, Mani I. An open label study on the supplementation of Gymnema sylvestre in type 2 diabetics. J Diet Suppl, 2010; 7(3):273–82. CrossRef
Kumar S, Mittal A, Babu D, Mittal A. Herbal medicines for diabetes management and its secondary complications. Curr Diabetes Rev, 2021; 17(4):437–56. CrossRef
Lacher SE, Lee JS, Wang X, Campbell MR, Bell DA, Slattery M. Beyond antioxidant genes in the ancient Nrf2 regulatory network. Free Radic Biol Med, 2015; 88(Pt B):452–65. CrossRef
Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, Ye JM, Lee CH, Oh WK, Kim CT, Hohnen-Behrens C, Gosby A, Kraegen EW, James DE, Kim JB. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes, 2006; 55(8):2256–64. CrossRef
Leng S, Lu F, Xu L. Therapeutic effects of berberine in impaired glucose tolerance rats and its influence on insulin secretion. Acta Pharmacol Sin, 2004; 25(4):496–502.
Li W, Zhang M, Gu J, Meng Z, Zhao LC, Zheng YN, Chen L, Yang GL. Hypoglycemic effect of protopanaxadiol-type ginsenosides and compound K on type 2 diabetes mice induced by high-fat diet combining with streptozotocin via suppression of hepatic gluconeogenesis. Fitoterapia, 2012; 83(1):192–8. CrossRef
Liu Z, Li W, Li X, Zhang M, Chen L, Zheng Y, Sun GZ, Ruan CC. Antidiabetic effects of malonyl ginsenosides from Panax ginseng on type 2 diabetic rats induced by high-fat diet and streptozotocin. J Ethnopharmacol, 2013; 145(1):233–40. CrossRef
Liu Y, Mu S, Chen W, Liu S, Cong Y, Liu J, Jia N. Saponins of Momordica charantia increase insulin secretion in INS-1 pancreatic β-cells via the PI3K/Akt/FoxO1 signaling pathway. Endocrinol Diabetes Nutr, 2021; 68(5):329–37. CrossRef
Liu W, Son DO, Lau HK, Zhou Y, Prud’homme GJ, Jin T, Wang Q. Combined oral administration of GABA and DPP-4 inhibitor prevents beta cell damage and promotes beta cell regeneration in mice. Front Pharmacol, 2017; 8:362. CrossRef
Liu J, Yin F, Xiao H, Guo L, Gao X. Glucagon-like peptide 1 receptor plays an essential role in geniposide attenuating lipotoxicity-induced β-cell apoptosis. Toxicol In Vitro, 2012; 26(7):1093–7. CrossRef
Liu D, Zhen W, Yang Z, Carter JD, Si H, Reynolds KA. Genistein acutely stimulates insulin secretion in pancreatic beta-cells through a cAMP-dependent protein kinase pathway. Diabetes, 2006; 55(4):1043–50. CrossRef
Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents wogonin, baicalein and baicalin. Cancer Treat Rev, 2009; 35(1):57–68. CrossRef
Lu MC, Ji JA, Jiang ZY, You QD. The keap1-Nrf2-ARE pathway as a potential preventive and therapeutic target: an update. Med Res Rev, 2016; 36(5):924–63. CrossRef
Lucas EA, Dumancas GG, Smith BJ, Clarke SL, Arjmandi BH. Health benefits of bitter melon (Momordica charantia). Bioactive foods in promoting health. Elsevier, Amsterdam, The Netherlands, pp 525–49, 2010. CrossRef
Luo JZ, Luo L. American ginseng stimulates insulin production and prevents apoptosis through regulation of uncoupling protein-2 in cultured beta cells. Evid Based Complement Alternat Med, 2006; 3(3):365–72. CrossRef
Lupi R, Del Guerra S, Marselli L, Bugliani M, Boggi U, Mosca F, Marchetti P, Del Prato S. Rosiglitazone prevents the impairment of human islet function induced by fatty acids: evidence for a role of PPARgamma2 in the modulation of insulin secretion. Am J Physiol Endocrinol Metab, 2004; 286(4):E560–7. CrossRef
Malekshahi H, Bahrami G, Miraghaee S, Ahmadi SA, Sajadimajd S, Hatami R, Mohammadi B, Keshavarzi S. Momordica charantia reverses type II diabetes in rat. J Food Biochem, 2019; 43(11):e13021. CrossRef
Marrano N, Spagnuolo R, Biondi G, Cignarelli A, Perrini S, Vincenti L, Laviola L, Giorgino F, Natalicchio A. Effects of extra virgin olive oil polyphenols on beta-cell function and survival. Plants, 2021; 10(2):286. CrossRef
Mathijs I, Da Cunha DA, Himpe E, Ladriere L, Chellan N, Roux CR, Joubert E, Muller C, Cnop M, Louw J, Bouwens L. Phenylpropenoic acid glucoside augments pancreatic beta cell mass in high-fat diet-fed mice and protects beta cells from ER stress-induced apoptosis. Mol Nutr Food Res, 2014; 58(10):1980–90. CrossRef
Mhaskar KS, Caius JF. A study of Indian medicinal plants. II. Gymnema sylvestre. Br Indian Med Res Memoirs, 1930 (Memoir No. 16):1–49.
