The Multifaceted Role of Curcumin in Advanced Nanocurcumin Form in the Treatment and Management of Chronic Disorders
Abstract
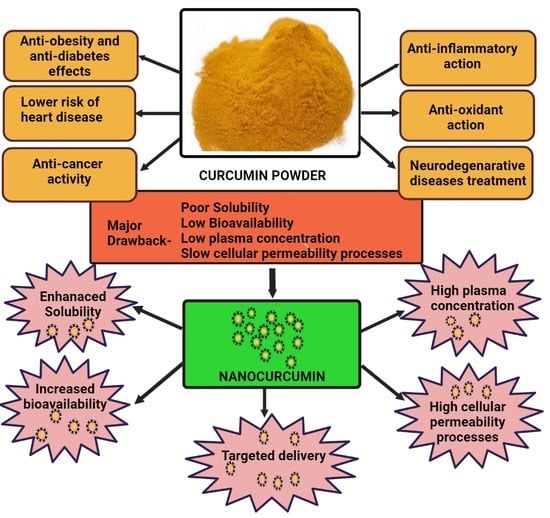
:1. Introduction
2. Review Methodology
3. Bioavailability of Curcumin
4. Therapeutic Applications of Nanocurcumin over Curcumin
4.1. Antioxidant Effects
4.2. Anti-Inflammatory Effects
4.3. Antibacterial Effects
4.4. Hepatoprotective Effects
4.5. Lower Risk of Heart Disease
4.6. Nanoencapsulated Curcumin’s Potential in Crossing the Blood–Brain Barrier (BBB)
4.6.1. Boost Brain-Derived Neurotrophic Factor
4.6.2. Potential Role in the Treatment of Neurodegenerative Disease
Potential Role in AD
Potential in the Treatment of GBM
4.7. Anticancer Effects
4.7.1. Activation of Tumor Apoptosis
4.7.2. Antiangiogenesis
4.8. Antidiabetic Effects
5. Various Nano Drug Delivery Systems for Curcumin
5.1. Liposomes
5.2. Micelles
5.3. Nanoemulsions
5.4. Solid Lipid Nanoparticles
5.5. Niosomes
5.6. Dendrimers
5.7. Nanogels
5.8. Cyclodextrins
6. Clinical Studies and Patent Reviews
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TLC | Thin-layer chromatography |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| TNF- | Tumor necrosis factor |
| NF-ĸB | Nuclear factor kappa B |
| ROS | Reactive oxygen species |
| iNOS | Nitric oxide synthase |
| NO | Nitric oxide |
| BDNF | Brain-derived neurotrophic factor |
| CDGSH iron sulfur domain 2 | CISD2 |
| BP | Blood pressure |
| CVD | Cardiovascular disease |
| APP | Amyloid precursor protein |
| BACE-1 | β-site amyloid precursor protein cleaving enzyme 1 |
| GSK-3 | Glycogen synthase kinase-3 |
| BBB | Blood–brain barrier |
| BBTB | Blood–brain tumor barrier |
| Ecs | Endothelial cells |
| CNS | Central nervous system |
| GBM | Glioblastoma |
| RMT | Receptor-mediated transcytosis |
| AMT | Adsorptive-mediated transcytosis |
| EPR | Extended permeability and retention |
| MDA | Malondialdehyde |
| Alg-Np-Cur | Curcumin–alginate nanoparticles |
| mPEG-PCL | Methoxy polyethylene glycol polycaprolactone |
| SLCP | Solid lipid nanoparticles |
| C-LNCs | Curcumin-loaded lipid-core nanocapsules |
| DNC | Dendrosomal curcumin |
| HA | Hyaluronic acid |
| Lf-Cur-PSNPs | Curcumin–lactoferrin conjugated |
| EAC | Ehrlich ascites carcinoma |
| STZ | Streptozotocin |
| CurLIP | Curcumin liposomal formulations |
| AIP | Aluminum phosphide |
| TNBC | Triple-negative breast cancer |
| DSPN | Diabetic sensorimotor polyneuropathy |
| DMSO | Dimethyl sulfoxide |
| ALT | Alanine transaminase |
| AST | Aspartate aminotransferase |
| ALP | Alkaline phosphatase |
| HDL | High-density lipoprotein |
| ELISA | Enzyme-linked immunosorbent assays |
| Cur-M | Curcumin encapsulated polymeric micelles |
| SLMPs | Solid lipid microparticles |
| PAMAMs | Polyamidoamines |
| CYP3A4 | Cytochrome P4503A4 |
| HEK 293 | Human embryonic kidney |
| hERG | Human ether-related gene |
| DSTNs | Dendritic silica/titania mesoporous nanoparticles |
| PEI–FA | Polyethylenimine–folic acid groups |
References
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phytother. Res. 2018, 32, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Tanvir, E.M.; Hossen, S.; Hossain, F.; Afroz, R.; Gan, S.H.; Khalil, I.; Karim, N. Antioxidant Properties of Popular Turmeric (Curcuma longa)Varieties from Bangladesh. J. Food Qual. 2017, 2017, 8471785. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, S.; Mishra, R.; Gupta, A.K.; Gupta, A. Turmeric: Isolation and synthesis of important biological molecules. In Synthesis of Medicinal Agents; Plants Elsevier: Amsterdam, The Netherlands, 2018; pp. 105–125. [Google Scholar]
- Kannigadu, C.; N’Da, D. Recent Advances in the Synthesis and Development of Curcumin, its Combinations, Formulations and Curcumin-like Compounds as Anti-infective Agents. Curr. Med. Chem. 2021, 28, 5463–5497. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Ghosh, R.; Charcosset, C. Extraction, purification and applications of curcumin from plant materials-A comprehensive review. Trends Food Sci. Technol. 2021, 112, 419–430. [Google Scholar] [CrossRef]
- Sahne, F.; Mohammadi, M.; Najafpour, G.D.; Moghadamnia, A.A. Enzyme-assisted ionic liquid extraction of bioactive compound from turmeric (Curcuma longa L.): Isolation, purification and analysis of curcumin. Ind. Crop. Prod. 2017, 95, 686–694. [Google Scholar] [CrossRef]
- Santana, Á.L.; Meireles, M.A.A. Thin-layer chromatography profiles of non-commercial turmeric (Curcuma longa L.) products obtained via partial hydrothermal hydrolysis. Food Public Health 2016, 6, 15–25. [Google Scholar]
- Wangchuk, K.; Manochai, B.; Chulaka, P.; Wongchaochant, S.; Chintakovid, W.; Pumprasert, J. Monitoring of active constituents of turmeric (Curcuma longa L.) rhizome stored under supplemented white LED-light with different light intensities. Int. Forum Hortic. Prod. Qual. 2019, 131–138. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The essential medicinal chemistry of curcumin: Miniperspective. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Sabet, S.; Rashidinejad, A.; Melton, L.D.; McGillivray, D.J. Recent advances to improve curcumin oral bioavailability. Trends Food Sci. Technol. 2021, 110, 253–266. [Google Scholar] [CrossRef]
- Scazzocchio, B.; Minghetti, L.; D’Archivio, M. Interaction between gut microbiota and curcumin: A new key of under-standing for the health effects of curcumin. Nutrients 2020, 12, 2499. [Google Scholar] [CrossRef]
- Bolat, Z.B.; Islek, Z.; Demir, B.N.; Yilmaz, E.N.; Sahin, F.; Ucisik, M.H. Curcumin-and piperine-loaded emulsomes as combinational treatment approach enhance the anticancer activity of curcumin on HCT116 colorectal cancer model. Front. Bioeng. Biotechnol. 2020, 8, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Smart, J.D.; Pannala, A.S. Recent developments in formulation design for improving oral bioavailability of curcumin: A review. J. Drug Deliv. Sci. Technol. 2020, 60, 102082. [Google Scholar] [CrossRef]
- Huang, M.T.; Lou, Y.R.; Ma, W. Curcumin as a therapeutic agent: The evidence from in vitro, animal and human studies. Cancer Res. 1988, 48, 5941–5946. [Google Scholar] [PubMed]
- Atal, C.K.; Dubey, R.K.; Singh, J. Biochemical basis of enhanced drug bioavailability by piperine: Evidence that piperine is a potent inhibitor of drug metabolism. J. Pharmacol. Exp. Ther. 1985, 232, 258–262. [Google Scholar]
- Zeng, X.; Cai, D.; Zeng, Q.; Chen, Z.; Zhong, G.; Zhuo, J.; Gan, H.; Huang, X.; Zhao, Z.; Yao, N.; et al. Selective reduction in the expression of UGTs and SULTs, a novel mechanism by which piperine enhances the bioavailability of curcumin in rat. Biopharm. Drug Dispos. 2017, 38, 3–19. [Google Scholar] [CrossRef] [Green Version]
- Volak, L.P.; Ghirmai, S.; Cashman, J.R.; Court, M.H. Curcuminoids Inhibit Multiple Human Cytochromes P450, UDP-Glucuronosyltransferase, and Sulfotransferase Enzymes, whereas Piperine Is a Relatively Selective CYP3A4 Inhibitor. Drug Metab. Dispos. 2008, 36, 1594–1605. [Google Scholar] [CrossRef] [Green Version]
- Pandey, M.; Choudhury, H.; Gunasegaran, T.A.P.; Nathan, S.S.; Shadab; Gorain, B.; Tripathy, M.; Hussain, Z. Hyaluronic acid-modified betamethasone encapsulated polymeric nanoparticles: Fabrication, characterisation, in vitro release kinetics, and dermal targeting. Drug Deliv. Transl. Res. 2019, 9, 520–533. [Google Scholar] [CrossRef]
- Biswas, A.K.; Islam, M.R.; Choudhury, Z.S.; Mostafa, A.; Kadir, M.F. Nanotechnology based approaches in cancer therapeutics. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 043001. [Google Scholar] [CrossRef]
- Flora, G.; Gupta, D.; Tiwari, A. Nanocurcumin: A Promising Therapeutic Advancement over Native Curcumin. Crit. Rev. Ther. Drug Carr. Syst. 2013, 30, 331–368. [Google Scholar] [CrossRef]
