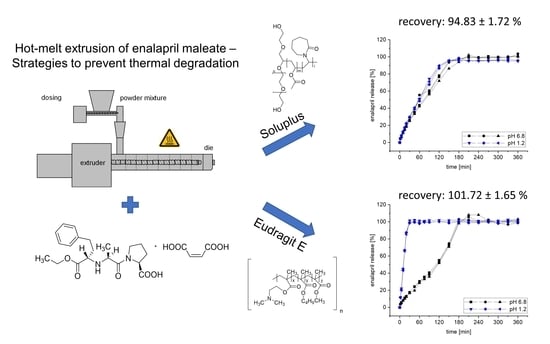
Hot-Melt Extrusion of the Thermo-Sensitive Peptidomimetic Drug Enalapril Maleate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Screening Experiments for HME
2.3. Formulations for HME at Reduced Temperatures
2.4. Particle Size Distribution
2.5. Scanning Electron Microscopy (SEM) Imaging
2.6. Thermal Analysis
2.7. Drug Content of Extrudates
2.7.1. Screening Experiments
2.7.2. Samples from Extrusion at Reduced Temperatures
2.8. FT-IR Spectra Measurements
2.9. Dissolution
3. Results and Discussion
3.1. Raw Material Properties
3.1.1. Particle Size Distribution
3.1.2. Scanning Electron Microscopy (SEM) Imaging
3.1.3. Thermal Analysis
3.2. Polymer Selection for HME
3.2.1. Screening Experiments
3.2.2. Optimized Process Conditions
3.3. Drug Content of Extrudates
3.3.1. Content Uniformity of the Extrudates of the Screening Experiments
3.3.2. Content Uniformity for the Formulations under Optimized Conditions
3.4. FT-IR Spectra Measurements
3.5. Dissolution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACEI | angiotensin-converting enzyme inhibitor |
| Ala | alanine |
| BCS | Biopharmaceutics Classification System |
| bPMMA | basic butylated methacrylate copolymer |
| CRS | chemical reference standard |
| CV | coefficient of variation |
| DKP | diketopiperazine derivative |
| DSC | Dynamic Scanning Calorimetry |
| DTG | Derivative Thermogravimetric Analysis |
| EM | enalapril maleate |
| ENP | enalapril |
| FDM | Fused Deposition Modeling |
| FT-IR | Fourier transform-infrared spectroscopy |
| HME | hot melt extrusion |
| HPLC | High Performance Liquid Chromatography |
| HPMC | hydroxypropyl methyl cellulose, hypromellose |
| Imp | impurity |
| K | Kollidon |
| LOD | limit of detection |
| LOQ | limit of quantification |
| PF | Pyrogen-free |
| Phe | phenylalanine |
| Pro | proline |
| SD | standard deviation |
| SEM | Scanning Electron Microscopy |
| SiO2 | fumed silica |
| SOL | Soluplus |
| Tg | glass transition temperature |
| TGA | thermogravimetric analysis |
| VA | poly(vinylpyrrolidone-vinyl acetate)-copolymer |
| WHO | World Health Organization |
References
- WHO Model List of Essential Medicines—22nd list. 2021. Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02 (accessed on 24 July 2022).
- Ip, D.P.; Brenner, G.S. Enalapril maleate. In Analytical Profiles of Drug Substances; Florey, K., Ed.; Academic Press: Cambridge, MA, USA, 1987; Volume 16, pp. 207–243. [Google Scholar]
- Steinhilber, D.; Schubert-Zsilavecz, M.; Roth, H.J. Medizinische Chemie: Targets, Arzneistoffe, Chemische Biologie; Deutscher Apotheker Verlag: Stuttgart, Germany, 2010; pp. 214–253. [Google Scholar]
- Verbeeck, R.K.; Kanfer, I.; Löbenberg, R.; Abrahamsson, B.; Cristofoletti, R.; Groot, D.W.; Langguth, P.; Polli, J.E.; Parr, A.; Shah, V.P.; et al. Biowaiver monographs for immediate-release solid oral dosage forms: Enalapril. J. Pharm. Sci. 2017, 106, 1933–1943. [Google Scholar] [CrossRef]
- Commentary on Ph. Eur. 9.0, 2017. 57th supply, enalapril maleate. In Commentary on the European Pharmacopoeia; Wissenschaftliche Verlagsgesellschaft mbH: Stuttgart, Germany; Govi-Verlag: Eschborn, Germany, 2017.