Misawa E, Tanaka M, Nomaguchi K, Yamada M, Toida T, Takase M, Iwatsuki K, Kawada T. Administration of phytosterols isolated from Aloe vera gel reduce visceral fat mass and improve hyperglycemia in Zucker diabetic fatty (ZDF) rats. Obes Res Clin Pract, 2008; 2(4):I–II. CrossRef
Montanya E, Nacher V, Biarnés M, Soler J. Linear correlation between beta-cell mass and body weight throughout the lifespan in Lewis rats: role of beta-cell hyperplasia and hypertrophy. Diabetes, 2000; 49(8):1341–6. CrossRef
Muller CJF, Joubert E, Pheiffer C, Ghoor S, Sanderson M, Chellan N, Fey SJ, Louw J. Z-2-(β-D-glucopyranosyloxy)-3-phenylpropenoic acid, an α-hydroxy acid from rooibos (Aspalathus linearis) with hypoglycemic activity. Mol Nutr Food Res, 2013; 57(12):2216–22. CrossRef
Murakami N, Murakami T, Kadoya M, Matsuda H, Yamahara J, Yoshikawa M. New hypoglycemic constituents in “gymnemic acid” from Gymnema sylvestre. Chem Pharm Bull, 1996; 44(2):469–71. CrossRef
Ni Y, Turner D, Yates KM, Tizard I. Isolation and characterization of structural components of Aloe vera L. leaf pulp. Int Immunopharmacol, 2004; 4(14):1745–55. CrossRef
Nie J, Lilley BN, Pan YA, Faruque O, Liu X, Zhang W, Sanes JR, Han X, Shi Y. SAD-A potentiates glucose-stimulated insulin secretion as a mediator of glucagon-like peptide 1 response in pancreatic β cells. Mol Cell Biol, 2013; 33(13):2527–34. CrossRef
Njume C, Donkor O, McAinch AJ. Predisposing factors of type 2 diabetes mellitus and the potential protective role of native plants with functional properties. J Funct Foods, 2019; 53:115–24. CrossRef
Noor A, Gunasekaran S, Manickam AS, Vijayalakshmi MA. Antidiabetic activity of Aloe vera and histology of organs in streptozotocin-induced diabetic rats. Curr Sci, 2008; 94(8):1070–6.