- Zou, P.; Zhang, J.; Xia, Y.; Kanchana, K.; Guo, G.; Chen, W.; Huang, Y.; Wang, Z.; Yang, S.; Liang, G. ROS generation mediates the anti-cancer effects of WZ35 via activating JNK and ER stress apoptotic pathways in gastric cancer. Oncotarget 2015, 6, 5860. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Shayeganpour, A.; Brocks, D.R.; Lavasanifar, A.; Samuel, J. High-performance liquid chromatography analysis of curcu-min in rat plasma: Application to pharmacokinetics of polymeric micellar formulation of curcumin. Biomed. Chromatogr. 2007, 21, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.M.; Basirun, W.J.; Seyfoddin, A. Nanoantioxidants: Recent Trends in Antioxidant Delivery Applications. Antioxidants 2019, 9, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enumo, A., Jr.; Pereira, C.I.D.; Parize, A.L. Temperature evaluation of curcumin keto–enolic kinetics and its interaction with two pluronic copolymers. J. Phys. Chem. B 2019, 123, 5641–5650. [Google Scholar] [CrossRef] [PubMed]
- Tagde, P.; Khan, F.; Gandhare, B. In Vitro Antioxidant Activity of Ipoema Biloba. Int. J. Phytopharm. 2012, 1, 50–54. [Google Scholar] [CrossRef]
- Mandal, M.; Jaiswal, P.; Mishra, A. Role of curcumin and its nanoformulations in neurotherapeutics: A comprehensive review. J. Biochem. Mol. Toxicol. 2020, 34, e22478. [Google Scholar] [CrossRef]
- Tsuda, T. Curcumin as a functional food-derived factor: Degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2018, 9, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Siddique, Y.H.; Khan, W.; Singh, B.R.; Naqvi, A.H. Synthesis of Alginate-Curcumin Nanocomposite and Its Protective Role in Transgenic Drosophila Model of Parkinson’s Disease. ISRN Pharmacol. 2013, 2013, 794582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajasekar, A. Facile synthesis of curcumin nanocrystals and validation of its antioxidant activity against circulatory toxici-ty in Wistar rats. J. Nanosci. Nanotechnol. 2015, 15, 4119–4125. [Google Scholar]
- Ranjbar, A.; Gholami, L.; Ghasemi, H.; Kheiripour, N.E. Effects of nano-curcumin and curcumin on the oxidant and antioxidant system of the liver mitochondria in aluminum phosphide-induced experimental toxicity. Nanomed. J. 2019, 7, 58–64. [Google Scholar]
- Nakamura, Y.; Park, J.-H.; Hayakawa, K. Therapeutic use of extracellular mitochondria in CNS injury and disease. Exp. Neurol. 2020, 324, 113114. [Google Scholar] [CrossRef]
- Sharma, A.; Khan, H.; Singh, T.G.; Grewal, A.K.; Najda, A.; Kawecka-Radomska, M.; Kamel, M.; Altyar, A.E.; Del-Daim, M.M. Pharmacological Modulation of Ubiquitin-Proteasome Pathways in Oncogenic Signaling. Int. J. Mol. Sci. 2021, 22, 11971. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Thomas, C.J.; Radcliffe, J.; Itsiopoulos, C. Omega-3 Fatty Acids in Early Prevention of Inflammatory Neurodegenerative Disease: A Focus on Alzheimer’s Disease. BioMed Res. Int. 2015, 2015, 172801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Luo, X.; Long, X.; Jiang, P.; Jiang, Q.; Guo, H.; Chen, Z. Potential role of mitochondria in synoviocytes. Clin. Rheumatol. 2021, 40, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Chiang, T.-H.; Chen, W.-J.; Sun, Y.-Y.; Lee, Y.-H.; Lin, M.-S. CISD2 serves a novel role as a suppressor of nitric oxide signalling and curcumin increases CISD2 expression in spinal cord injuries. Injury 2015, 46, 2341–2350. [Google Scholar] [CrossRef]
- Liao, H.-Y.; Liao, B.; Zhang, H.-H. CISD2 plays a role in age-related diseases and cancer. Biomed. Pharmacother. 2021, 138, 111472. [Google Scholar] [CrossRef]
- Xu, H.D.; Qin, Z.H. Beclin 1, Bcl-2 and autophagy. Autophagy Biol. Dis. 2019, 109–126. [Google Scholar]
- Mollazadeh, H.; Cicero, A.F.G.; Blesso, C.N.; Pirro, M.; Majeed, M.; Sahebkar, A. Immune modulation by curcumin: The role of interleukin-10. Crit. Rev. Food Sci. Nutr. 2019, 59, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Shao, H.; Zhu, W. Function of tumor necrosis factor alpha before and after mutation in gastric cancer. Saudi J. Biol. Sci. 2017, 24, 1920–1924. [Google Scholar] [CrossRef]
- Li, Y. Copper homeostasis: Emerging target for cancer treatment. IUBMB Life 2020, 72, 1900–1908. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, R.; Xie, Q.; Li, A.; Xiao, Y.; Li, K.; Liu, H.; Cui, D.; Chen, Y.; Wang, S. Enhanced bioavailability and efficiency of cur-cumin for the treatment of asthma by its formulation in solid lipid nanoparticles. Int. J. Nanomed. 2012, 7, 3667. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Lin, J.; Liao, G.; Tian, Y.; Liang, Y.; Li, R.; Liu, M.; Yuan, Y. ALK Inhibitors in the Treatment of ALK Positive NSCLC. Front. Oncol. 2019, 8, 557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karthikeyan, A.; Senthil, N.; Min, T. Nanocurcumin: A Promising Candidate for Therapeutic Applications. Front. Pharmacol. 2020, 11, 487. [Google Scholar] [CrossRef]
- Sarawi, W.S.; Alhusaini, A.M.; Fadda, L.M.; Alomar, H.A.; Albaker, A.B.; Aljrboa, A.S.; Alotaibi, A.M.; Hasan, I.H.; Mahmoud, A.M. Curcumin and Nano-Curcumin Mitigate Copper Neurotoxicity by Modulating Oxidative Stress, Inflammation, and Akt/GSK-3β Signaling. Molecules 2021, 26, 5591. [Google Scholar] [CrossRef]
- Sinjari, B.; Pizzicannella, J.; D’Aurora, M.; Zappacosta, R.; Gatta, V.; Fontana, A.; Trubiani, O.; Diomede, F. Curcumin/Liposome Nanotechnology as Delivery Platform for Anti-inflammatory Activities via NFkB/ERK/pERK Pathway in Human Dental Pulp Treated With 2-HydroxyEthyl MethAcrylate (HEMA). Front. Physiol. 2019, 10, 633. [Google Scholar] [CrossRef]
- Betts, J.W.; Wareham, D.W. In vitro activity of curcumin in combination with epigallocatechin gallate (EGCG) versus multi-drug-resistant Acinetobacter baumannii. BMC Microbiol. 2014, 14, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyagi, P.; Singh, M.; Kumari, H.; Kumari, A.; Mukhopadhyay, K. Bactericidal Activity of Curcumin I Is Associated with Damaging of Bacterial Membrane. PLoS ONE 2015, 10, e0121313. [Google Scholar] [CrossRef] [Green Version]
- Qian, T.; Wang, M.; Wang, J.; Zhu, R.; He, X.; Sun, X.; Sun, D.; Wang, Q.; Wang, S. Transient spectra study on photo-dynamics of curcumin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 166, 38–43. [Google Scholar] [CrossRef]
- Bhawana; Basniwal, R.K.; Buttar, H.S.; Jain, V.K.; Jain, N. Curcumin Nanoparticles: Preparation, Characterization, and Antimicrobial Study. J. Agric. Food Chem. 2011, 59, 2056–2061. [Google Scholar] [CrossRef]
- No, D.S.; AlGburi, A.; Huynh, P.; Moret, A.; Ringard, M.; Comito, N.; Drider, D.; Takhistov, P.; Chikindas, M.L. Antimicrobial efficacy of curcumin nanoparticles against Listeria monocytogenes is mediated by surface charge. J. Food Saf. 2017, 37, e12353. [Google Scholar] [CrossRef]
- Shariati, A.; Asadian, E.; Fallah, F.; Azimi, T.; Hashemi, A.; Sharahi, J.Y.; Moghadam, M.T. Evaluation of Nano-curcumin effects on expression levels of virulence genes and biofilm production of multidrug-resistant Pseudomonas aeruginosa isolated from burn wound infection in Tehran, Iran. Infect. Drug Resist. 2019, 12, 2223–2235. [Google Scholar] [CrossRef] [Green Version]
- Maghsoudi, A.; Yazdian, F.; Shahmoradi, S.; Ghaderi, L.; Hemati, M.; Amoabediny, G. Curcumin-loaded polysaccharide nano-particles: Optimization and anticariogenic activity against Streptococcus mutans. Mater. Sci. Eng. C 2017, 75, 1259–1267. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.; Gao, Y.; Zhang, Y.; Cheng, T.; Ou, H.; Yang, L.; Liu, J.; Shi, L.; Liu, J. Silver-Decorated Polymeric Micelles Combined with Curcumin for Enhanced Antibacterial Activity. ACS Appl. Mater. Interfaces 2017, 9, 16880–16889. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Niu, R.; Dong, L.; Gao, L.; Zhang, J.; Zheng, Y.; Shi, M.; Liu, Z.; Li, K. Nanoencapsulation of Curcumin and Its Protective Effects against CCl4-Induced Hepatotoxicity in Mice. J. Nanomater. 2019, 2019, 7140132. [Google Scholar] [CrossRef]
- Alhusaini, A.; Hasan, I.; AlDowsari, N.; Alsaadan, N. Prophylactic Administration of Nanocurcumin Abates the Incidence of Liver Toxicity Induced by an Overdose of Copper Sulfate: Role of CYP4502E1, NF-κB and Bax Expressions. Dose-Response 2018, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maghsoumi, F.; Bidgoli, S.A. Hepatoprotective Effects of Curcumin Nanomicells in Alcohol-induced Liver Injury: Comparison with Curcumin and Silymarin in Mice Model. J. Med. Plants 2020, 4, 64–77. [Google Scholar] [CrossRef]