- Lick, I.D.; Villalba, M.L.; Gavernet, L. Synthesis of diketopiperazine: A kinetic study by means of thermoanalytical methods. Thermochim. Acta 2012, 527, 143–147. [Google Scholar] [CrossRef]
- Sadia, M.; Isreb, A.; Abbadi, I.; Isreb, M.; Aziz, D.; Selo, A.; Timmins, P.; Alhnan, M.A. From ‘fixed dose combinations’ to ‘a dynamic dose combiner’: 3D printed bi-layer antihypertensive tablets. Eur. J. Pharm. Sci. 2018, 123, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cao, F.; Zhang, C.; Ping, Q. Use of polymer combinations in the preparation of solid dispersions of a thermally unstable drug by hot-melt extrusion. Acta Pharm. Sin. B 2013, 3, 263–272. [Google Scholar] [CrossRef]
- Simões, M.F.; Pinto, R.M.A.; Simões, S. Hot-Melt Extrusion: A roadmap for product development. AAPS PharmSciTech 2021, 22, 184. [Google Scholar] [CrossRef]
- Breitenbach, J. Melt extrusion: From process to drug delivery technology. Eur. J. Pharm. Biopharm. 2002, 54, 107–117. [Google Scholar] [CrossRef]
- Almeida, A.; Possemiers, S.; Boone, M.; De Beer, T.; Quinten, T.; Van Hoorebeke, L.; Remon, J.P.; Vervaet, C. Ethylene vinyl acetate as matrix for oral sustained release dosage forms produced via hot-melt extrusion. Eur. J. Pharm. Biopharm. 2011, 77, 297–305. [Google Scholar] [CrossRef]
- Verhoeven, E.; De Beer, T.; Schacht, E.; Van den Mooter, G.; Remon, J.; Vervaet, C. Influence of polyethylene glycol/polyethylene oxide on the release characteristics of sustained-release ethylcellulose mini-matrices produced by hot-melt extrusion: In vitro and in vivo evaluations. Eur. J. Pharm. Biopharm. 2009, 72, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Gryczke, A.; Schminke, S.; Maniruzzaman, M.; Beck, J.; Douroumis, D. Development and evaluation of orally disintegrating tablets (ODTs) containing Ibuprofen granules prepared by hot melt extrusion. Colloids Surf. B 2011, 86, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Witzleb, R.; Kanikanti, V.-R.; Hamann, H.-J.; Kleinebudde, P. Solid lipid extrusion with small die diameters—Electrostatic charging, taste masking and continuous production. Eur. J. Pharm. Biopharm. 2011, 77, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Djemai, A.; Gendron, C.M.; Xi, H.; Smith, M.; Kogan, J.; Li, L. Development of a HPMC-based controlled release formulation with hot melt extrusion (HME). Drug Dev. Ind. Pharm. 2013, 39, 1070–1083. [Google Scholar] [CrossRef] [PubMed]
- Crowley, M.M.; Zhang, F.; Repka, M.A.; Thumma, S.; Upadhye, S.B.; Kumar Battu, S.; McGinity, J.W.; Martin, C. Pharmaceutical Applications of Hot-Melt Extrusion: Part I. Drug Dev. Ind. Pharm. 2007, 33, 909–926. [Google Scholar] [CrossRef]
- Ghosh, I.; Vippagunta, R.; Li, S.; Vippagunta, S. Key considerations for optimization of formulation and melt-extrusion process parameters for developing thermosensitive compound. Pharm. Dev. Technol. 2012, 17, 502–510. [Google Scholar] [CrossRef] [PubMed]
- DiNunzio, J.C.; Brough, C.; Hughey, J.R.; Miller, D.A.; Williams, R.O.; McGinity, J.W. Fusion production of solid dispersions containing a heat-sensitive active ingredient by hot melt extrusion and Kinetisol® dispersing. Eur. J. Pharm. Biopharm. 2010, 74, 340–351. [Google Scholar] [CrossRef]
- Huang, S.; O’Donnell, K.P.; Delpon de Vaux, S.M.; O’Brien, J.; Stutzman, J.; Williams, R.O. Processing thermally labile drugs by hot-melt extrusion: The lesson with gliclazide. Eur. J. Pharm. Biopharm. 2017, 119, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Xie, Z.; Rao, Q.; Liamas, E.; Pan, P.; Guan, S.; Zhang, Z.J.; Lu, M.; Li, Q. Hot melt extrusion of heat-sensitive and high melting point drug: Inhibit the recrystallization of the prepared amorphous drug during extrusion to improve the bioavailability. Int. J. Pharm. 2019, 565, 316–324. [Google Scholar] [CrossRef]