Noor A, Gunasekaran S, Vijayalakshmi MA. Improvement of insulin secretion and pancreatic β-cell function in streptozotocin-induced diabetic rats treated with Aloe vera extract. Pharmacognosy Res, 2017; 9(Suppl 1):S99–104. CrossRef
Oh MR, Park SH, Kim SY, Back HI, Kim MG, Jeon JY, Ha KC, Na WT, Cha YS, Park BH, Park TS, Chae SW. Postprandial glucose-lowering effects of fermented red ginseng in subjects with impaired fasting glucose or type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. BMC Complement Altern Med, 2014; 14:237. CrossRef
Ohno T, Kato N, Ishii C, Shimizu M, Ito Y, Tomono S, Kawazu S. Genistein augments cyclic adenosine 3’5’-monophosphate(cAMP) accumulation and insulin release in MIN6 cells. Endocr Res, 1993; 19(4):273–85. CrossRef
Pamunuwa G, Karunaratne DN, Waisundara VY. Antidiabetic properties, bioactive constituents, and other therapeutic effects of Scoparia dulcis. Evid Based Complement Alternat Med, 2016; 2016:8243215. CrossRef
Park S, Ahn IS, Kwon DY, Ko BS, Jun WK. Ginsenosides Rb1 and Rg1 suppress triglyceride accumulation in 3T3-L1 adipocytes and enhance beta-cell insulin secretion and viability in Min6 cells via PKA-dependent pathways. Biosci Biotechnol Biochem, 2008; 72(11):2815–23. CrossRef
Park EY, Kim HJ, Kim YK, Park SU, Choi JE, Cha JY, Jun HS. Increase in insulin secretion induced by Panax ginseng berry extracts contributes to the amelioration of hyperglycemia in streptozotocininduced diabetic mice. J Ginseng Res, 2012; 36(2):153–60. CrossRef
Park SH, Oh MR, Choi EK, Kim MG, Ha KC, Lee SK, Kim YG, Park BH, Kim DS, Chae SW. An 8-wk, randomized, double-blind, placebo-controlled clinical trial for the antidiabetic effects of hydrolyzed ginseng extract. J Ginseng Res, 2014; 38(4):239–43. CrossRef
Patel MB, Mishra S. Hypoglycemic activity of alkaloidal fraction of Tinospora cordifolia. Phytomedicine, 2011; 18(12):1045–52. CrossRef
Persaud SJ, Al-Majed H, Raman A, Jones PM. Gymnema sylvestre stimulates insulin release in vitro by increased membrane permeability. J Endocrinol, 1999a; 163(2):207–12. CrossRef
Persaud SJ, Harris TE, Burns CJ, Jones PM. Tyrosine kinases play a permissive role in glucose-induced insulin secretion from adult rat islets. J Mol Endocrinol, 1999b; 22(1):19–28. CrossRef
Prasannaraja C, Kamalanathan AS, Vijayalakshmi MA, Venkataraman K. A dipyrrole derivative from Aloe vera inhibits an anti-diabetic drug target dipeptidyl peptidase (DPP)-IV in vitro. Prep Biochem Biotechnol, 2020; 50(5):511–20. CrossRef
Pu P, Wang XA, Salim M, Zhu LH, Wang L, Chen KJ, Xiao JF, Deng W, Shi HW, Jiang H, Li HL. Baicalein, a natural product, selectively activating AMPKα(2) and ameliorates metabolic disorder in diet-induced mice. Mol Cell Endocrinol, 2012; 362(1–2):128–38. CrossRef
Purwana I, Zheng J, Li X, Deurloo M, Son DO, Zhang Z, Liang C, Shen E, Tadkase A, Feng ZP, Li Y, Hasilo C, Paraskevas S, Bortell R, Greiner DL, Atkinson M, Prud’homme GJ, Wang Q. GABA promotes human β-cell proliferation and modulates glucose homeostasis. Diabetes, 2014; 63(12):4197–205. CrossRef
Qadir MMF, Álvarez-Cubela S, Klein D, Lanzoni G, García-Santana C, Montalvo A, Pláceres-Uray F, Mazza EMC, Ricordi C, Inverardi LA, Pastori RL, Domínguez-Bendala J. P2RY1/ALK3-Expressing cells within the adult human exocrine pancreas are BMP-7 expandable and exhibit progenitor-like characteristics. Cell Rep, 2018; 22(9):2408–20. CrossRef
Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab, 2008; 10(Suppl 4):32–42. CrossRef
Rajalakshmi M, Anita R. β-cell regenerative efficacy of a polysaccharide isolated from methanolic extract of Tinospora cordifolia stem on streptozotocin-induced diabetic Wistar rats. Chem Biol Interact, 2016; 243:45–53. CrossRef
Rajalakshmi M, Eliza J, Priya C, Nirmala A, Daisy P. Anti-diabetic properties of Tinospora cordifolia stem extracts on streptozotocin- induced diabetic rats. Afr J Pharm Pharmacol, 2009; 3(5):171–80.
