Scale-Up Strategy in Quality by Design Approach for Pharmaceutical Blending Process with Discrete Element Method Simulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
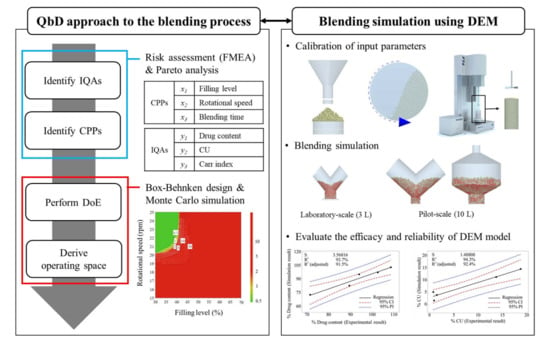
2.2. Application of QbD Approach
2.2.1. Definitions of IQAs and CPPs for the Blending Process
2.2.2. DoE for Blending Process
2.2.3. Measurement of IQAs
2.3. Application of DEM to Blending Process
2.3.1. Contact Model
Hertz–Mindlin (No Slip) Model
Hertz–Mindlin + JKR
2.3.2. Calibration of Input Parameters for Amlodipine Formulation
Direct Measurement of Input Parameters
Angle of Repose for Calibration of Input Parameters
BFE Test for Calibration of Input Parameters
2.3.3. Blending Simulation at Laboratory and Pilot-Scales
3. Results and Discussion
3.1. Results and Discussion of QbD Application in the Blending Process
3.1.1. Risk Assessment
3.1.2. Analysis of DoE Results
Effect of Control Factors on Drug Content (y1)
Effect of Control Factors on CU (y2)
Optimization for Blending Process Parameters
3.2. Results and Discussion of DEM Application to Blending Process
3.2.1. Definition of Input Parameters
3.2.2. Validation of Developed DEM Model
3.2.3. Evaluation of Change of Operating Space through Developed DEM Model
3.2.4. Comparison of Blending Behavior at Laboratory- and Pilot-Scale
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barrasso, D.; Eppinger, T.; Pereira, F.E.; Aglave, R.; Debus, K.; Bermingham, S.K.; Ramachandran, R. A multi-scale, mechanistic model of a wet granulation process using a novel bi-directional PBM–DEM coupling algorithm. Chem. Eng. Sci. 2015, 123, 500–513. [Google Scholar] [CrossRef]
- Djuris, J.; Djuric, Z. Modeling in the quality by design environment: Regulatory requirements and recommendations for design space and control strategy appointment. Int. J. Pharm. 2017, 533, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, M.; Van Dyck, W. Transforming Industrialization, a New Paradigm for Pharmaceutical Development; IBM Institute for Business Value, Publication G510-3997-00; IBM Corp.: Somers, NY, USA, 2005; p. 438. [Google Scholar]
- Lawrence, X.Y. Pharmaceutical quality by design: Product and process development, understanding, and control. Pharm. Res. 2008, 25, 781–791. [Google Scholar]
- Adam, S.; Suzzi, D.; Radeke, C.; Khinast, J.G. An integrated quality by design (QbD) approach towards design space definition of a blending unit operation by discrete element method (DEM) simulation. Eur. J. Pharm. Sci. 2011, 42, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Dumarey, M.; Talwar, S.; Yahyah, M.; Peterson, J. Empirical modelling to support scale up of primary pharmaceutical processes. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2016; Volume 38, pp. 2241–2246. [Google Scholar]
- Björn, I.N.; Jansson, A.; Karlsson, M.; Folestad, S.; Rasmuson, A. Empirical to mechanistic modelling in high shear granulation. Chem. Eng. Sci. 2005, 60, 3795–3803. [Google Scholar] [CrossRef]
- Ketterhagen, W.R.; am Ende, M.T.; Hancock, B.C. Process modeling in the pharmaceutical industry using the discrete element method. J. Pharm. Sci. 2009, 98, 442–470. [Google Scholar] [CrossRef] [PubMed]
- Wassgren, C.; Curtis, J.S. The application of computational modeling to pharmaceutical materials science. MRS Bull. 2006, 31, 900–904. [Google Scholar] [CrossRef]
- Pandey, P.; Bharadwaj, R. Predictive Modeling of Pharmaceutical Unit Operations; Woodhead Publishing: Sawston/Cambridge, UK, 2016. [Google Scholar]