- Ng, P.Q.; Ling, L.S.; Chellian, J.; Madheswaran, T.; Panneerselvam, J.; Kunnath, A.P.; Gupta, G.; Satija, S.; Mehta, M.; Hansbro, P.M.; et al. Applications of nanocarriers as drug delivery vehicles for active phytoconstituents. Curr. Pharm. Des. 2020, 26, 4580–4590. [Google Scholar] [CrossRef]
- Oliver, J.M.; Stoner, L.; Rowlands, D.S.; Caldwell, A.R.; Sanders, E.; Kreutzer, A.; Mitchell, J.B.; Purpura, M.; Jäger, R. Novel form of curcumin improves endothelial function in young, healthy individuals: A double-blind placebo controlled study. J. Nutr. Metab. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.P.; Wang, Z.F.; Tootle, S.; Philip, T.; Zhao, Z.Q. Curcumin promotes cardiac repair and ameliorates cardiac dysfunction following myocardial infarction. Br. J. Pharmacol. 2012, 167, 1550–1562. [Google Scholar] [CrossRef] [Green Version]
- Banez, M.J.; Geluz, M.I.; Chandra, A.; Hamdan, T.; Biswas, O.S.; Bryan, N.S.; Von Schwarz, E.R. A systemic review on the antioxidant and anti-inflammatory effects of resveratrol, curcumin, and dietary nitric oxide supplementation on human cardiovascular health. Nutr. Res. 2020, 78, 11–26. [Google Scholar] [CrossRef]
- Qin, S.; Huang, L.; Gong, J.; Shen, S.; Huang, J.; Ren, H.; Hu, H. Efficacy and safety of turmeric and curcumin in lowering blood lipid levels in patients with cardiovascular risk factors: A meta-analysis of randomized controlled trials. Nutr. J. 2017, 16, 68. [Google Scholar] [CrossRef]
- Sarraf, P.; Parohan, M.; Javanbakht, M.H.; Ranji-Burachaloo, S.; Djalali, M. Short-term curcumin supplementation enhances serum brain-derived neurotrophic factor in adult men and women: A systematic review and dose-response meta-analysis of randomized controlled trials. Nutr. Res. 2019, 69, 31279955. [Google Scholar] [CrossRef] [PubMed]
- Tiekou, L.H.; Fitzsimons, O.; Mursaleen, L.; Renshaw, D.; Begum, G.; Zariwala, M.G. Co-administration of iron and a bioavailable curcumin supplement increases serum BDNF levels in healthy adults. Antioxidants 2020, 9, 645. [Google Scholar] [CrossRef]
- Xiong, J.; Zhang, Z.; Sun, R.; Yan, M.A.; Liu, Q. Curcumin combined with electroacupuncture promotes the expression of brain-derived neurotrophic factor and nerve growth factor after cerebral infarction. Chin. J. Phys. Med. Rehabil. 2017, 39, 170–174. [Google Scholar]
- Nardi, J. Increasing Brain Derived Neurotrophic Factor with Traumatic Brain Injuries. Nutr. Perspect. J. Counc. Nutr. 2020, 43. [Google Scholar]
- Yavarpour-Bali, H.; Ghasemi-Kasman, M.; Pirzadeh, M. Curcumin-loaded nanoparticles: A novel therapeutic strategy in treatment of central nervous system disorders. Int. J. Nanomed. 2019, 14, 4449–4460. [Google Scholar] [CrossRef] [Green Version]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Panzarini, E.; Mariano, S.; Tacconi, S.; Carata, E.; Tata, A.M.; Dini, L. Novel Therapeutic Delivery of Nanocurcumin in Central Nervous System Related Disorders. Nanomater 2020, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, J.J.; Cunnane, E.M.; Ross, A.M.; Duskey, J.T.; Tosi, G.; Grabrucker, A.M. Drug delivery across the blood–brain barrier: Re-cent advances in the use of nanocarriers. Nanomedicine 2020, 15, 205–214. [Google Scholar] [CrossRef]
- Bhaskar, S.; Tian, F.; Stoeger, T.; Kreyling, W.; de la Fuente, J.M.; Grazú, V.; Borm, P.; Estrada, G.; Ntziachristos, V.; Razansky, D. Multifunctional Nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: Perspectives on tracking and neuroimaging. Part. Fibre Toxicol. 2010, 7, 3. [Google Scholar] [CrossRef] [Green Version]
- Meng, F.; Asghar, S.; Gao, S.; Su, Z.; Song, J.; Huo, M.; Meng, W.; Ping, Q.; Xiao, Y. A novel LDL-mimic nanocarrier for the targeted delivery of curcumin into the brain to treat Alzheimer’s disease. Colloids Surf. B Biointerfaces 2015, 134, 88–97. [Google Scholar] [CrossRef]
- Ahmed, T.; Enam, S.A.; Gilani, A.H. Curcuminoids enhance memory in an amyloid-infused rat model of Alzheimer’s disease. Neuroscience 2010, 169, 1296–1306. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug and gene targeting to the brain with molecular trojan horses. Nat. Rev. Drug Discov. 2002, 1, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Kim, B.; Ramalingam, P.; Karthivashan, G.; Revuri, V.; Park, S.; Choi, D.K. Antineuroinflammatory activities and neurotoxicological assessment of curcumin loaded solid lipid nanoparticles on LPS-stimulated BV-2 microglia cell models. Molecules 2019, 24, 1170. [Google Scholar] [CrossRef] [Green Version]
- Shabbir, U.; Rubab, M.; Tyagi, A.; Oh, D.-H. Curcumin and Its Derivatives as Theranostic Agents in Alzheimer’s Disease: The Implication of Nanotechnology. Int. J. Mol. Sci. 2021, 22, 196. [Google Scholar] [CrossRef] [PubMed]
- Chainoglou, E.; Hadjipavlou-Litina, D. Curcumin in Health and Diseases: Alzheimer’s Disease and Curcumin Analogues, Derivatives, and Hybrids. Int. J. Mol. Sci. 2020, 21, 1975. [Google Scholar] [CrossRef] [Green Version]
- de la Monte, M.S. Brain insulin resistance and deficiency as therapeutic targets in Alzheimer’s disease. Curr. Alzheimer Res. 2012, 9, 35–66. [Google Scholar] [CrossRef] [PubMed]
- Zanforlin, E.; Zagotto, G.; Ribaudo, G. An overview of new possible treatments of Alzheimer’s disease, based on natural products and semi-synthetic compounds. Curr. Med. Chem. 2017, 24, 3749–3773. [Google Scholar] [CrossRef]
- Serafini, M.M.; Catanzaro, M.; Rosini, M.; Racchi, M.; Lanni, C. Curcumin in Alzheimer’s disease: Can we think to new strategies and perspectives for this molecule? Pharmacol. Res. 2017, 124, 146–155. [Google Scholar] [CrossRef]
- Farooqi, A.A.; Desai, N.N.; Qureshi, M.Z.; Nogueira-Librelotto, D.R.; Gasparri, M.L.; Bishayee, A.; Nabavi, S.M.; Curti, V.; Daglia, M. Exosome biogenesis, bioactivities and functions as new delivery systems of natural compounds. Biotechnol. Adv. 2018, 36, 328–334. [Google Scholar] [CrossRef]
- Sambon, M.; Wins, P.; Bettendorff, L. Neuroprotective Effects of Thiamine and Precursors with Higher Bioavailability: Focus on Benfotiamine and Dibenzoylthiamine. Int. J. Mol. Sci. 2021, 22, 5418. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Nagesh, P.K.B.; Jaggi, M.; Chauhan, S.C. Therapeutic Applications of Curcumin Nanoformulations. AAPSJ 2015, 17, 1341–1356. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Mahajan, S.D.; Kutscher, H.L.; Kim, S.; Prasad, P.N. Curcumin-Pluronic Nanoparticles: A Theranostic Nanoformulation for Alzheimer’s Disease. Crit. Rev. Biomed. Eng. 2020, 48, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Tagde, P.; Tagde, P.; Tagde, S.; Bhattacharya, T.; Garg, V.; Akter, R.; Rahman, H.; Najda, A.; Albadrani, G.M.; Sayed, A.A.; et al. Natural bioactive molecules: An alternative approach to the treatment and control of glioblastoma multiforme. Biomed. Pharmacother. 2021, 141, 111928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; van Os, W.L.; Tian, X.; Zu, G.; Ribovski, L.; Bron, R.; Bussmann, J.; Kros, A.; Liu, Y.; Zuhorn, I.S. Development of curcu-min-loaded zein nanoparticles for transport across the blood–brain barrier and inhibition of glioblastoma cell growth. Biomater. Sci. 2021, 9, 7092–7103. [Google Scholar] [CrossRef]
- Hesari, A.; Rezaei, M.; Rezaei, M.; Dashtiahangar, M.; Fathi, M.; Rad, J.G.; Momeni, F.; Avan, A.; Ghasemi, F. Effect of curcumin on glioblastoma cells. J. Cell. Physiol. 2019, 234, 10281–10288. [Google Scholar] [CrossRef] [PubMed]
- Sadeghizadeh, M.; Mirgani, M.T.; Isacchi, B.; Marra, F.; Bilia, A.R.; Mowla, S.J.; Najafi, F.; Babaei, E. Dendrosomal curcumin nanoformulation downregulates pluripotency genes via miR-145 activation in U87MG glioblastoma cells. Int. J. Nanomed. 2014, 9, 403–417. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Asghar, S.; Yang, L.; Li, H.; Wang, Z.; Ping, Q.; Xiao, Y. Lactoferrin-coated polysaccharide nanoparticles based on chitosan hydrochloride/hyaluronic acid/PEG for treating brain glioma. Carbohydr. Polym. 2017, 157, 419–428. [Google Scholar] [CrossRef]