Rajappa R, Sireesh D, Salai MB, Ramkumar KM, Sarvajayakesavulu S, Madhunapantula SV. Treatment with Naringenin elevates the activity of transcription factor Nrf2 to protect pancreatic β-cells from streptozotocin-induced diabetes in vitro and in vivo. Front Pharmacol, 2018; 9:1562. CrossRef
Rajasekaran S, Sivagnanam K, Ravi K, Subramanian S. Hypoglycemic effect of Aloe vera gel on streptozotocin-induced diabetes in experimental rats. J Med Food, 2004; 7(1):61–6. CrossRef
Reeds DN, Patterson BW, Okunade A, Holloszy JO, Polonsky KS, Klein S. Ginseng and ginsenoside re do not improve β-cell function or insulin sensitivity in overweight and obese subjects with impaired glucose tolerance or diabetes. Diabetes Care, 2011; 34(5):1071–6. CrossRef
Reilly JM, Thompson MP. Dietary fatty acids up-regulate the expression of UCP2 in 3T3-L1 preadipocytes. Biochem Biophys Res Commun, 2000; 277(3):541–5. CrossRef
Rizvi SI, Mishra N. Traditional Indian medicines used for the management of diabetes mellitus. J Diabetes Res, 2013; 2013:712092. CrossRef
Sahu PK, Giri DD, Singh R, Pandey P, Gupta S, Shrivastava AK, Kumar A, Dev Pandey K. Therapeutic and medicinal uses of Aloe vera: a review. Pharmacol Pharm, 2013; 04(08):599–610. CrossRef
Salehi B, Ata A, Anil Kumar NV, Sharopov F, Ramírez-Alarcón K, Ruiz-Ortega A, Ayatollahi SA, Fokou PVT, Kobarfard F, Zakaria ZA, Iriti M, Taheri Y, Martorell M, Sureda A, Setzer WN, Durazzo A, Lucarini M, Santini A, Capasso R, Ostrander EA, Rahman A, Choudhary MI, Cho WC, Sharifi-Rad J. Antidiabetic potential of medicinal plants and their active components. Biomolecules, 2019a; 9(10):551. CrossRef
Salehi B, Venditti A, Sharifi-Rad M, Kr?giel D, Sharifi-Rad J, Durazzo A, Lucarini M, Santini A, Souto EB, Novellino E, Antolak H, Azzini E, Setzer WN, Martins N. The therapeutic potential of apigenin. Int J Mol Sci, 2019b; 20(6):1305. CrossRef
Sangeetha MK, Balaji Raghavendran HR, Gayathri V, Vasanthi HR. Tinospora cordifolia attenuates oxidative stress and distorted carbohydrate metabolism in experimentally induced type 2 diabetes in rats. J Nat Med, 2011; 65(3–4):544–50. CrossRef
Senthilkumar M. Phytochemical screening and antibacterial activity of Gymnema sylvestre r. br. ex schult. Int J Pharm Sci Res, 2015; 6(6) :2496.
Seo E, Kim S, Lee SJ, Oh BC, Jun HS. Ginseng berry extract supplementation improves age-related decline of insulin signaling in mice. Nutrients, 2015; 7(4):3038–53. CrossRef
Shanmugasundaram ER, Gopinath KL, Radha Shanmugasundaram K, Rajendran VM. Possible regeneration of the islets of Langerhans in streptozotocin-diabetic rats given Gymnema sylvestre leaf extracts. J Ethnopharmacol, 1990a; 30(3):265–79. CrossRef
Shanmugasundaram ER, Rajeswari G, Baskaran K, Rajesh Kumar BR, Radha Shanmugasundaram K, Kizar Ahmath B. Use of Gymnema sylvestre leaf extract in the control of blood glucose in insulin-dependent diabetes mellitus. J Ethnopharmacol, 1990b; 30(3):281–94. CrossRef
Sharma R, Amin H, Galib, Prajapati PK. Antidiabetic claims of Tinospora cordifolia (Willd.) Miers: critical appraisal and role in therapy. Asian Pac J Trop Biomed, 2015; 5(1):68–78. CrossRef
Sharma BR, Park CM, Kim HA, Kim HJ, Rhyu DY. Tinospora cordifolia preserves pancreatic beta cells and enhances glucose uptake in adipocytes to regulate glucose metabolism in diabetic rats. Phytother Res, 2019; 33(10):2765–74. CrossRef
Shay J, Elbaz HA, Lee I, Zielske SP, Malek MH, Hüttemann M. Molecular mechanisms and therapeutic effects of (-)-epicatechin and other polyphenols in cancer, inflammation, diabetes, and neurodegeneration. Oxid Med Cell Longev, 2015; 2015:181260. CrossRef
Shen W, Taylor B, Jin Q, Nguyen-Tran V, Meeusen S, Zhang YQ, Kamireddy A, Swafford A, Powers AF, Walker J, Lamb J, Bursalaya B, DiDonato M, Harb G, Qiu M, Filippi CM, Deaton L, Turk CN, Suarez-Pinzon WL, Liu Y, Hao X, Mo T, Yan S, Li J, Herman AE, Hering BJ, Wu T, Seidel HM, McNamara P, Glynne R, Laffitte B. Inhibition of DYRK1A and GSK3B induces human β-cell proliferation. Nat Commun, 2015; 6:8372. CrossRef
Shu L, Matveyenko AV, Kerr-Conte J, Cho JH, McIntosh CHS, Maedler K. Decreased TCF7L2 protein levels in type 2 diabetes mellitus correlate with downregulation of GIP- and GLP-1 receptors and impaired beta-cell function. Hum Mol Genet, 2009; 18(13):2388–99. CrossRef
Shu L, Zien K, Gutjahr G, Oberholzer J, Pattou F, Kerr-Conte J, Maedler K. TCF7L2 promotes beta cell regeneration in human and mouse pancreas. Diabetologia, 2012; 55(12):3296–307. CrossRef
Singh N, Gupta M. Regeneration of beta cells in islets of Langerhans of pancreas of alloxan diabetic rats by acetone extract of Momordica charantia (Linn.) (bitter gourd) fruits. Indian J Exp Biol, 2007; 45(12):1055–62.
