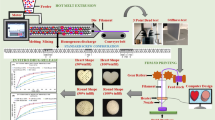
Abstract
Purpose
Fabrication of immediate release (IR) tablet formulations with rapid release profile via fused deposition modeling 3D printing (FDM 3DP) is a challenge. The aims of this study were to prepare IR tablets with different dissolution profiles and to increase their in vitro dissolution rates by making physical modifications on them. Pramipexole was used as the model low-dose drug.
Methods
Polymeric filaments were prepared with six different combinations of Eudragit EPO and poly(ethylene) oxide by hot melt extrusion and 3D tablets were produced using an FDM printer. Characterization studies for the filaments and tablets were carried out. The printability of the filaments was also evaluated using a novel mechanical characterization method. Tablet formulation with optimum dissolution profile was chosen and physical modifications (infill %, shape change and thickness) on this formulation were made.
Results
Low-dose pramipexole loading filaments and 3D tablets were homogenously prepared. The printability of the filaments was related to their flexibility. With the physical modifications, the drug release completion time of the tablets reduced to 5 min.
Conclusions
The same rapid release profiles with conventional IR tablets can be reached by making only physical changes on 3D tablets without using any filling or disintegrating agents.









Similar content being viewed by others
Abbreviations
- 3DP:
-
3D printing
- ABS:
-
Acrylonitrile butadiene styrene
- AM:
-
Additive manufacturing
- API:
-
Active pharmaceutical ingredient
- CAD:
-
Computer aided design
- DSC:
-
Differential scanning calorimetry
- FDM:
-
Fused deposition modeling
- HME:
-
Hot melt extrusion
- IR:
-
Immediate release
- PC:
-
Polycarbonate
- PCL:
-
Poly(ε-caprolactone)
- PLA:
-
Polylactic acid
- PPD:
-
Pramipexole dihydrochloride monohydrate
- PVA:
-
Polyvinyl alcohol
- RP:
-
Rapid prototyping
- SEM:
-
Scanning electron microscopy
References
Gross BC, Erkal JL, Lockwood SY, Chen C, Spence DM. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal Chem. 2014;86(7):3240–53.
Melocchi A, Parietti F, Loreti G, Maroni A, Gazzaniga A, Zema L. 3D printing by fused deposition modeling (FDM) of a swellable/erodible capsular device for oral pulsatile release of drugs. J Drug Deliv Sci Tec. 2015;30:360–7.
Norman J, Madurawe RD, Moore CM, Khan MA, Khairuzzaman A. A new chapter in pharmaceutical manufacturing 3D-printed drug products. Adv Drug Deliv Rev. 2017;108:39–50.
Voelker R. The printed pill. JAMA. 2015;314:1108.
Gioumouxouzis CI, Katsamenis OL, Bouropoulos N, Fatouros DG. 3D printed oral solid dosage forms containing hydrochlorothiazide for controlled drug delivery. J Drug Deliv Sci Tec. 2017;40:164–71.
Wang J, Goyanes A, Gaisford S, Basit AW. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int J Pharm. 2016;503(1–2):207–12.
Nandwana P, Elliott AM, Siddel D, Merriman A, Peter WH, Babu SS. Powder bed binder jet 3D printing of Inconel 718: densification, microstructural evolution and challenges. Curr Opin Solid St M. 2017;21(4):207–18.
Lee JY, An J, Chua CK. Fundamentals and applications of 3D printing for novel materials. Appl Mater Today. 2017;7:120–33.
Goyanes A, Kobayashi M, Martínez-Pacheco R, Gaisford S, Basit AW. Fused-filament 3D printing of drug products: microstructure analysis and drug release characteristics of PVA-based caplets. Int J Pharm. 2016;514(1):290–5.
Awad A, Trenfield SJ, Goyanes A, Gaisford S, Basit AW. Reshaping drug development using 3D printing. Drug Discov Today. 2018;23:1547–55.
Novakova-Marcincinova L, Kuric I. Basic and advanced materials for fused deposition modeling rapid prototyping technology. Manuf and Ind Eng. 2012;11(1):24–7.
Genina N, Holländer J, Jukarainen H, Mäkilä E, Salonen J, Sandler N. Ethylene vinyl acetate (EVA) as a new drug carrier for 3D printed medical drug delivery devices. Eur J Pharm Sci. 2016;90:53–63.
Repka MA, Battu SK, Upadhye SB, Thumma CMM, Zhang F, Martin C, et al. Pharmaceutical applications of hot-melt extrusion: part II. Drug Dev Ind Pharm. 2007;33(10):1043–57.