- Willenbacher, E.; Khan, S.Z.; Mujica, S.C.A.; Trapani, D.; Hussain, S.; Wolf, D.; Willenbacher, W.; Spizzo, G.; Seeber, A. Curcumin: New Insights into an Ancient Ingredient against Cancer. Int. J. Mol. Sci. 2019, 20, 1808. [Google Scholar] [CrossRef] [Green Version]
- Hesari, A.; Azizian, M.; Sheikhi, A.; Nesaei, A.; Sanaei, S.; Mahinparvar, N.; Derakhshani, M.; Hedayt, P.; Ghasemi, F.; Mirzaei, H. Chemopreventive and therapeutic potential of curcumin in esophageal cancer: Current and future status. Int. J. Cancer 2019, 144, 1215–1226. [Google Scholar] [CrossRef]
- Elbialy, N.S.; Aboushoushah, S.F.; Sofi, B.F.; Noorwali, A. Multifunctional curcumin-loaded mesoporous silica nanoparticles for cancer chemoprevention and therapy. Microporous Mesoporous Mater. 2020, 291, 109540. [Google Scholar] [CrossRef]
- Termini, D.; Hartogh, D.J.D.; Jaglanian, A.; Tsiani, E. Curcumin against Prostate Cancer: Current Evidence. Biomolecules 2020, 10, 1536. [Google Scholar] [CrossRef]
- Guesmi, F.; Prasad, S.; Tyagi, A.K.; Landoulsi, A. Antinflammatory and anticancer effects of terpenes from oily fractions of Teucruim alopecurus, blocker of IκBα kinase, through downregulation of NF-κB activation, potentiation of apoptosis and suppression of NF-κB-regulated gene expression. Biomed. Pharmacother. 2017, 95, 1876–1885. [Google Scholar] [CrossRef]
- McGlorthan, L.; Paucarmayta, A.; Casablanca, Y.; Maxwell, G.L.; Syed, V. Progesterone induces apoptosis by activation of caspase-8 and calcitriol via activation of caspase-9 pathways in ovarian and endometrial cancer cells in vitro. Apoptosis 2021, 26, 184–194. [Google Scholar] [CrossRef]
- Jayakumar, T.; Hou, S.M.; Chang, C.C.; Fong, T.H.; Hsia, C.W.; Chen, Y.J.; Huang, W.C.; Saravanabhavan, P.; Manubolu, M.; Sheu, J.R.; et al. Columbianadin Dampens In Vitro Inflammatory Actions and Inhibits Liver Injury via Inhibition of NF-κB/MAPKs: Impacts on OH° Radicals and HO-1 Expression. Antioxidants 2021, 10, 553. [Google Scholar] [CrossRef] [PubMed]
- Radomska-Leśniewska, D.; Białoszewska, A.; Kamiński, P. Angiogenic Properties of NK Cells in Cancer and Other Angiogenesis-Dependent Diseases. Cells 2021, 10, 1621. [Google Scholar] [CrossRef] [PubMed]
- Tagde, P.; Kulkarni, G.T.; Mishra, D.K.; Kesharwani, P. Recent advances in folic acid engineered nanocarriers for treatment of breast cancer. J. Drug Deliv. Sci. Technol. 2020, 56, 101613. [Google Scholar] [CrossRef]
- Zahariah, S.; Winarsih, S.; Baktiyani, S.C.; Rahardjo, B.; Kalsum, U. The Effect of Turmeric Decoctum to the Angiogenic Molecules Expression on Chicken Embryo. J. Trop. Life Sci. 2017, 7, 61–65. [Google Scholar]
- Barui, A.K.; Kotcherlakota, R.; Bollu, V.S.; Nethi, S.K.; Patra, C.R. Biomedical and drug delivery applications of functionalized inorganic nanomaterials. Biopolym. Based Compos. 2017, 2017, 325–379. [Google Scholar] [CrossRef]
- Mogharrabi, M.; Rahimi, H.R.; Hasanzadeh, S.; Dastani, M.; Kazemi-Oskuee, R.; Akhlaghi, S.; Soukhtanloo, M. The effects of nanomicelle of curcumin on the matrix metalloproteinase (MMP-2, 9) activity and expression in patients with coronary artery disease (CAD): A randomized controlled clinical trial. ARYA Atheroscler. 2020, 16, 136–145. [Google Scholar]
- Amin, S.A.; Adhikari, N.; Jha, T. Design of Aminopeptidase N Inhibitors as Anti-cancer Agents. J. Med. Chem. 2018, 61, 6468–6490. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shishodia, S.; Sethi, G.; Aggarwal, B.B. Curcumin: Getting Back to the Roots. Ann. N. Y. Acad. Sci. 2005, 1056, 206–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, B.L.; Norhaizan, M.E. Curcumin Combination Chemotherapy: The Implication and Efficacy in Cancer. Molecules 2019, 24, 2527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basniwal, R.K.; Khosla, R.; Jain, N. Improving the Anticancer Activity of Curcumin Using Nanocurcumin Dispersion in Water. Nutr. Cancer 2014, 66, 1015–1022. [Google Scholar] [CrossRef]
- Baghi, N.; Bakhshinejad, B.; Keshavarz, R.; Babashah, S.; Sadeghizadeh, M. Dendrosomal nanocurcumin and exogenous p53 can act synergistically to elicit anticancer effects on breast cancer cells. Gene 2018, 670, 55–62. [Google Scholar] [CrossRef]
- Noce, A.; Di Lauro, M.; Di Daniele, F.; Zaitseva, A.P.; Marrone, G.; Borboni, P.; Di Daniele, N. Natural Bioactive Compounds Useful in Clinical Management of Metabolic Syndrome. Nutrients 2021, 13, 630. [Google Scholar] [CrossRef]
- Saad, B.; Zaid, H.; Shanak, S.; Kadan, S. Anti-diabetes and Anti-obesity Medicinal Plants and Phytochemicals. Anti-Diabetes Anti-Obes. Med. Plants Phytochem. 2017, 59–93. [Google Scholar] [CrossRef]
- Kumar, G.; Mittal, S.; Sak, K.; Tuli, H.S. Molecular mechanisms underlying chemopreventive potential of curcumin: Current challenges and future perspectives. Life Sci. 2016, 148, 313–328. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2016, 174, 1325–1348. [Google Scholar] [CrossRef] [Green Version]
- Wojcik, M.; Krawczyk, M.; Wojcik, P.; Cypryk, K.; Wozniak, L.A. Molecular Mechanisms Underlying Curcumin-Mediated Therapeutic Effects in Type 2 Diabetes and Cancer. Oxidative Med. Cell. Longev. 2018, 2018, 9698258. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, H.R.; Nedaeinia, R.; Shamloo, A.S.; Nikdoust, S.; Oskuee, R.K. Novel delivery system for natural products: Nano-curcumin formulations. Avicenna J. Phytomed. 2016, 6, 383, PMID: 27516979; PMCID: PMC4967834. [Google Scholar]
- Mohiti-Ardekani, J.; Asadi, S.; Ardakani, A.M.; Rahimifard, M.; Baeeri, M.; Momtaz, S. Curcumin increases insulin sensitivity in C2C12 muscle cells via AKT and AMPK signaling pathways. Cogent Food Agric. 2019, 5. [Google Scholar] [CrossRef]
- Faraji, A.H.; Wipf, P. Nanoparticles in cellular drug delivery. Bioorg. Med. Chem. 2009, 17, 2950–2962. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Shin, D.M. CCR 20th Anniversary Commentary: Prospects and Challenges of Therapeutic Nanoparticles in Cancer. Clin. Cancer Res. 2015, 21, 4499–4501. [Google Scholar] [CrossRef] [Green Version]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M.V. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013, 334, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Witika, B.; Makoni, P.; Matafwali, S.; Mweetwa, L.; Shandele, G.; Walker, R. Enhancement of Biological and Pharmacological Properties of an Encapsulated Polyphenol: Curcumin. Molecules 2021, 26, 4244. [Google Scholar] [CrossRef]
- Rahman, S.; Cao, S.; Steadman, K.J.; Wei, M.; Parekh, H.S. Native and β-cyclodextrin-enclosed curcumin: Entrapment within liposomes and their in vitro cytotoxicity in lung and colon cancer. Drug Deliv. 2012, 19, 346–353. [Google Scholar] [CrossRef]
- Fischer, M.; Zimmerman, A.; Zhang, E.; Kolis, J.; Dickey, A.; Burdette, M.K.; Weick, J.P. Biodistribution and inflam-matory response to intracranial delivery of scintillating nanoparticles. bioRxiv 2019, 609354. [Google Scholar]
- Gao, X.X.; Shi, H.-S.; Li, D.; Zhang, Q.-W.; Wang, Y.-S.; Zheng, Y.; Cai, L.-L.; Zhong, R.-M.; Rui, A.; Li, Z.-Y.; et al. A systemic administration of liposomal curcumin inhibits radiation pneumonitis and sensitizes lung carcinoma to radiation. Int. J. Nanomed. 2012, 7, 2601–2611. [Google Scholar] [CrossRef] [Green Version]
- Matabudul, D.; Pucaj, K.; Bolger, G.; Vcelar, B.; Majeed, M.; Helson, L. Tissue distribution of (Lipocurc™) liposomal cur-cumin and tetrahydrocurcumin following two-and eight-hour infusions in beagle dogs. Anticancer. Res. 2012, 32, 4359–4364. [Google Scholar]
- Dhule, S.S.; Penfornis, P.; Frazier, T.; Walker, R.; Feldman, J.; Tan, G.; Pochampally, R. Curcumin-loaded γ-cyclodextrin liposomal nanoparticles as delivery vehicles for osteosarcoma. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 440–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, A. Cyclodextrin-based nanoparticles for pharmaceutical applications: A review. Environ. Chem. Lett. 2021, 1–14. [Google Scholar] [CrossRef]
- De Silva, L.; Goh, B.H.; Lee, L.H.; Chuah, L.H. Curcumin-loaded nanoparticles and their potential as anticancer agents in breast cancer. Nat. Bioact. Compd. 2019, 147–178. [Google Scholar]