- Rogers, A.; Ierapetritou, M. Challenges and opportunities in modeling pharmaceutical manufacturing processes. Comput. Chem. Eng. 2015, 81, 32–39. [Google Scholar] [CrossRef]
- Cundall, P.A.; Strack, O.D. A discrete numerical model for granular assemblies. Geotechnique 1979, 29, 47–65. [Google Scholar] [CrossRef]
- Norton, T.; Sun, D.-W. Computational fluid dynamics (CFD)—An effective and efficient design and analysis tool for the food industry: A review. Trends Food Sci. Technol. 2006, 17, 600–620. [Google Scholar] [CrossRef]
- Zheng, Q.J.; Yu, A.B. Modelling the granular flow in a rotating drum by the eulerian finite element method. Powder Technol. 2015, 286, 361–370. [Google Scholar] [CrossRef]
- Diarra, H.; Mazel, V.; Busignies, V.; Tchoreloff, P. Fem simulation of the die compaction of pharmaceutical products: Influence of visco-elastic phenomena and comparison with experiments. Int. J. Pharm. 2013, 453, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Ishihara, S.; Kano, J. Evaluation of particle density effect for mixing behavior in a rotating drum mixer by DEM simulation. Adv. Powder Technol. 2016, 27, 864–870. [Google Scholar] [CrossRef]
- Börner, M.; Michaelis, M.; Siegmann, E.; Radeke, C.; Schmidt, U. Impact of impeller design on high-shear wet granulation. Powder Technol. 2016, 295, 261–271. [Google Scholar] [CrossRef]
- Freireich, B.; Kumar, R.; Ketterhagen, W.; Su, K.; Wassgren, C.; Zeitler, J.A. Comparisons of intra-tablet coating variability using DEM simulations, asymptotic limit models, and experiments. Chem. Eng. Sci. 2015, 131, 197–212. [Google Scholar] [CrossRef] [Green Version]
- Vanarase, A.U.; Osorio, J.G.; Muzzio, F.J. Effects of powder flow properties and shear environment on the performance of continuous mixing of pharmaceutical powders. Powder Technol. 2013, 246, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Mahdi, Y.; Mouhi, L.; Guemras, N.; Daoud, K. Coupling the image analysis and the artificial neural networks to predict a mixing time of a pharmaceutical powder. J. Fundam. Appl. Sci. 2016, 8, 655–670. [Google Scholar] [CrossRef]
- Moakher, M.; Shinbrot, T.; Muzzio, F.J. Experimentally validated computations of flow, mixing and segregation of non-cohesive grains in 3d tumbling blenders. Powder Technol. 2000, 109, 58–71. [Google Scholar] [CrossRef]
- Cleary, P.W.; Sinnott, M.D. Assessing mixing characteristics of particle-mixing and granulation devices. Particuology 2008, 6, 419–444. [Google Scholar] [CrossRef]
- Mendez, A.S.L.; de Carli, G.; Garcia, C.V. Evaluation of powder mixing operation during batch production: Application to operational qualification procedure in the pharmaceutical industry. Powder Technol. 2010, 198, 310–313. [Google Scholar] [CrossRef]
- Chaudhuri, B.; Mehrotra, A.; Muzzio, F.J.; Tomassone, M.S. Cohesive effects in powder mixing in a tumbling blender. Powder Technol. 2006, 165, 105–114. [Google Scholar] [CrossRef]
- Arratia, P.E.; Duong, N.-h.; Muzzio, F.J.; Godbole, P.; Lange, A.; Reynolds, S. Characterizing mixing and lubrication in the bohle bin blender. Powder Technol. 2006, 161, 202–208. [Google Scholar] [CrossRef]
- Dubey, A.; Vanarase, A.U.; Muzzio, F.J. Impact of process parameters on critical performance attributes of a continuous blender—A DEM-based study. AIChE J. 2012, 58, 3676–3684. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Guidance for Industry: Q8 (R2) Pharmaceutical Development; Food and Drug Administration: White Oak, MD, USA, 2006.