- Kulkarni, C.; Kelly, A.L.; Gough, T.; Jadhav, V.; Singh, K.; Paradkar, A. Application of hot melt extrusion for improving bioavailability of artemisinin a thermolabile drug. Drug Dev. Ind. Pharm. 2018, 44, 206–214. [Google Scholar] [CrossRef]
- Bhardwaj, S.P.; Singh, S. Study of forced degradation behavior of enalapril maleate by LC and LC–MS and development of a validated stability-indicating assay method. J. Pharm. Biomed. Anal. 2008, 46, 113–120. [Google Scholar] [CrossRef]
- Koppala, S.; Ranga Reddy, V.; Anireddy, S. User-Friendly HPLC Method Development and Validation for Determination of Enalapril Maleate and Its Impurities in Enalapril Tablets. J. Chromatogr. Sci. 2017, 55, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Šalamoun, J.; Šlais, K. Elimination of peak splitting in the liquid chromatography of the proline-containing drug enalapril maleate. J. Chromatogr. A 1991, 537, 249–257. [Google Scholar] [CrossRef]
- Tidau, M.; Kwade, A.; Finke, J.H. Influence of High, Disperse API Load on Properties along the Fused-Layer Modeling Process Chain of Solid Dosage Forms. Pharmaceutics 2019, 11, 194. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Wang, S.-L.; Chen, T.-F.; Hu, T.-C. Intramolecular cyclization of diketopiperazine formation in solid-state enalapril maleate studied by thermal FT-IR microscopic system. Eur. J. Pharm. Biopharm. 2002, 54, 249–254. [Google Scholar] [CrossRef]
- mez Pineda, E.A.; Martins Ferrarezi, A.D.; Ferrarezi, J.G.; Winkler Hechenleitner, A.A. Thermal decomposition of enalapril maleate studied by dynamic isoconversional method. J. Therm. Anal. Calorim. 2005, 79, 259–262. [Google Scholar] [CrossRef]
- de Souza, S.M.M.; e Melo Franco, P.I.B.; Leles, M.I.G.; da Conceição, E.C. Evaluation of thermal stability of enalapril maleate tablets using thermogravimetry and differential scanning calorimetry. J. Therm. Anal. Calorim. 2016, 123, 1943–1949. [Google Scholar] [CrossRef]
- BASF, Meeting Formulation Challenges for Poorly Soluble Drugs. Available online: https://ipecamericas.org/sites/default/files/ef12april24-hall.b%233-nigel.langley(basf).pdf (accessed on 24 July 2022).
- Gupta, S.S.; Meena, A.; Parikh, T.; Serajuddin, A.T.M. Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion—I: Polyvinylpyrrolidone and related polymers. J. Excip. Food Chem. 2014, 5, 32–45. [Google Scholar]
- O’Donnell, K.P.; Woodward, W.H.H. Dielectric spectroscopy for the determination of the glass transition temperature of pharmaceutical solid dispersions. Drug Dev. Ind. Pharm. 2015, 41, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Ilyés, K.; Kovács, N.K.; Balogh, A.; Borbás, E.; Farkas, B.; Casian, T.; Marosi, G.; Tomuță, I.; Nagy, Z.K. The applicability of pharmaceutical polymeric blends for the fused deposition modelling (FDM) 3D technique: Material considerations–printability–process modulation, with consecutive effects on in vitro release, stability and degradation. Eur. J. Pharm. Sci. 2019, 129, 110–123. [Google Scholar] [CrossRef]
- Parikh, T.; Gupta, S.S.; Meena, A.; Serajuddin, A.T. Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion-III: Polymethacrylates and polymethacrylic acid based polymers. J. Excip. Food Chem. 2016, 5, 1003. [Google Scholar]