- Li, L.; Braiteh, F.S.; Kurzrock, R. Liposome-encapsulated curcumin: In vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2005, 104, 1322–1331. [Google Scholar] [CrossRef]
- Kong, Z.L.; Kuo, H.P.; Johnson, A.; Wu, L.C.; Chang, K.L.B. Curcumin-loaded mesoporous silica nanoparticles mark-edly enhanced cytotoxicity in hepatocellular carcinoma cells. Int. J. Mol. Sci. 2019, 20, 2918. [Google Scholar] [CrossRef] [Green Version]
- Kabir, M.; Rahman, M.; Akter, R.; Behl, T.; Kaushik, D.; Mittal, V.; Abdel-Daim, M.M. Potential role of curcumin and its nanoformulations to treat various types of cancers. Biomolecules 2021, 11, 392. [Google Scholar] [CrossRef] [PubMed]
- Hosseini-Zare, M.S.; Sarhadi, M.; Zarei, M.; Thilagavathi, R.; Selvam, C. Synergistic effects of curcumin and its analogs with other bioactive compounds: A comprehensive review. Eur. J. Med. Chem. 2021, 210, 113072. [Google Scholar] [CrossRef]
- Wang, D.; Veena, M.S.; Stevenson, K.; Tang, C.; Ho, B.; Suh, J.D.; Wang, M.B. Liposome-encapsulated curcumin sup-presses growth of head and neck squamous cell carcinoma in vitro and in xenografts through the inhibition of nuclear factor κB by an AKT-independent pathway. Clin. Cancer Res. 2008, 14, 6228–6236. [Google Scholar] [CrossRef] [Green Version]
- Feng, T.; Wei, Y.; Lee, R.J.; Zhao, L. Liposomal curcumin and its application in cancer. Int. J. Nanomed. 2017, 12, 6027. [Google Scholar] [CrossRef] [Green Version]
- Deljoo, S.; Rabiee, N.; Rabiee, M. Curcumin-hybrid nanoparticles in drug delivery system. Asian J. Nanosci. Mater. 2019, 2, 66–91. [Google Scholar]
- Ghalandarlaki, N.; Alizadeh, A.M.; Ashkani-Esfahani, S. Nanotechnology-applied curcumin for different diseases ther-apy. BioMed Res. Int. 2014, 2014, 394264. [Google Scholar] [CrossRef] [Green Version]
- Karthika, C.; Hari, B.; Mano, V.; Radhakrishnan, A.; Janani, S.K.; Akter, R.; Kaushik, D.; Rahman, M.H. Curcumin as a great contributor for the treatment and mitigation of colorectal cancer. Exp. Gerontol. 2021, 152, 111438. [Google Scholar] [CrossRef]
- Zheng, B.; McClements, D.J. Formulation of more efficacious curcumin delivery systems using colloid science: En-hanced solubility, stability, and bioavailability. Molecules 2020, 25, 2791. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, Y. Effects of length and unsaturation of the alkyl chain on the hydrophobic binding of curcumin with Tween micelles. Food Chem. 2018, 246, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sun, L.; Wu, Q.; Guo, W.; Li, L.; Chen, Y.; Li, Y.; Gong, C.; Qian, Z.; Wei, Y. Curcumin loaded polymeric micelles inhibit breast tumor growth and spontaneous pulmonary metastasis. Int. J. Pharm. 2013, 443, 175–182. [Google Scholar] [CrossRef]
- Na, Q.; Xiyou, D.; Ji, J.; Zhai, G. A review of stimuli-responsive polymeric micelles for tumor-targeted delivery of cur-cumin. Drug Dev. Ind. Pharm. 2021, 47, 839–856. [Google Scholar]
- Chang, T.; Trench, D.; Putnam, J.; Stenzel, M.H.; Lord, M.S. Curcumin-Loading-Dependent Stability of PEGMEMA-Based Micelles Affects Endocytosis and Exocytosis in Colon Carcinoma Cells. Mol. Pharm. 2016, 13, 924–932. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal communication: When i’m calling you, will you answer too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef]
- Ma, W.; Guo, Q.; Li, Y.; Wang, X.; Wang, J.; Tu, P. Co-assembly of doxorubicin and curcumin targeted micelles for synergistic delivery and improving anti-tumor efficacy. Eur. J. Pharm. Biopharm. 2017, 112, 209–223. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, N.; Cheng, R.; Zhao, C.; Liu, Z.; Li, X.; Liu, J.; Tian, Z. pH multistage responsive micellar system with charge-switch and PEG layer detachment for co-delivery of paclitaxel and curcumin to synergistically eliminate breast cancer stem cells. Biomaterials 2017, 147, 53–67. [Google Scholar] [CrossRef]
- Jiang, T.; Liao, W.; Charcosset, C. Recent advances in encapsulation of curcumin in nanoemulsions: A review of encapsu-lation technologies, bioaccessibility and applications. Food Res. Int. 2020, 132, 109035. [Google Scholar] [CrossRef]
- Choi, S.J.; McClements, D.J. Nanoemulsions as delivery systems for lipophilic nutraceuticals: Strategies for improving their formulation, stability, functionality and bioavailability. Food Sci. Biotechnol. 2020, 29, 149–168. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Coimbra, M.A.; Vicente, A.A. In vitro behaviour of curcumin nanoemulsions stabilized by biopolymer emulsifiers—Effect of interfacial composition. Food Hydrocoll. 2016, 52, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Salvia-Trujillo, L.; Soliva-Fortuny, R.; Rojas-Graü, M.A.; McClements, D.J.; Martín-Belloso, O. Edible Nanoemulsions as Carriers of Active Ingredients: A Review. Annu. Rev. Food Sci. Technol. 2017, 8, 439–466. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ahmad, R.; Al-Qudaihi, A.; Alaseel, S.E.; Fita, I.Z.; Khalid, M.S.; Pottoo, F.H. Preparation of a novel cur-cumin nanoemulsion by ultrasonication and its comparative effects in wound healing and the treatment of inflammation. RSC Adv. 2019, 9, 20192–20206. [Google Scholar] [CrossRef] [Green Version]
- Beloqui, A.; Memvanga, P.B.; Coco, R.; Reimondez-Troitino, S.; Alhouayek, M.; Muccioli, G.G.; Préat, V. A compara-tive study of curcumin-loaded lipid-based nanocarriers in the treatment of inflammatory bowel disease. Colloids Surf. B Biointerfaces 2016, 143, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, Z.; Nejatian, M.; Daeihamed, M.; Jafari, S.M. Application of different nanocarriers for encapsulation of curcumin. Crit. Rev. Food Sci. Nutr. 2019, 59, 3468–3497. [Google Scholar] [CrossRef]
- Sari, T.P.; Mann, B.; Kumar, R.; Singh, R.R.B.; Sharma, R.; Bhardwaj, M.; Athira, S. Preparation and characterization of nanoemulsion encapsulating curcumin. Food Hydrocoll. 2015, 43, 540–546. [Google Scholar] [CrossRef]
- Nikolić, I.; Mitsou, E.; Damjanović, A.; Papadimitriou, V.; Antić-Stanković, J.; Stanojević, B.; Savić, S. Curcumin-loaded low-energy nanoemulsions: Linking EPR spectroscopy-analysed microstructure and antioxidant potential with in vitro evaluated biological activity. J. Mol. Liq. 2020, 301. [Google Scholar] [CrossRef]
- Guerrero, S.; Inostroza-Riquelme, M.; Contreras-Orellana, P.; Diaz-Garcia, V.; Lara, P.; Vivanco-Palma, A.; Oyarzun-Ampuero, F. Curcumin-loaded nanoemulsion: A new safe and effective formulation to prevent tumor reinci-dence and metastasis. Nanoscale 2018, 10, 22612–22622. [Google Scholar] [CrossRef]
- Ekambaram, P.; Sathali, A.A.H. Formulation and Evaluation of Solid Lipid Nanoparticles of Ramipril. J. Young-Pharm. 2011, 3, 216–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakkar, V.; Singh, S.; Singla, D.; Kaur, I.P. Exploring solid lipid nanoparticles to enhance the oral bioavailability of curcumin. Mol. Nutr. Food Res. 2011, 55, 495–503. [Google Scholar] [CrossRef]
- Kakkar, V.; Muppu, S.K.; Chopra, K.; Kaur, I.P. Curcumin loaded solid lipid nanoparticles: An efficient formulation ap-proach for cerebral ischemic reperfusion injury in rats. Eur. J. Pharm. Biopharm. 2013, 85, 339–345. [Google Scholar] [CrossRef]
- Yadav, V.R.; Suresh, S.; Devi, K.; Yadav, S. Novel formulation of solid lipid microparticles of curcumin for an-ti-angiogenic and anti-inflammatory activity for optimization of therapy of inflammatory bowel disease. J. Pharm. Pharmacol. 2009, 61, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, T.; Xu, H.; Ren, B.; Cheng, X.; Qi, R.; Liu, H.; Wang, Y.; Yan, L.; Chen, S.; et al. Curcumin-Loaded Solid Lipid Nanoparticles Enhanced Anticancer Efficiency in Breast Cancer. Molecules 2018, 23, 1578. [Google Scholar] [CrossRef] [Green Version]
- Fathy Abd-Ellatef, G.-E.; Gazzano, E.; Chirio, D.; Hamed, A.R.; Belisario, D.C.; Zuddas, C.; Peira, E.; Rolando, B.; Kopecka, J.; Assem Said Marie, M.J.P. Curcumin-loaded solid lipid nanoparticles bypass P-glycoprotein mediated doxorubicin resistance in triple negative breast cancer cells. Pharmaceutics 2020, 12, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazi, K.M.; Mandal, A.S.; Biswas, N.; Guha, A.; Chatterjee, S.; Behera, M.; Kuotsu, K. Niosome: A future of targeted drug delivery systems. J. Adv. Pharm. Technol. Res. 2010, 1, 374. [Google Scholar]