Singh N, Gupta M, Sirohi P, Varsha. Effects of alcoholic extract of Momordica charantia (Linn.) whole fruit powder on the pancreatic islets of alloxan diabetic albino rats. J Environ Biol, 2008; 29(1):101–6.
Solar M, Cardalda C, Houbracken I, Martín M, Maestro MA, De Medts N, Xu X, Grau V, Heimberg H, Bouwens L, Ferrer J. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell, 2009; 17(6):849–60. CrossRef
Soltani N, Qiu H, Aleksic M, Glinka Y, Zhao F, Liu R, Li Y, Zhang N, Chakrabarti R, Ng T, Jin T, Zhang H, Lu WY, Feng ZP, Prud’homme GJ, Wang Q. GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc Natl Acad Sci USA, 2011; 108(28):11692–7. CrossRef
Son DO, Liu W, Li X, Prud’homme GJ, Wang Q. Combined effect of GABA and glucagon-like peptide-1 receptor agonist on cytokine-induced apoptosis in pancreatic β-cell line and isolated human islets. J Diabetes, 2019; 11(7):563–72. CrossRef
Sorenson RL, Brelje TC, Roth C. Effect of tyrosine kinase inhibitors on islets of Langerhans: evidence for tyrosine kinases in the regulation of insulin secretion. Endocrinology, 1994; 134(4):1975–8. CrossRef
Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet, 2005; 365(9467):1333–46. CrossRef
Sudha P, Zinjarde SS, Bhargava SY, Kumar AR. Potent α-amylase inhibitory activity of Indian ayurvedic medicinal plants. BMC Complement Altern Med, 2011; 11:5. CrossRef
Sugihara Y, Nojima H, Matsuda H, Murakami T, Yoshikawa M, Kimura I. Antihyperglycemic effects of gymnemic acid IV, a compound derived from Gymnema sylvestre leaves in streptozotocin-diabetic mice. J Asian Nat Prod Res, 2000; 2(4):321–7. CrossRef
Sumathi R, Tamizharasi S, Sivakumar T. Bio-dynamic activity of Naringenin–a review. Int J Curr Adv Res, 2015; 4(8):234–6.
Surya S, Salam AD, Tomy DV, Carla B, Kumar RA, Sunil C. Diabetes mellitus and medicinal plants-a review. Asian Pac J Trop Dis, 2014; 4(5):337–47. CrossRef
Suzuki A, Nakauchi H, Taniguchi H. Glucagon-like peptide 1 (1-37) converts intestinal epithelial cells into insulin-producing cells. Proc Natl Acad Sci USA, 2003; 100(9):5034–9. CrossRef
Tahira S, Hussain F. Antidiabetic evaluation of Momordica charantia L fruit extracts. West Indian Med J, 2014; 63(4):294–9. CrossRef
Tanaka M, Misawa E, Ito Y, Habara N, Nomaguchi K, Yamada M, Toida T, Hayasawa H, Takase M, Inagaki M, Higuchi R. Identification of five phytosterols from Aloe vera gel as anti-diabetic compounds. Biol Pharm Bull, 2006; 29(7):1418–22. CrossRef
Tiwari P, Mishra BN, Sangwan NS. Phytochemical and pharmacological properties of Gymnema sylvestre: an important medicinal plant. Biomed Res Int, 2014; 2014:830285. CrossRef
Tokui Y, Kozawa J, Yamagata K, Zhang J, Ohmoto H, Tochino Y, Okita K, Iwahashi H, Namba M, Shimomura I, Miyagawa J. Neogenesis and proliferation of beta-cells induced by human betacellulin gene transduction via retrograde pancreatic duct injection of an adenovirus vector. Biochem Biophys Res Commun, 2006; 350(4):987–93. CrossRef
Tomita T. Apoptosis in pancreatic β-islet cells in type 2 diabetes. Bosn J Basic Med Sci, 2016; 16(3):162–79. CrossRef
Tripathi UN, Chandra D. Anti-hyperglycemic and anti-oxidative effect of aqueous extract of Momordica charantia pulp and Trigonella foenum graecum seed in alloxan-induced diabetic rats. Indian J Biochem Biophys, 2010; 47(4):227–33.