- Sudah, O.S.; Coffin-Beach, D.; Muzzio, F.J. Effects of blender rotational speed and discharge on the homogeneity of cohesive and free-flowing mixtures. Int. J. Pharm. 2002, 247, 57–68. [Google Scholar] [CrossRef]
- Aksu, B.; Paradkar, A.; de Matas, M.; Özer, Ö.; Güneri, T.; York, P. A quality by design approach using artificial intelligence techniques to control the critical quality attributes of ramipril tablets manufactured by wet granulation. Pharm. Dev. Technol. 2013, 18, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.R.; Kwon, S.Y.; Choi, D.H.; Park, E.S. Quality by design (QbD) approach to optimize the formulation of a bilayer combination tablet (Telmiduo®) manufactured via high shear wet granulation. Int. J. Pharm. 2017, 534, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Charoo, N.A.; Shamsher, A.A.A.; Zidan, A.S.; Rahman, Z. Quality by design approach for formulation development: A case study of dispersible tablets. Int. J. Pharm. 2012, 423, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Bonate, P.L. A brief introduction to monte carlo simulation. Clin. Pharmacokinet. 2001, 40, 15–22. [Google Scholar] [CrossRef]
- Cogdill, R.P.; Drennen, J.K. Risk-based quality by design (QbD): A taguchi perspective on the assessment of product quality, and the quantitative linkage of drug product parameters and clinical performance. J. Pharm. Innov. 2008, 3, 23–29. [Google Scholar] [CrossRef]
- Escotet-Espinoza, M.S.; Foster, C.J.; Ierapetritou, M. Discrete element modeling (DEM) for mixing of cohesive solids in rotating cylinders. Powder Technol. 2018, 335, 124–136. [Google Scholar] [CrossRef]
- Zhu, H.P.; Zhou, Z.Y.; Yang, R.Y.; Yu, A.B. Discrete particle simulation of particulate systems: Theoretical developments. Chem. Eng. Sci. 2007, 62, 3378–3396. [Google Scholar] [CrossRef]
- Kremer, D.; Hancock, B. Process simulation in the pharmaceutical industry: A review of some basic physical models. J. Pharm. Sci. 2006, 95, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Ketterhagen, W.R. Modeling the motion and orientation of various pharmaceutical tablet shapes in a film coating pan using DEM. Int. J. Pharm. 2011, 409, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Hertz, H.R. Uber die beruhrung fester elastischer korper und uber die harte. Verh. Des Ver. Zur Beford. Des GewerbefleibesBerl. 1882, 92, 156–171. [Google Scholar]
- Mindlin, R.D. Elastic spheres in contact under varying oblique forces. J. Appl. Mech. 1953, 20, 327–344. [Google Scholar]
- Xiao, X.; Tan, Y.; Zhang, H.; Deng, R.; Jiang, S. Experimental and DEM studies on the particle mixing performance in rotating drums: Effect of area ratio. Powder Technol. 2017, 314, 182–194. [Google Scholar] [CrossRef]
- Bortolotti, C.T.; Santos, K.G.; Francisquetti, M.C.; Duarte, C.R.; Barrozo, M.A. Hydrodynamic study of a mixture of west indian cherry residue and soybean grains in a spouted bed. Can. J. Chem. Eng. 2013, 91, 1871–1880. [Google Scholar] [CrossRef]
- Cunha, R.N.; Santos, K.G.; Lima, R.N.; Duarte, C.R.; Barrozo, M.A.S. Repose angle of monoparticles and binary mixture: An experimental and simulation study. Powder Technol. 2016, 303, 203–211. [Google Scholar] [CrossRef]
- Johnson, K.L.; Kendall, K.; Roberts, A. Surface energy and the contact of elastic solids. Proc. R. Soc. Lond. A 1971, 324, 301–313. [Google Scholar] [CrossRef] [Green Version]
- Horabik, J.; Molenda, M. Parameters and contact models for DEM simulations of agricultural granular materials: A review. Biosyst. Eng. 2016, 147, 206–225. [Google Scholar] [CrossRef]