- Kempin, W.; Domsta, V.; Grathoff, G.H.; Brecht, I.; Semmling, B.; Tillmann, S.; Weitschies, W.; Seidlitz, A. Immediate Release 3D-Printed Tablets Produced Via Fused Deposition Modeling of a Thermo-Sensitive Drug. Pharm. Res. 2018, 35, 124. [Google Scholar] [CrossRef]
- Kollamaram, G.; Croker, D.M.; Walker, G.M.; Goyanes, A.; Basit, A.W.; Gaisford, S. Low temperature fused deposition modeling (FDM) 3D printing of thermolabile drugs. Int. J. Pharm. Pharm. Sci. 2018, 545, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, I.; Snyder, J.; Vippagunta, R.; Alvine, M.; Vakil, R.; Tong, W.-Q.; Vippagunta, S. Comparison of HPMC based polymers performance as carriers for manufacture of solid dispersions using the melt extruder. Int. J. Pharm. Pharm. Sci. 2011, 419, 12–19. [Google Scholar] [CrossRef]
- Gupta, S.S.; Solanki, N.; Serajuddin, A.T.M. Investigation of Thermal and Viscoelastic Properties of Polymers Relevant to Hot Melt Extrusion, IV: Affinisol™ HPMC HME Polymers. AAPS PharmSciTech 2016, 17, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; O’Donnell, K.P.; Keen, J.M.; Rickard, M.A.; McGinity, J.W.; Williams, R.O. A New Extrudable Form of Hypromellose: AFFINISOL™ HPMC HME. AAPS PharmSciTech 2016, 17, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Prasad, E.; Islam, M.T.; Goodwin, D.J.; Megarry, A.J.; Halbert, G.W.; Florence, A.J.; Robertson, J. Development of a hot-melt extrusion (HME) process to produce drug loaded Affinisol™ 15LV filaments for fused filament fabrication (FFF) 3D printing. Addit. Manuf. 2019, 29, 100776. [Google Scholar] [CrossRef]
- Gültekin, H.E.; Tort, S.; Acartürk, F. An Effective Technology for the Development of Immediate Release Solid Dosage Forms Containing Low-Dose Drug: Fused Deposition Modeling 3D Printing. Pharm. Res. 2019, 36, 128. [Google Scholar] [CrossRef]
- BASF, Technical Information Soluplus®. Available online: https://pharma.basf.com/products/soluplus (accessed on 24 July 2022).
- Meena, A.; Parikh, T.; Gupta, S.S.; Serajuddin, A.T. Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion-II: Cellulosic polymers. J. Excip. Food Chem. 2016, 5, 1002. [Google Scholar]
- Bhattarai, P.; Katuwal, T. Spectroscopic study of Enalapril Maleate and Hydrochlorothiazide. Int. J. Phys. Appl. 2021, 9, 11–14. [Google Scholar]
- Widjaja, E.; Lim, G.H.; Chow, P.S.; Tan, S. Multivariate data analysis as a tool to investigate the reaction kinetics of intramolecular cyclization of enalapril maleate studied by isothermal and non-isothermal FT-IR microscopy. Eur. J. Pharm. Sci. 2007, 32, 349–356. [Google Scholar]
- Evonik, Technical Information EUDRAGIT® E 100, EUDRAGIT® E PO and EUDRAGIT® E 12,5. Available online: www.pharmaexcipients.com/wp-content/uploads/attachments/TI-EUDRAGIT-E-100-E-PO-E-12-5-EN.pdf?t=1508413942 (accessed on 24 July 2022).
- Liu, J.; Zou, M.; Piao, H.; Liu, Y.; Tang, B.; Gao, Y.; Ma, N.; Cheng, G. Characterization and pharmacokinetic study of aprepitant solid dispersions with Soluplus®. Molecules 2015, 20, 11345–11356. [Google Scholar] [CrossRef] [PubMed]
- BASF; Yidan, L.; Shaukat, A.; Nigel, L. Characterization of Soluplus® by FTIR and Raman Spectroscopy. In Proceedings of the CRS 2010 Annual Conference, Portland, OR, USA, 10–14 July 2010. [Google Scholar]
- Joshi, Y.; Muppalaneni, S.; Omidian, A.; Mastropietro, D.J.; Omidian, H. Determining abuse deterrence performance of poly (ethylene oxide) using a factorial design. Adv. Pharm. Bull. 2018, 8, 495. [Google Scholar] [CrossRef]