- Jain, S.; Singh, P.; Mishra, V.; Vyas, S.P. Mannosylatedniosomes as adjuvant–carrier system for oral genetic immuniza-tion against Hepatitis B. Immunol. Lett. 2005, 101, 41–49. [Google Scholar] [CrossRef]
- Rungphanichkul, N.; Nimmannit, U.; Muangsiri, W.; Rojsitthisak, P. Preparation of curcuminoid niosomes for enhance-ment of skin permeation. Die Pharm. Int. J. Pharm. Sci. 2011, 66, 570–575. [Google Scholar]
- Davletshina, R.; Ivanov, A.; Shamagsumova, R.; Evtugyn, V.; Evtugyn, G. Electrochemical Biosensor Based on Polyelec-trolyte Complexes with Dendrimer for the Determination of Reversible Inhibitors of Acetylcholinesterase. Anal. Lett. 2021, 54, 1709–1728. [Google Scholar] [CrossRef]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, R.W.; Wilson, O.M.; Crooks, R.M. Characterization, and applications of dendrimer-encapsulated nanoparticles. J. Phys. Chem. B 2005, 109, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Yang, H. Targeted nanosystems: Advances in targeted dendrimers for cancer therapy. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 309–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malekmohammadi, S.; Hadadzadeh, H.; Farrokhpour, H.; Amirghofran, Z. Immobilization of gold nanoparticles on folate-conjugated dendritic mesoporous silica-coated reduced graphene oxide nanosheets: A new nanoplatform for curcumin pH-controlled and targeted delivery. Soft Matter 2018, 14, 2400–2410. [Google Scholar] [CrossRef]
- Wang, L.; Xu, X.; Zhang, Y.; Zhang, Y.; Zhu, Y.; Shi, J.; Sun, Y.; Huang, Q. Encapsulation of curcumin within poly(amidoamine) dendrimers for delivery to cancer cells. J. Mater. Sci. Mater. Electron. 2013, 24, 2137–2144. [Google Scholar] [CrossRef]
- Qin, W.; Yang, K.; Tang, H.; Tan, L.; Xie, Q.; Ma, M.; Yao, S. Improved GFP gene transfection mediated by polyami-doamine dendrimer-functionalized multi-walled carbon nanotubes with high biocompatibility. Colloids Surf. B Biointerfaces 2011, 84, 206–213. [Google Scholar] [CrossRef]
- Akhtar, N.; Mohammed, S.A.; Singh, V.; Abdellatif, A.A.; Mohammad, H.A.; Ahad, A.; Yusuf, M.; Khadri, H.; Naz, M.; Khan, O.J.P.P.A. Liposome-based drug delivery of various anticancer agents of synthetic and natural product origin: A patent over-view. Pharm. Pat. Anal. 2020, 9, 87–116. [Google Scholar] [CrossRef]
- Guo, G.; Fu, S.; Zhou, L.; Liang, H.; Fan, M.; Luo, F.; Qian, Z.; Wei, Y. Preparation of curcumin loaded poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) nanofibers and their in vitro antitumor activity against Glioma 9L cells. Nanoscale 2011, 3, 3825–3832. [Google Scholar] [CrossRef]
- Babaei, E.; Sadeghizadeh, M.; Hassan, Z.M.; Feizi, M.A.H.; Najafi, F.; Hashemi, S.M. Dendrosomal curcumin signifi-cantly suppresses cancer cell proliferation in vitro and in vivo. Int. Immunopharmacol. 2012, 12, 226–234. [Google Scholar] [CrossRef]
- Tahmasebi, B.M.; Erfani, M.V.; Babaei, E.; Najafi, F.; Zamani, M.; Shariati, M.; Sadeghizadeh, M. Dendrosomal Nano-curcumin, the Novel Formulation to Improve the Anticancer Properties of Curcumin. Prog. Biol. Sci. 2015, 5, 143–158. [Google Scholar]
- Malekmohammadi, S.; Hadadzadeh, H.; Rezakhani, S.; Amirghofran, Z. Design and synthesis of gatekeeper coated dendritic silica/titania mesoporous nanoparticles with sustained and controlled drug release properties for targeted syn-ergetic chemo-sonodynamic therapy. ACS Biomater. Sci. Eng. 2019, 5, 4405–4415. [Google Scholar] [CrossRef]
- Wang, S.; Ha, Y.; Huang, X.; Chin, B.; Sim, W.; Chen, R. A New Strategy for Intestinal Drug Delivery via pH-Responsive and Membrane-Active Nanogels. ACS Appl. Mater. Interfaces 2018, 10, 36622–36627. [Google Scholar] [CrossRef] [PubMed]
- Reeves, A.; Vinogradov, S.V.; Morrissey, P.; Chernin, M.; Ahmed, M.M. Curcumin-encapsulating nanogels as an effective anticancer formulation for intracellular uptake. Mol. Cell. Pharmacol. 2015, 7, 25. [Google Scholar] [PubMed]
- Dandekar, P.P.; Jain, R.; Patil, S.; Dhumal, R.; Tiwari, D.; Sharma, S.; Patravale, V. Curcumin-loaded hydrogel nano-particles: Application in anti-malarial therapy and toxicological evaluation. J. Pharm. Sci. 2010, 99, 4992–5010. [Google Scholar] [CrossRef]
- Zhang, Y.; Rauf Khan, A.; Fu, M.; Zhai, Y.; Ji, J.; Bobrovskaya, L.; Zhai, G. Advances in curcumin-loaded nanopreparations: Improving bioavailability and overcoming inherent drawbacks. J. Drug Target. 2019, 27, 917–931. [Google Scholar] [CrossRef]
- Kesharwani, P.; Jain, A.; Srivastava, A.K.; Keshari, M.K. Systematic development and characterization of curcumin-loaded nanogel for topical application. Drug Dev. Ind. Pharm. 2020, 46, 1443–1457. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, G.N.K.; Singh, M.K.; Datri, S.; Karri, V.V.S.R. Design and Development of Curcumin Nanogel for Squamous Cell Carcinoma. J. Pharm. Sci. Res. 2019, 11, 1683. [Google Scholar]
- Khosropanah, M.H.; Dinarvand, A.; Nezhadhosseini, A.; Haghighi, A.; Hashemi, S.; Nirouzad, F.; Dehghani, H. Anal-ysis of the antiproliferative effects of curcumin and nanocurcumin in MDA-MB231 as a breast cancer cell line. Iran. J. Pharm. Res. IJPR 2016, 15, 231. [Google Scholar]
- Chopra, H.; Dey, P.S.; Das, D.; Bhattacharya, T.; Shah, M.; Mubin, S.; Alamri, B.M. Curcumin Nanoparticles as Promising Therapeutic Agents for Drug Targets. Molecules 2021, 26, 4998. [Google Scholar] [CrossRef]
- Menuel, S.; Joly, J.P.; Courcot, B.; Elysée, J.; Ghermani, N.E.; Marsura, A. Synthesis and inclusion ability of a bis-β-cyclodextrin pseudo-cryptand towards Busulfan anticancer agent. Tetrahedron 2007, 63, 1706–1714. [Google Scholar] [CrossRef]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen, H.H.; Másson, M.; Loftsson, T. Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: Solubility, chemical and photochemical stability. Int. J. Pharm. 2002, 244, 127–135. [Google Scholar] [CrossRef]
- Tomren, M.A.; Masson, M.; Loftsson, T.; Tønnesen, H.H. Studies on curcumin and curcuminoids: XXXI. Symmetric and asymmetric curcuminoids: Stability, activity and complexation with cyclodextrin. Int. J. Pharm. 2007, 338, 27–34. [Google Scholar] [CrossRef]
- Darandale, S.S.; Vavia, P.R. Cyclodextrin-based nanosponges of curcumin: Formulation and physicochemical character-ization. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 315–322. [Google Scholar] [CrossRef]
- Sesarman, A.; Tefas, L.; Sylvester, B.; Licarete, E.; Rauca, V.; Luput, L.; Porfire, A. Anti-angiogenic and an-ti-inflammatory effects of long-circulating liposomes co-encapsulating curcumin and doxorubicin on C26 murine colon cancer cells. Pharmacol. Rep. 2018, 70, 331–339. [Google Scholar] [CrossRef]
- Chen, Y.; Du, Q.; Guo, Q.; Huang, J.; Liu, L.; Shen, X.; Peng, J. AW/O emulsion mediated film dispersion method for cur-cumin encapsulated pH-sensitive liposomes in the colon tumor treatment. Drug Dev. Ind. Pharm. 2019, 45, 282–291. [Google Scholar] [CrossRef]
- Huo, X.; Zhang, Y.; Jin, X.; Li, Y.; Zhang, L. A novel synthesis of selenium nanoparticles encapsulated PLGA nanospheres with curcumin molecules for the inhibition of amyloid β aggregation in Alzheimer’s disease. J. Photochem. Photobiol. B Biol. 2019, 190, 98–102. [Google Scholar] [CrossRef]
- Reddy, A.S.; Lakshmi, B.A.; Kim, S.; Kim, J. Synthesis and characterization of acetyl curcumin-loaded core/shell lipo-some nanoparticles via an electrospray process for drug delivery, and theranostic applications. Eur. J. Pharma-Ceutics Biopharm. 2019, 142, 518–530. [Google Scholar] [CrossRef]
- Raveendran, R.; Bhuvaneshwar, G.S.; Sharma, C.P. In vitro cytotoxicity and cellular uptake of curcumin-loaded Pluron-ic/Polycaprolactone micelles in colorectal adenocarcinoma cells. J. Biomater. Appl. 2013, 27, 811–827. [Google Scholar] [CrossRef]