- Coetzee, C.J. Review: Calibration of the discrete element method. Powder Technol. 2017, 310, 104–142. [Google Scholar] [CrossRef]
- Liu, X.Y.; Specht, E.; Mellmann, J. Experimental study of the lower and upper angles of repose of granular materials in rotating drums. Powder Technol. 2005, 154, 125–131. [Google Scholar] [CrossRef]
- Lommen, S.; Schott, D.; Lodewijks, G. Dem speedup: Stiffness effects on behavior of bulk material. Particuology 2014, 12, 107–112. [Google Scholar] [CrossRef]
- Frankowski, P.; Morgeneyer, M. Calibration and Validation of DEM Rolling and Sliding Friction Coefficients in Angle of Repose and Shear Measurements; AIP Conference Proceedings; AIP: Sydney, Australia, 2013; pp. 851–854. [Google Scholar]
- Derakhshani, S.M.; Schott, D.L.; Lodewijks, G. Micro–macro properties of quartz sand: Experimental investigation and DEM simulation. Powder Technol. 2015, 269, 127–138. [Google Scholar] [CrossRef]
- Freeman, R. Measuring the flow properties of consolidated, conditioned and aerated powders—A comparative study using a powder rheometer and a rotational shear cell. Powder Technol. 2007, 174, 25–33. [Google Scholar] [CrossRef]
- Yan, Z.; Wilkinson, S.K.; Stitt, E.H.; Marigo, M. Investigating mixing and segregation using discrete element modelling (DEM) in the Freeman FT4 rheometer. Int. J. Pharm. 2016, 513, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Pantaleev, S.; Yordanova, S.; Janda, A.; Marigo, M.; Ooi, J.Y. An experimentally validated DEM study of powder mixing in a paddle blade mixer. Powder Technol. 2017, 311, 287–302. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, S.; Turnbull, S.; Yan, Z.; Stitt, E.H.; Marigo, M. A parametric evaluation of powder flowability using a freeman rheometer through statistical and sensitivity analysis: A discrete element method (DEM) study. Comput. Chem. Eng. 2017, 97, 161–174. [Google Scholar] [CrossRef]
- Coetzee, C. Particle upscaling: Calibration and validation of the discrete element method. Powder Technol. 2019, 344, 487–503. [Google Scholar] [CrossRef]
- Remy, B.; Glasser, B.J.; Khinast, J.G. The effect of mixer properties and fill level on granular flow in a bladed mixer. AIChE J. 2010, 56, 336–353. [Google Scholar] [CrossRef]
- Brone, D.; Alexander, A.; Muzzio, F. Quantitative characterization of mixing of dry powders in V-blenders. AIChE J. 1998, 44, 271–278. [Google Scholar] [CrossRef]
- Alexander, A.; Shinbrot, T.; Johnson, B.; Muzzio, F.J. V-blender segregation patterns for free-flowing materials: Effects of blender capacity and fill level. Int. J. Pharm. 2004, 269, 19–28. [Google Scholar] [CrossRef]
- Lemieux, M.; Bertrand, F.; Chaouki, J.; Gosselin, P. Comparative study of the mixing of free-flowing particles in a V-blender and a bin-blender. Chem. Eng. Sci. 2007, 62, 1783–1802. [Google Scholar] [CrossRef]
- Portillo, P.M.; Ierapetritou, M.; Tomassone, S.; Mc Dade, C.; Clancy, D.; Avontuur, P.P.; Muzzio, F.J. Quality by design methodology for development and scale-up of batch mixing processes. J. Pharm. Innov. 2008, 3, 258–270. [Google Scholar] [CrossRef]
- Arntz, M.; den Otter, W.K.; Briels, W.J.; Bussmann, P.; Beeftink, H.; Boom, R. Granular mixing and segregation in a horizontal rotating drum: A simulation study on the impact of rotational speed and fill level. AIChE J. 2008, 54, 3133–3146. [Google Scholar] [CrossRef]