Upadhyay AK, Kumar K, Kumar A, Mishra HS. Tinospora cordifolia (Willd.) Hook. f. and Thoms. (Guduchi)- validation of the ayurvedic pharmacology through experimental and clinical studies. Int J Ayurveda Res, 2010; 1(2):112–21. CrossRef
Venkatesan V, Gopurappilly R, Goteti SK, Dorisetty RK, Bhonde RR. Pancreatic progenitors: the shortest route to restore islet cell mass. Islets, 2011; 3(6):295–301. CrossRef
Von Eckardstein A, Sibler RA. Possible contributions of lipoproteins and cholesterol to the pathogenesis of diabetes mellitus type 2. Curr Opin Lipidol, 2011; 22(1):26–32. CrossRef
Vuksan V, Sievenpiper JL, Koo VY, Francis T, Beljan-Zdravkovic U, Xu Z, Vidgen E. American ginseng (Panax quinquefolius L) reduces postprandial glycemia in nondiabetic subjects and subjects with type 2 diabetes mellitus. Arch Intern Med, 2000; 160(7):1009–13. CrossRef
Vuksan V, Sung MK, Sievenpiper JL, Stavro PM, Jenkins AL, Di Buono M, Lee KS, Leiter LA, Nam KY, Arnason JT, Choi M, Naeem A. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutr Metab Cardiovasc Dis, 2008; 18(1):46–56. CrossRef
Waisundara VY, Hsu A, Tan BKH, Huang D. Baicalin reduces mitochondrial damage in streptozotocin-induced diabetic Wistar rats. Diabetes Metab Res Rev, 2009; 25(7):671–7. CrossRef
Wakitani S, Takaoka K, Hattori T, Miyazawa N, Iwanaga T, Takeda S, Watanabe TK, Tanigami A. Embryonic stem cells injected into the mouse knee joint form teratomas and subsequently destroy the joint. Rheumatology, 2003; 42(1):162–5. CrossRef
Wållberg M, Cooke A. Immune mechanisms in type 1 diabetes. Trends Immunol, 2013; 34(12):583–91. CrossRef
Wang N, Yi WJ, Tan L, Zhang JH, Xu J, Chen Y, Qin M, Yu S, Guan J, Zhang R. Apigenin attenuates streptozotocin-induced pancreatic β cell damage by its protective effects on cellular antioxidant defense. In Vitro Cell Dev Biol Anim, 2017; 53(6):554–63. CrossRef
Watson RR, Preedy VR. Bioactive food as dietary interventions for diabetes: bioactive foods in chronic disease states. Academic Press,Cambridge, MA, 2012.
Wei S, Li W, Yu Y, Yao F, Lixiang A, Lan X, Guan F, Zhang M, Chen L. Ginsenoside compound K suppresses the hepatic gluconeogenesis via activating adenosine-5’monophosphate kinase: a study in vitro and in vivo. Life Sci, 2015; 139:8–15. CrossRef
Weir GC. Glucolipotoxicity, β-cells, and diabetes: the emperor has no clothes. Diabetes, 2020; 69(3):273–8. CrossRef
Xiang L, Huang X, Chen L, Rao P, Ke L. The reparative effects of Momordica Charantia Linn. extract on HIT-T15 pancreatic beta-cells. Asia Pac J Clin Nutr, 2007; 16(Suppl 1):249–52.