- Ramírez-Rigo, M.V.; Olivera, M.E.; Rubio, M.; Manzo, R.H. Enhanced intestinal permeability and oral bioavailability of enalapril maleate upon complexation with the cationic polymethacrylate Eudragit E100. Eur. J. Pharm. Sci. 2014, 55, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-L.; Lin, S.-Y.; Chen, T.-F.; Cheng, W.-T. Eudragit E accelerated the diketopiperazine formation of enalapril maleate determined by thermal FTIR Microspectroscopic technique. Pharm. Res. 2004, 21, 2127–2132. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Wang, S.-L. Advances in simultaneous DSC–FTIR microspectroscopy for rapid solid-state chemical stability studies: Some dipeptide drugs as examples. Adv. Drug Deliv. Rev. 2012, 64, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pang, H.; Guo, Z.; Lin, L.; Dong, Y.; Li, G.; Lu, M.; Wu, C. Interactions between drugs and polymers influencing hot melt extrusion. J. Pharm. Pharmacol. 2014, 66, 148–166. [Google Scholar] [CrossRef]
- Lima, D.M.; dos Santos, L.D.; Lima, E.M. Stability and in vitro release profile of enalapril maleate from different commercially available tablets: Possible therapeutic implications. J. Pharm. Biomed. Anal. 2008, 47, 934–937. [Google Scholar] [CrossRef]











| Formulation | Matrix (%) | Plasticizer (%) | Glidant (%) | |||||
|---|---|---|---|---|---|---|---|---|
| F1 | K 12 PF | 74.5 | PEG 6.000 | 15 | SiO2 | 0.5 | ||
| F2 | HPMC | 74.5 | bPMMA | 10 | PEG 6.000 | 5 | SiO2 | 0.5 |
| F3 | K 12 PF | 30 | K VA 64 | 30 | PEG 6.000 | 29.5 | SiO2 | 0.5 |
| F4 | HPMC | 84.5 | PEG 6.000 | 5 | SiO2 | 0.5 | ||
| F5 | bPMMA | 44 | PEO | 44 | SiO2 | 2.0 | ||
| F6 | SOL | 44.75 | PEO | 44.75 | SiO2 | 0.5 | ||
| Formulation | Powder Feed Rate (g/h) | Screw Speed (1/min) | Temperature (°C) |
|---|---|---|---|
| F1 | 50 | 50 | 130 |
| F2 | 50 | 25 | 130 |
| F3 | 50 | 25 | 140 |
| F4 | 50 | 35 | 140 |
| F5 | 100 | 35 | 150 |
| F6 | 100 | 25 | 100 |
| Formulation | Zone 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Die |
|---|---|---|---|---|---|---|---|---|---|
| F5 | 20 | 20 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| F5 | 20 | 20 | 70 | 70 | 70 | 70 | 70 | 70 | 70 |
| F6 | 20 | 20 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Time [min] | Acetonitrile (% v/v) | Buffer (% v/v) |
|---|---|---|
| 0–5.0 | 5 | 95 |
| 5.0–8.0 | 5 → 25 | 95 → 75 |
| 8.0–16.0 | 25 | 75 |
| 16.0–24.0 | 25 → 55 | 75 → 45 |
| 24.0–26.0 | 55 | 45 |
| 26.0–26.1 | 55 → 95 | 45 → 5 |
| 26.1–28.0 | 95 | 5 |
| 28.0–28.1 | 95 → 5 | 5 → 95 |
| 28.1–30.0 | 5 | 95 |
| Time [min] | Acetonitrile (% v/v) | Buffer (% v/v) |
|---|---|---|
| 0–1.0 | 2 | 98 |
| 1.0–1.2 | 2 → 25 | 98 → 75 |
| 1.2–5.0 | 25 | 75 |
| 5.0–7.5 | 25 → 40 | 75 → 60 |
| 7.5–9.0 | 40 → 75 | 60 → 25 |
| 9.0–11.0 | 75 → 95 | 25 → 5 |
| 11.0–12.5 | 95 | 5 |
| 12.5–12.6 | 95 → 2 | 5 → 98 |
| 12.6–15.0 | 2 | 98 |
| Substance | x10 (µm) | x50 (µm) | x90 (µm) |
|---|---|---|---|
| Enalapril maleate | 6.2 ± 0.1 | 47.2 ± 1.3 | 181 ± 6.9 |
| bPMMA | 3.8 ± 0.1 | 9.7 ± 0.1 | 37.3 ± 26.9 |
| SOL | 191 ± 4.6 | 308 ± 6.1 | 483 ± 5.6 |
| PEO | 14 ± 0.6 | 111 ± 8.6 | 320 ± 15.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffmann, L.; Breitkreutz, J.; Quodbach, J. Hot-Melt Extrusion of the Thermo-Sensitive Peptidomimetic Drug Enalapril Maleate. Pharmaceutics 2022, 14, 2091. https://doi.org/10.3390/pharmaceutics14102091
Hoffmann L, Breitkreutz J, Quodbach J. Hot-Melt Extrusion of the Thermo-Sensitive Peptidomimetic Drug Enalapril Maleate. Pharmaceutics. 2022; 14(10):2091. https://doi.org/10.3390/pharmaceutics14102091
Chicago/Turabian StyleHoffmann, Lena, Jörg Breitkreutz, and Julian Quodbach. 2022. "Hot-Melt Extrusion of the Thermo-Sensitive Peptidomimetic Drug Enalapril Maleate" Pharmaceutics 14, no. 10: 2091. https://doi.org/10.3390/pharmaceutics14102091