- Yu, H.; Li, J.; Shi, K.; Huang, Q. Structure of modified ε-polylysine micelles and their application in improving cellular antioxidant activity of curcuminoids. Food Funct. 2011, 2, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Podaralla, S.; Averineni, R.; Alqahtani, M.; Perumal, O. Synthesis of novel biodegradable methoxy poly (ethylene gly-col)–zein micelles for effective delivery of curcumin. Mol. Pharm. 2012, 9, 2778–2786. [Google Scholar] [CrossRef]
- Song, Z.; Feng, R.; Sun, M.; Guo, C.; Gao, Y.; Li, L.; Zhai, G. Curcumin-loaded PLGA-PEG-PLGA triblock copolymeric micelles: Preparation, pharmacokinetics and distribution in vivo. J. Colloid Interface Sci. 2011, 354, 116–123. [Google Scholar] [CrossRef]
- Maran, A.; Yaszemski, M.J.; Kohut, A.; Voronov, A. Curcumin and osteosarcoma: Can invertible polymeric micelles help? Materials 2016, 9, 520. [Google Scholar] [CrossRef] [PubMed]
- Javadi, S.; Rostamizadeh, K.; Hejazi, J.; Parsa, M.; Fathi, M. Curcumin mediated down-regulation of αVβ3 integrin and up-regulation of pyruvate dehydrogenase kinase 4 (PDK4) in Erlotinib resistant SW480 colon cancer cells. Phytother. Res. 2018, 32, 355–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Li, Q.; Li, H.; Yang, L.; Yi, J.-Z.; Xie, M.; Zhang, L.-M. Long-circulating zein-polysulfobetaine conjugate-based nanocarriers for enhancing the stability and pharmacokinetics of curcumin. Mater. Sci. Eng. C 2020, 109, 110636. [Google Scholar] [CrossRef] [PubMed]
- Lazăr, L.F.; Olteanu, E.D.; Iuga, R.; Burz, C.; Achim, M.; Clichici, S.; Tefas, L.R.; Nenu, I.; Tudor, D.; Baldea, I.; et al. Solid lipid nanoparticles: Vital characteristics and prospective applications in cancer treatment. Crit. Rev. Ther. Drug Carr. Syst. 2019, 36, 537–581. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Su, X.; Ding, B.; He, X.; Liu, X.; Yu, A.; Lou, H.; Zhai, G. Advances in nanotechnology-based delivery systems for curcumin. Nanomedicine 2012, 7, 1085–1100. [Google Scholar] [CrossRef]
- Bhatt, H.; Rompicharla, S.V.; Komanduri, N.; Aashma, S.; Paradkar, S.; Ghosh, B.; Biswas, S. Development of curcu-min-loaded solid lipid nanoparticles utilizing glyceryl monostearate as single lipid using QbD approach: Characterization and evaluation of anticancer activity against human breast cancer cell line. Curr. Drug Deliv. 2018, 15, 1271–1283. [Google Scholar] [CrossRef]
- Li, Z.; Shi, M.; Li, N.; Xu, R. Application of functional biocompatible nanomaterials to improve curcumin bioavailability. Front. Chem. 2020, 8, 929. [Google Scholar] [CrossRef]
- Liu, J.; Chen, S.; Lv, L.; Song, L.; Guo, S.; Huang, S. Recent progress in studying curcumin and its nano-preparations for cancer therapy. Curr. Pharm. Des. 2013, 19, 1974–1993. [Google Scholar] [CrossRef]
- Debnath, S.; Saloum, D.; Dolai, S.; Sun, C.; Averick, S.; Raja, K.; Fata, J.E. Dendrimer-Curcumin Conjugate: A Water Soluble and Effective Cytotoxic Agent Against Breast Cancer Cell Lines. Anti-Cancer Agents Med. Chem. 2013, 13, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.C.; Yallapu, M.M.; Ebeling, M.C.; Chauhan, N.; Jaggi, M. Interaction of curcumin nanoformulations with human plasma proteins and erythrocytes. Int. J. Nanomed. 2011, 6, 2779–2790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.; Zhang, H.; Wang, Y.; Yang, J.; Jiang, F. Investigation on the interaction behavior between curcumin and PAMAM dendrimer by spectral and docking studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 108, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Mollazade, M.; Zarghami, N.; Nasiri, M.; Nejati, K.; Rahmati, M.; Pourhasan, M. Polyamidoamine (PAMAM) encapsulat-ed curcumin inhibits telomerase activity in breast cancer cell line. Clin. Biochem. 2011, 13, S217. [Google Scholar] [CrossRef]
- Falconieri, M.C.; Adamo, M.; Monasterolo, C.; Bergonzi, M.C.; Coronnello, M.; Bilia, A.R. New dendrimer-based na-noparticles enhance curcumin solubility. Planta Med. 2017, 83, 420–425. [Google Scholar] [PubMed]
- Elmi, T.; Ardestani, M.S.; Hajialiani, F.; Motevalian, M.; Mohamadi, M.; Sadeghi, S.; Tabatabaie, F. Novel chloroquine loaded curcumin based anionic linear globular dendrimer G2: A metabolomics study on Plasmodium falciparum in vitro using 1H NMR spectroscopy. Parasitology 2020, 147, 747–759. [Google Scholar]
- Mangalathillam, S.; Rejinold, N.S.; Nair, A.; Lakshmanan, V.K.; Nair, S.V.; Jayakumar, R. Curcumin loaded chitin nanogels for skin cancer treatment via the transdermal route. Nanoscale 2012, 4, 239–250. [Google Scholar] [CrossRef]
- Wei, X.; Senanayake, T.H.; Warren, G.; Vinogradov, S.V. Hyaluronic acid-based nanogel–drug conjugates with en-hanced anticancer activity designed for the targeting of CD44-positive and drug-resistant tumors. Bioconj. Chem. 2013, 24, 658–668. [Google Scholar] [CrossRef] [Green Version]
- Amanlou, N.; Parsa, M.; Rostamizadeh, K.; Sadighian, S.; Moghaddam, F. Enhanced cytotoxic activity of curcumin on cancer cell lines by incorporating into gold/chitosan nanogels. Mater. Chem. Phys. 2019, 226, 151–157. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, R.X.; Zhang, C.; Dai, C.; Ju, X.; He, R. Fabrication of Stable and Self-Assembling Rapeseed Protein Nanogel for Hydrophobic Curcumin Delivery. J. Agric. Food Chem. 2019, 67, 887–894. [Google Scholar] [CrossRef]
- Priya, P.; Raj, R.M.; Vasanthakumar, V.; Raj, V. Curcumin-loaded layer-by-layer folic acid and casein coated carbox-ymethyl cellulose/casein nanogels for treatment of skin cancer. Arab. J. Chem. 2020, 13, 694–708. [Google Scholar] [CrossRef]
- Nabih Maria, D.; Mishra, S.R.; Wang, L.; Helmy Abd-Elgawad, A.E.; Abd-Elazeem Soliman, O.; Salah El-Dahan, M.; Jablonski, M. Water-soluble complex of curcumin with cyclodextrins: Enhanced physical properties for ocular drug delivery. Curr. Drug Deliv. 2017, 14, 875–886. [Google Scholar]
- Guo, S. Encapsulation of curcumin into β-cyclodextrins inclusion: A review. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2019; Volume 131, p. 1100. [Google Scholar]
- Parohan, M.; Sarraf, P.; Javanbakht, M.H.; Foroushani, A.R.; Ranji-Burachaloo, S.; Djalali, M. The synergistic effects of nano-curcumin and coenzyme Q10 supplementation in migraine prophylaxis: A randomized, placebo-controlled, double-blind trial. Nutr. Neurosci. 2021, 24, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Honarvar, N.M.; Soveid, N.; Abdolahi, M.; Djalali, M.; Hatami, M.; Karzar, N.H. Anti-Neuroinflammatory Properties of n-3 Fatty Acids and Nano-Curcumin on Migraine Patients from Cellular to Clinical Insight: A Randomized, Double-Blind and Pla-cebo-Controlled Trial. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 365–373. [Google Scholar]
- Karimi, A.; Mahmoodpoor, A.; Kooshki, F.; Niazkar, H.R.; Shoorei, H.; Tarighat-Esfanjani, A. Effects of nanocurcumin on in-flammatory factors and clinical outcomes in critically ill patients with sepsis: A pilot randomized clinical trial. Eur. J. Integr. Med. 2020, 36, 101122. [Google Scholar] [CrossRef]
- Talebi, S.; Safarian, M.; Jaafari, M.R.; Sayedi, S.J.; Abbasi, Z.; Ranjbar, G.; Kianifar, H.R. The effects of nano-curcumin as a nutritional strategy on clinical and inflammatory factors in children with cystic fibrosis: The study protocol for a randomized con-trolled trial. Trials 2021, 22, 292. [Google Scholar] [CrossRef]
- Cheragh-Birjandi, S.; Moghbeli, M.; Haghighi, F.; Safdari, M.R.; Baghernezhad, M.; Akhavan, A.; Ganji, R. Impact of resistance ex-ercises and nano-curcumin on synovial levels of collagenase and nitric oxide in women with knee osteoarthritis. Transl. Med. Commun. 2020, 5, 3. [Google Scholar] [CrossRef]
- Bakhshi, M.; Gholami, S.; Mahboubi, A.; Jaafari, M.R.; Namdari, M. Combination Therapy with 1% Nanocurcumin Gel and 0.1% Triamcinolone Acetonide Mouth Rinse for Oral Lichen Planus: A Randomized Double-Blind Placebo Controlled Clinical Trial. Dermatol. Res. Pract. 2020, 2020, 4298193. [Google Scholar] [CrossRef]
- Dolati, S.; Babaloo, Z.; Ayromlou, H.; Ahmadi, M.; Rikhtegar, R.; Rostamzadeh, D.; Roshangar, L.; Nouri, M.; Mehdizadeh, A.; Younesi, V.; et al. Nanocurcumin improves regulatory T-cell frequency and function in patients with multiple sclero-sis. J. Neuroimmunol. 2019, 327, 15–21. [Google Scholar] [CrossRef]