Goyanes A, Chang H, Sedough D, Hatton GB, Wang J, Buanz A, et al. Fabrication of controlled-release budesonide tablets via desktop (FDM) 3D printing. Int J Pharm. 2015;496(2):414–20.
Holländer J, Genina N, Jukarainen H, Khajeheian M, Rosling A, Mäkilä E, et al. Three-dimensional printed PCL-based implantable prototypes of medical devices for controlled drug delivery. J Pharm Sci. 2016;105(9):2665–76.
Lim SH, Chia SMY, Kang L, Yap KYL. Three-dimensional printing of carbamazepine sustained-release scaffold. J Pharm Sci. 2016;105(7):2155–63.
Jamróz W, Kurek M, Łyszczarz E, Szafraniec J, Knapik-Kowalczuk J, Syrek K, et al. 3D printed orodispersible films with aripiprazole. Int J Pharm. 2017;533(2):413–20.
Okwuosa TC, Soares C, Gollwitzer V, Habashy R, Timmins P, Alhnan MA. On demand manufacturing of patient-specific liquid capsules via co-ordinated 3D printing and liquid dispensing. Eur J Pharm Sci. 2018;118:134–43.
Melocchi A, Parietti F, Maccagnan S, Ortenzi MA, Antenucci S, Briatico-Vangosa F, et al. Industrial development of a 3D-printed nutraceutical delivery platform in the form of a multicompartment HPC capsule. AAPS Pharm Sci Tech. 2018;19(8):3343–54.
Gioumouxouzis CI, Baklavaridis A, Katsamenis OL, Markopoulou CK, Bouropoulos N, Tzetzis D, et al. A 3D printed bilayer oral solid dosage form combining metformin for prolonged and glimepiride for immediate drug delivery. Eur J Pharm Sci. 2018;120:40–52.
Muwaffak Z, Goyanes A, Clark V, Basit AW, Hilton ST, Gaisford S. Patient-specific 3D scanned and 3D printed antimicrobial polycaprolactone wound dressings. Int J Pharm. 2017;527:161–70.
Chai X, Chai H, Wang X, Yang J, Li J, Zhao Y, et al. Fused deposition modeling (FDM) 3D printed tablets for intragastric floating delivery of domperidone. Sci Rep. 2017;7:2829.
Kempin W, Domsta V, Grathoff G, Brecht I, Semmling B, Tillmann S, et al. Immediate release 3D-printed tablets produced via fused deposition modeling of a Thermo-sensitive drug. Pharm Res. 2018;35(6):124.
Sadia M, Arafat B, Ahmed W, Forbes RT, Alhnan MA. Channelled tablets: An innovative approach to accelerating drug release from 3D printed tablets. J Control Release. 2018;269:355–63.
Arafat B, Wojsz M, Isreb A, Forbes RT, Isreb M, Ahmed W, et al. Tablet fragmentation without a disintegrant: a novel design approach for accelerating disintegration and drug release from 3D printed cellulosic tablets. Eur J Pharm Sci. 2018;118:191–9.
Goyanes A, Buanz AB, Hatton GB, Gaisford S, Basit AW. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur J Pharm Biopharm. 2015;89:157–62.
Pietrzak K, Isreb A, Alhnan MA. A flexible-dose dispenser for immediate and extended release 3D printed tablets. Eur J Pharm Biopharm. 2015;96:380–7.
Skowyra J, Pietrzak K, Alhnan MA. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur J Pharm Sci. 2015;68:11–7.
Pawar SM, Khatal LD, Gabhe SY, Dhaneshwar SR. Establishment of inherent stability of pramipexole and development of validated stability indicating LC–UV and LC–MS method. J Pharm Anal. 2013;3(2):109–17.
CHMP Assessment Report.; accessed 22 January 2019 . Available from: https://www.ema.europa.eu/documents/assessment-report/pramipexole-teva-epar-public-assessment-report_en.pdf.
Pramipexole Accord, EMA.; accessed 7 January 2019. Available from: https://www.ema.europa.eu/documents/assessment-report/pramipexole-accord-epar-public-assessment-report_en.pdf.
Putri RSI, Setiawati E, Aziswan SA, Ong F, Tjandrawinata RR, Susanto LW. A comparative pharmacokinetics study of the anti-parkinsonian drug Pramipexole. Sci Pharm. 2016;84(4):715–23.
Mirapex® - FDA.; accessed 22 January 2019. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020667s014s017s018lbl.pdf.