- Barrios, G.K.; de Carvalho, R.M.; Kwade, A.; Tavares, L.M. Contact parameter estimation for DEM simulation of iron ore pellet handling. Powder Technol. 2013, 248, 84–93. [Google Scholar] [CrossRef]
- Zhou, Y.C.; Xu, B.H.; Yu, A.B.; Zulli, P. An experimental and numerical study of the angle of repose of coarse spheres. Powder Technol. 2002, 125, 45–54. [Google Scholar] [CrossRef]
- Combarros, M.; Feise, H.J.; Zetzener, H.; Kwade, A. Segregation of particulate solids: Experiments and DEM simulations. Particuology 2014, 12, 25–32. [Google Scholar] [CrossRef]
- Pandey, P.; Song, Y.; Kayihan, F.; Turton, R. Simulation of particle movement in a pan coating device using discrete element modeling and its comparison with video-imaging experiments. Powder Technol. 2006, 161, 79–88. [Google Scholar] [CrossRef]
- Rajkhowa, R.; Kafi, A.; Zhou, Q.T.; Kondor, A.; Morton, D.A.; Wang, X. Relationship between processing, surface energy and bulk properties of ultrafine silk particles. Powder Technol. 2015, 270, 112–120. [Google Scholar] [CrossRef]
- Alian, M.; Ein-Mozaffari, F.; Upreti, S.R. Analysis of the mixing of solid particles in a plowshare mixer via discrete element method (DEM). Powder Technol. 2015, 274, 77–87. [Google Scholar] [CrossRef]
- Marigo, M.; Stitt, E.H. Discrete element method (DEM) for industrial applications: Comments on calibration and validation for the modelling of cylindrical pellets. Kona Powder Part. J. 2015, 32, 236–252. [Google Scholar] [CrossRef]
- Muzzio, F.J.; Alexander, A.W. Scale up of powder-blending operations. Pharm. Technol. 2005, 26, 34–41. [Google Scholar]
- Brone, D.; Muzzio, F.J. Enhanced mixing in double-cone blenders. Powder Technol. 2000, 110, 179–189. [Google Scholar] [CrossRef]












| Blender Geometry | Laboratory-Scale | Pilot-Scale | |
|---|---|---|---|
| 3 L V-Blender | 10 L V-Blender | 10 L Double-Cone Blender | |
| Actual geometry |  |  |  |
| Graphical geometry |  |  |  |
| Blending Process Parameters | IQAs | S | P | D | RPN | Risk Level | Justification |
|---|---|---|---|---|---|---|---|
| Filling level (%) | Carr index | 5 | 7 | 3 | 105 | Medium | The amount of mixture can have a noticeable effect on the Carr index. Adding too much or too little mixture may result in inhomogeneity of mixture. This may also lead to undesirable flowability. Therefore, the filling level poses a medium risk to the Carr index. |
| Drug content | 7 | 7 | 7 | 343 | High | For desirable blending, it is preferable to add appropriate amounts of the mixture to the volume of blending vessel. If too much or too little mixture is added, the mixture homogeneity cannot be ensured. Therefore, the filling level poses high risk to the drug content. | |
| CU | 7 | 7 | 7 | 343 | High | It is desirable to add appropriate amounts of mixture to the volume of the blending vessel. Adding too much or too little mixture may result in inhomogeneity of mixture. This is also directly correlated to the CU. Therefore, the filling level poses a high risk to the CU. | |
| Rotational speed (rpm) | Carr index | 5 | 7 | 3 | 105 | Medium | Rotational speed can have a noticeable effect on the Carr index. Excessive or sluggish rotational speed can result in inhomogeneity of mixture. This may also lead to undesirable flowability. Therefore, the rotational speed poses a medium risk to the Carr index. |
| Drug content | 7 | 7 | 7 | 343 | High | Rotational speed is directly related to homogeneity of mixture, which should be ensured in the blending process. This homogeneity is explained by the drug content. Therefore, rotational speed poses a high risk to the drug content. | |