Xu M, Sun B, Li D, Mao R, Li H, Li Y, Wang J. Beneficial effects of small molecule oligopeptides isolated from Panax ginseng meyer on pancreatic beta-cell dysfunction and death in diabetic rats. Nutrients, 2017; 9(10):1061. CrossRef
Yagi A, Hamano S, Tanaka T, Kaneo Y, Fujioka T, Mihashi K. Biodisposition of FITC-labeled aloemannan in mice. Planta Med, 2001; 67(4):297–300. CrossRef
Yagi A, Hegazy S, Kabbash A, Wahab EAE. Possible hypoglycemic effect of Aloe vera L. high molecular weight fractions on type 2 diabetic patients. Saudi Pharm J, 2009; 17(3):209–15. CrossRef
Yamamoto K, Miyagawa J, Waguri M, Sasada R, Igarashi K, Li M, Nammo T, Moriwaki M, Imagawa A, Yamagata K, Nakajima H, Namba M, Tochino Y, Hanafusa T, Matsuzawa Y. Recombinant human betacellulin promotes the neogenesis of beta-cells and ameliorates glucose intolerance in mice with diabetes induced by selective alloxan perfusion. Diabetes, 2000; 49(12):2021–7. CrossRef
Yang Z, Huang W, Zhang J, Xie M, Wang X. Baicalein improves glucose metabolism in insulin resistant HepG2 cells. Eur J Pharmacol, 2019; 854:187–93. CrossRef
Yang MS, Wu MY. Chinese ginseng. Nutraceuticals. Elsevier, Amsterdam, The Netherlands, pp 693–705, 2016. CrossRef
Yao DD, Yang L, Wang Y, Liu C, Wei YJ, Jia XB, Yin W, Shu L. Geniposide promotes beta-cell regeneration and survival through regulating β-catenin/TCF7L2 pathway. Cell Death Dis, 2015; 6:e1746. CrossRef
Yin J, Zhang H, Ye J. Traditional Chinese medicine in treatment of metabolic syndrome. Endocr Metab Immune Disord Drug Targets, 2008; 8(2):99–111. CrossRef
Yoon JW, Kang SM, Vassy JL, Shin H, Lee YH, Ahn HY, Choi SH, Park KS, Jang HC, Lim S. Efficacy and safety of ginsam, a vinegar extract from Panax ginseng, in type 2 diabetic patients: results of a double-blind, placebo-controlled study. J Diabetes Investig, 2012; 3(3):309–17. CrossRef
Yongchaiyudha S, Rungpitarangsi V, Bunyapraphatsara N, Chokechaijaroenporn O. Antidiabetic activity of Aloe vera L. juice. I. Clinical trial in new cases of diabetes mellitus. Phytomedicine, 1996; 3(3):241–3. CrossRef
Zanker KS, Mang B, Wolters M, Hahn A. Personalized diabetes and cancer medicine: a rationale for anti-diabetic nutrition (Bitter Melon) in a supportive setting. Curr Cancer Ther Rev, 2012; 8(1):66–77. CrossRef
Zhang Z, Hu Y, Xu N, Zhou W, Yang L, Chen R, Yang R, Sun J, Chen H. A new way for beta cell neogenesis: transdifferentiation from alpha cells induced by glucagon-like peptide 1. J Diabetes Res, 2019; 2019:2583047. CrossRef
Zhang Y, Liu W, Liu D, Zhao T, Tian H. Efficacy of Aloe Vera supplementation on prediabetes and early non-treated diabetic patients: a systematic review and meta-analysis of randomized controlled trials. Nutrients, 2016; 8(7):388. CrossRef
Zhang D, Shen M, Mikita A, Zhang W, Liu Y, Liu Q, Dai Y, Zhang C, Zheng S, Zheng XX. Targeting uncoupling protein-2 improves islet graft function. Cell Transplant, 2011; 20(3):421–9. CrossRef
Zhou L, Wang X, Shao L, Yang Y, Shang W, Yuan G, Jiang B, Li F, Tang J, Jing H, Chen M. Berberine acutely inhibits insulin secretion from beta-cells through 3’,5’-cyclic adenosine 5’-monophosphate signaling pathway. Endocrinology, 2008; 149(9):4510–8. CrossRef
Zhou J, Zhou S, Tang J, Zhang K, Guang L, Huang Y, Xu Y, Ying Y, Zhang L, Li D. Protective effect of berberine on beta cells in streptozotocin- and high-carbohydrate/high-fat diet-induced diabetic rats. Eur J Pharmacol, 2009; 606(1–3):262–8. CrossRef