Khaled SA, Alexander MR, Irvine DJ, Wildman RD, Wallace MJ, Sharpe S, et al. Extrusion 3D printing of paracetamol tablets from a single formulation with tunable release profiles through control of tablet geometry. AAPS Pharm Sci Tech. 2018;19(8):3403–13.
Skalsky B, Petereit HU. Chemistry and application properties of polymethacrylate systems. In: Mc Ginity JW, Felton LA, editors. Aqueous polymeric coatings for pharmaceutical dosage forms, 3rd ed., NY: Informa healthcare USA; 2008. p. 237–78.
Pradhan R, Kim SY, Yong CS, Kim JO. Preparation and characterization of spray-dried valsartan-loaded Eudragit® E PO solid dispersion microparticles. Asian J Pharm. 2016;11(6):744–50.
Pawar HV, Tetteh J, Boateng JS. Preparation, optimisation and characterisation of novel wound healing film dressings loaded with streptomycin and diclofenac. Colloids Surf B: Biointerfaces. 2013;102:102–10.
POLYOX Water-Soluble Resins NF in Pharmaceutical Applications.; accessed 22 January 2019. Available from: http://msdssearch.dow.com/PublishedLiteratureDOWCOM/dh_0171/0901b80380171e3a.pdf?filepath=polyox/pdfs/noreg/326-00013.pdf%26fromPage=GetDoc.
Dow Answer Center.; accessed 22 January 2019. Available from: https://dowac.custhelp.com/app/answers/detail/a_id/1379.
Zhang J, Feng X, Patil H, Tiwari RV, Repka MA. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. Int J Pharm. 2017;519(1):186–97.
Goyanes A, Det-Amornrat U, Wang J, Basit AW, Gaisford S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J Control Release. 2016;234:41–8.
Sereewat P, Suthipinittham C, Sumathaluk S, Puttanlek C, Uttapap D, Rungsardthong V. Cooking properties and sensory acceptability of spaghetti made from rice flour and defatted soy flour. LWT-Food Sci Technol. 2015;60(2):1061–7.
Sadia M, Sośnicka A, Arafat B, Isreb A, Ahmed W, Kelarakis A, et al. Adaptation of pharmaceutical excipients to FDM 3D printing for the fabrication of patient-tailored immediate release tablets. Int J Pharm. 2016;513(1):659–68.
POLYOX Water-Soluble Resins.; accessed 6 January 2019. Available from: http://msdssearch.dow.com/PublishedLiteratureDOWCOM/dh_094e/0901b8038094e22f.pdf?filepath=/326-00001.pdf%26fromPage=GetDoc.
European Pharmacopoeia (Ph Eur). 2014. Strasbourg, France: Euro-pean Directorate for the Quality of Medicines, Council of Europe.
Okwuosa TC, Stefaniak D, Arafat B, Isreb A, Wan KW, Alhnan MA. A lower temperature FDM 3D printing for the manufacture of patient-specific immediate release tablets. Pharm Res. 2016;33:2704–12.
Solanki NG, Tahsin M, Shah AV, Serajuddin AT. Formulation of 3D printed tablet for rapid drug release by fused deposition modeling: screening polymers for drug release, drug-polymer miscibility and printability. J Pharm Sci. 2018;107(1):390–401.
Ajmal A, Meskarzadeh A, Genina N, Hirschberg C, Boetker JP, Rantanen J. The use of 3D printed molds to cast tablets with a designed disintegration profile. AAPS Pharm Sci Tech. 2019;20(3):127.
Dissolution Testing and Acceptance Criteria for Immediate-Release Solid Oral Dosage Form Drug Products Containing High Solubility Drug Substances.; accessed 15 May 2019. Available from: https://www.fda.gov/media/92988/download.
Acknowledgments and Disclosures
This study was supported by the Scientific and Technological Research Council of Turkey (TUBITAK) (grant number 217S456). The authors are thankful to Deva Pharmaceuticals (Turkey) for donating pramipexole dihydrochloride monohydrate, to Karadeniz Pharmaceutical Warehouse (Turkey) for providing Eudragit EPO and to Colorcon (Turkey) for providing POLYOXTM derivatives. None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gültekin, H.E., Tort, S. & Acartürk, F. An Effective Technology for the Development of Immediate Release Solid Dosage Forms Containing Low-Dose Drug: Fused Deposition Modeling 3D Printing. Pharm Res 36, 128 (2019). https://doi.org/10.1007/s11095-019-2655-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-019-2655-y