| CU | 7 | 7 | 7 | 343 | High | Rotational speed is directly related to homogeneity of mixture, which should be ensured in the blending process. Therefore, rotational speed poses a high risk to the CU. | |
| Order of input | Carr index | 3 | 3 | 3 | 27 | Low | The order of input for the API and excipients, excluding the lubricant, is not a critical process parameter in the blending process, as its effect on the IQAs is limited. |
| Drug content | 3 | 3 | 7 | 63 | Low | ||
| CU | 3 | 3 | 7 | 63 | Low | ||
| Blending time (min) | Carr index | 5 | 7 | 3 | 105 | Medium | The blending time can have a noticeable effect on the Carr index. Too long or too short blending time may lead to undesirable flowability. Therefore, the blending time poses a medium risk to the Carr index. |
| Drug content | 7 | 7 | 7 | 343 | High | Too long or short blending time can result in inhomogeneity of mixture. Therefore, the blending time poses high risk to the drug content. | |
| CU | 7 | 7 | 7 | 343 | High | For desirable homogeneity of mixture, appropriate blending time is required. Therefore, blending time poses a high risk to the CU. | |
| Manufacturing environment | Carr index | 3 | 3 | 3 | 27 | Low | The manufacturing environment, including temperature and humidity, may affect the IQAs. However, the manufacturing environment is kept at constant temperature and humidity. Therefore, this process parameter has negligible effect on the IQAs. |
| Drug content | 3 | 3 | 7 | 63 | Low | ||
| CU | 3 | 3 | 7 | 63 | Low |
| Run Order | Control Factors | Response Factors | ||||
|---|---|---|---|---|---|---|
| Filling Level (%) | Rotational Speed (rpm) | Blending Time (min) | Drug Content (%) | CU (%) | Carr Index | |
| x1 | x2 | x3 | y1 | y2 | y3 | |
| 1 | 70 | 15 | 16.5 | 96.77 | 6.90 | 19.63 |
| 2 | 70 | 20 | 9 | 92.86 | 6.19 | 18.03 |
| 3 | 50 | 25 | 9 | 92.21 | 9.01 | 18.50 |
| 4 | 30 | 20 | 24 | 95.70 | 2.58 | 18.54 |
| 5 | 50 | 25 | 24 | 94.56 | 1.67 | 19.00 |
| 6 | 70 | 20 | 24 | 94.41 | 7.13 | 18.52 |
| 7 | 70 | 25 | 16.5 | 90.04 | 5.18 | 18.14 |
| 8 | 50 | 15 | 9 | 94.70 | 2.26 | 18.84 |
| 9 | 50 | 15 | 24 | 95.36 | 7.45 | 18.92 |
| 10 | 50 | 20 | 16.5 | 94.13 | 5.08 | 18.40 |
| 11 | 30 | 25 | 16.5 | 95.62 | 4.18 | 18.00 |
| 12 | 50 | 20 | 16.5 | 94.42 | 5.14 | 18.03 |
| 13 | 30 | 20 | 9 | 93.86 | 6.15 | 19.01 |
| 14 | 50 | 20 | 16.5 | 94.13 | 5.05 | 19.58 |
| 15 | 30 | 15 | 16.5 | 92.87 | 4.02 | 17.94 |
| Response | Source | Sum of Squares 3 | DF 4 | Mean Square 5 | F Value 6 | p Value | R2 | Adjusted R2 | Predicted R2 |
|---|---|---|---|---|---|---|---|---|---|
| y1 | Model | 36.88 | 5 | 7.38 | 103.25 | <0.0001 | 0.98 | 0.97 | 0.93 |
| x1 | 1.97 | 1 | 1.97 | 27.58 | 0.0005 | - | - | - | |
| x2 | 6.61 | 1 | 6.1 | 92.48 | <0.0001 | - | - | - | |
| x3 | 5.12 | 1 | 5.12 | 71.67 | <0.0001 | - | - | - | |
| x1×2 | 22.47 | 1 | 22.47 | 314.50 | <0.0001 | - | - | - | |
| x2x3 | 0.71 | 1 | 0.71 | 10.00 | 0.0115 | - | - | - | |
| Residual 1 | 0.64 | 9 | 0.071 | - | - | - | - | - | |
| Cor Total 2 | 37.52 | 14 | - | - | - | - | - | - | |
| y2 | Model | 56.16 | 4 | 14.04 | 59.50 | <0.0001 | 0.96 | 0.94 | 0.90 |
| x1 | 8.97 | 1 | 8.97 | 38.00 | 0.0001 | - | - | - | |
| x2 | 0.04 | 1 | 0.04 | 0.17 | 0.6906 | - | - | - | |
| x3 | 2.86 | 2.86 | 12.10 | 0.0059 | 0.0059 | - | - | - | |
| x1x3 | 5.09 | 1 | 5.09 | 21.55 | 0.0009 | - | - | - | |
| x2x3 | 39.25 | 1 | 39.25 | 166.33 | <0.0001 | - | - | - | |
| Residual | 2.36 | 10 | 0.24 | - | - | - | - | - | |
| Cor Total | 58.52 | 14 | - | - | - | - | - | - |
| Optimal Setting | Response Factors | |||||||
|---|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | y1 | y2 | ||||
| Filling Level (%) | Rotational Speed (rpm) | Blending Time (min) | Drug Content (%) | CU (%) | ||||
| Optimal Solution | Experimental Results | Absolute Biases | Optimal Solution | Experimental Results | Absolute Biases | |||
| 32 | 24 | 24 | 96.67 | 96.82 | 0.15 | 0.07 | 0.98 | 0.91 |
| 40 | 24 | 24 | 95.72 | 95.53 | 0.19 | 0.94 | 1.54 | 0.6 |
| 36 | 23 | 24 | 95.96 | 96.25 | 0.29 | 1.15 | 1.88 | 0.73 |
| 32 | 22 | 24 | 96.01 | 95.70 | 0.31 | 1.35 | 2.14 | 0.79 |
| 40 | 22 | 24 | 95.44 | 95.51 | 0.07 | 2.23 | 2.75 | 0.52 |
| Materials | Static Angle of Repose | Dynamic Angle of Repose | BFE | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Experimental Results (°) | Simulation Results (°) | Relative Biases (%) | Experimental Results (°) | Simulation Results (°) | Relative Biases (%) | Experimental Results (mJ) | Simulation Results (mJ) | Relative Biases (%) | |
| Amlodipine besylate |  |  | 0.6 |  |  | 0.8 | 209.0 | 195.1 | 6.6 |
| 50.SMCC 90 |  |  | 1.5 |  |  | 2.3 | 235.0 | 228.3 | 2.8 |
| PVP K25 |  |  | 0.8 |  |  | 2.2 | 121.0 | 129.6 | 7.1 |
| CCM-Na |  |  | 1.8 |  |  | 0.8 | 341.0 | 330.7 | 3.0 |
| St-Mg |  |  | 0.8 |  |  | 1.4 | 16.7 | 17.4 | 4.2 |
| Materials | Input Parameters | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Material Properties | Interaction Parameters | |||||||||||||
| Particle Size (μm) | True Density (g/cm3) | Poisson’s Ratio | Shear Modulus (Pa) | Surface Energy (J/m2) | Coefficient of Restitution | Coefficient of Static Friction | Coefficient of Rolling Friction | |||||||
| D10 | D50 | D90 | P-P 1 | P-G 2 | P-P | P-G | P-P | P-G | ||||||
| Amlodipine formulation | Amlodipine besylate | 2.74 | 10.50 | 28.70 | 1.36 | 0.25 | 107 | 0.00 | 0.20 | 0.20 | 0.50 | 0.50 | 0.50 | 0.50 |
| SMCC 90 | 29.71 | 106.48 | 223.88 | 1.61 | 0.25 | 107 | 0.00 | 0.30 | 0.50 | 0.40 | 0.40 | 0.20 | 0.30 | |
| PVP K25 | 22.51 | 62.62 | 119.75 | 1.21 | 0.30 | 107 | 0.00 | 0.30 | 0.50 | 0.40 | 0.40 | 0.25 | 0.30 | |
| CCM-Na | 23.39 | 50.48 | 118.28 | 1.61 | 0.30 | 107 | 0.00 | 0.30 | 0.50 | 0.40 | 0.50 | 0.30 | 0.30 | |
| St-Mg | 1.09 | 5.52 | 24.99 | 1.11 | 0.30 | 107 | 0.02 | 0.30 | 0.30 | 0.50 | 0.50 | 0.45 | 0.40 | |
| Geometry | Stainless steel | - | - | - | 7.80 | 0.30 | 710 | - | - | - | - | - | - | - |
| Ti | Laboratory-Scale (3 L) | Pilot-Scale (10 L) | |
|---|---|---|---|
| V-Blender | V-Blender | Double-Cone Blender | |
| 1 |  |  |  |
| 2 |  |  |  |
| 3 |  |  |  |
| 4 |  |  |  |
| 5 |  |  |  |
| 6 |  |  |  |
| 7 |  |  |  |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeom, S.B.; Choi, D.H. Scale-Up Strategy in Quality by Design Approach for Pharmaceutical Blending Process with Discrete Element Method Simulation. Pharmaceutics 2019, 11, 264. https://doi.org/10.3390/pharmaceutics11060264
Yeom SB, Choi DH. Scale-Up Strategy in Quality by Design Approach for Pharmaceutical Blending Process with Discrete Element Method Simulation. Pharmaceutics. 2019; 11(6):264. https://doi.org/10.3390/pharmaceutics11060264
Chicago/Turabian StyleYeom, Su Bin, and Du Hyung Choi. 2019. "Scale-Up Strategy in Quality by Design Approach for Pharmaceutical Blending Process with Discrete Element Method Simulation" Pharmaceutics 11, no. 6: 264. https://doi.org/10.3390/pharmaceutics11060264