- Singh, P.K.; Prabhune, A.A.; Ogale, S.B. Curcumin-Sophorolipid Complex. U.S. Patent 9,931,309, 3 April 2018. [Google Scholar]
- Sezgin, V.C.; Bayraktar, O. Development of Curcumin and Piperine Loaded Double-Layered Biopolymer Based Nano Delivery Systems by Using Electrospray/Coating Method. U.S. Patent 10,398,650, 3 September 2019. [Google Scholar]
- Ranjan, A.P.; Mukerjee, A.; Vishwanatha, J.K. Solid in Oil/Water Emulsion-Diffusion-Evaporation Formulation for Preparing Curcumin-Loaded PLGA Nanoparticles. U.S. Patent 12/766,068, 18 November 2010. [Google Scholar]
- Haas, H.; Fattler, U. Liposomal Formulations of Lipophilic Compounds. U.S. Patent 10,413,511, 17 September 2019. [Google Scholar]
- Ranjan, A.P.; Mukerjee, A.; Vishwanatha, J.K.; Helson, L. Curcuminer, a Liposomal-PLGA Sustained Release Nanocurcumin for Minimizing QT Prolongation for Cancer Therapy. U.S. Patent 9,138,411, 22 September 2015. [Google Scholar]
- Kurzrock, R.; Li, L.; Mehta, K.; Aggarwal, B.B. Liposomal Curcumin for Treatment of Cancer. U.S. Patent 9,283,185, 15 March 2016. [Google Scholar]
- Di Martino, R.M.; Luppi, B.; Bisi, A.; Gobbi, S.; Rampa, A.; Abruzzo, A.; Belluti, F. Recent progress on curcumin-based therapeutics: A patent review (2012–2016). Part I: Curcumin. Expert Opin. Ther. Patents 2017, 27, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kesarla, R.; Chotai, N.; Misra, A.; Omri, A. Systematic Approach for the Formulation and Optimization of Solid Lipid Nanoparticles of Efavirenz by High Pressure Homogenization Using Design of Experiments for Brain Targeting and Enhanced Bioavailability. BioMed Res. Int. 2017, 2017, 5984014. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, M.E.; Vela Ramirez, J.E.; Peppas, N.A. 110th anniversary: Nanoparticle mediated drug delivery for the treatment of Alzheimer’s disease: Crossing the blood–brain barrier. Ind. Eng. Chem. Res. 2019, 58, 15079–15087. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Liu, Y.; Li, R.; Wu, W.; Fawcett, J.P.; Gu, J. Absorption, distribution, metabolism and excretion of the biomaterials used in Nanocarrier drug delivery systems. Adv. Drug Deliv. Rev. 2019, 143, 97–114. [Google Scholar] [CrossRef] [PubMed]





| Mechanisms Involved in Degeneration in AD | Effects of Curcumin |
|---|---|
| β-amyloid | |
|
|
|
|
|
|
|
|
|
|
|
|
| Oxidative stress | |
|
|
|
|
|
|
|
|
| Types | Form | Size (nm) | Study Models | Models | Outcomes | References |
|---|---|---|---|---|---|---|
| Liposome | Globular | 25–205 | Breast cancer, lung cancer, renal ischemia, and malaria | In vivo, in vitro | Antitumor and antiangiogenesis effects were improved; antimelanoma, anti-inflammatory, and antimalarial activities were demonstrated | [188,189,190] |
| Micelle | Spherical | 10–100 | Lung, colorectal, and breast cancer | In vivo, in vitro | Improved antioxidative and anticancer properties; enhanced solubility and bioavailability; longer circulation period and enhanced fluorescence impact | [191,192,193,194,195,196,197] |
| Solid lipid nanoparticles | Spherical | 50–1000 | Ischemia of the brain, colitis, allergies, and breast cancer | In vivo, in vitro | Improved anti-inflammatory properties; enhanced blood circulation; enhanced brain delivery | [198,199,200,201,202] |
| Niosome | Lamellar | 190–1140 | Cancer cells | In vivo, in vitro | Improved fluorescence intensity; anticancer properties | [158] |
| Dendrimer | Globular polymer | 15–150 | Breast cancer and colon cancer | In vivo, in vitro | Enhanced antitumor and antiproliferative effects; improved stability | [203,204,205,206,207,208] |
| Nanogel | Network of cross-linked polymers | 10–200 | Melanoma and breast, pancreatic, colorectal, andskin cancer | In vitro | Improved fluorescence effects; improved bioavailability; increased anticancer activity; more regulated release; extended half-life; improved melanoma therapy | [209,210,211,212,213] |
| Cyclodextrin | Cyclic | 150–500 | Cancers of the bowel, breast, lung, pancreas, and prostate | In vivo, in vitro | Increased solubility; stronger antiproliferation effects; improved anticancer and anti-inflammatory properties; Improved bioavailability | [214,215] |
| S. No. | Clinical Studies Identifier | Study Title | Interventions | Phase, Recruitment Status | Place Intended for Study | References |
|---|---|---|---|---|---|---|
| 1 | IRCT2017080135444N1 | The synergistic effects of nanocurcumin and coenzyme Q10 supplementation in migraine prophylaxis: a randomized, placebo-controlled, double-blind trial | Migraine-related impairment | Phase 2 and 3, completed | Tehran University of Medical Sciences, Tehran, Iran | [216] |
| 2 | NCT02532023 | Effects of nanocurcumin on inflammatory factors and clinical outcomes in critically ill patients with sepsis: A pilot randomized clinical trial | Patients with sepsis who are severely sick | Phase 4, completed | Tabriz University of Medical Sciences, Tabriz, Iran | [217] |
| 3 | IRCT20200705048018N1 | The effects of nanocurcumin as a nutritional strategy on clinical and inflammatory factors in children with cystic fibrosis: the study protocol for a randomized controlled trial | Cystic fibrosis | Phase 1, Recruiting | Akbar Children’s Hospital, Mashhad, Iran. | [218] |
| 4 | IRCT20161208031300N1 | Impact of resistance exercises and nanocurcumin on synovial levels of collagenase and nitric oxide in women with knee osteoarthritis | Osteoarthritis | Phase 3, completed | Imam Ali Hospital, Bojnourd, Iran | [219] |
| 5 | IRCT20190523043678N1 | Combination Therapy with 1% Nanocurcumin Gel and 0.1% Triamcinolone Acetonide Mouth Rinse for Oral Lichen Planus: A Randomized Double-Blind Placebo Controlled Clinical Trial | Oral lichen planus | Phase 3, completed | Shahid Beheshti University of Medical Sciences, Iran | [220] |
| 6 | NCT03150966 | The immunomodulatory effects of oral nanocurcumin in multiple sclerosis patients | Multiple sclerosis | Phase 2, completed | Tabriz University of Medical Sciences, Iran | [221] |
| 7 | NCT01201694 | Study on surface controlled water soluble curcumin | Cancer | Phase 1, completed | UT MD Anderson Cancer Center Houston, Texas, United States | [222] |
| S. No. | Patent No. | Study Title | Interventions | References |
|---|---|---|---|---|
| 1 | US 9, 931, 309 B2 | Complex curcumine-sophorolipids | Nanoencapsulated in acidic sophorolipids to enhance curcumin’s bioavailability and solubility to boost its pharmacological response, including cancer | [223] |
| 2 | US20180028447 | Development of curcumin and piperine loaded double-layered biopolymer based nano delivery systems by using electrospray/coating method | Curcumin was contained in the core network, which was made up of zein protein, and piperinewas encased in the outermost casing, which was chitosan. Although the precise method emphasizing the molecular mechanism of piperine for curcumin improvement was not defined, it was demonstrated that decreasing the efficiency of cytochrome P4503A4 (CYP3A4), which plays a role in curcumin metabolism, enhanced the residence duration of curcumin. | [224] |
| 3 | US20100290982A1 | Solid in oil/water emulsion-diffusion-evaporation formulation for preparing curcumin-loaded PLGA nanoparticles | Findings were obtained by producing the solid in oil/water emulsion diffusion evaporation composition for generating curcumin-loaded PLGA nanoparticles. | [225] |
| 4 | US10413511B2 | Liposomal formulations of lipophilic compounds | Revealed unique preparations for curing refractory and resistant pancreatic malignancies with a paclitaxel in a cationic liposomal form; gemcitabine, a ribonucleotide reductase inhibitor that prevents DNA synthesis in cancerous cells; and other anticancer drugs | [226] |
| 5 | US9138411B2 | Curcumin-ER, a liposomal-PLGA sustained release nanocurcumin for minimizing QT prolongation for cancer therapy | The bioactive substance curcumin and curcumin–PLGA analogues were utilized in the liposome, which consisted of a polymeric core with ground lipidic components. Human embryonic kidney (HEK 293) cell lines treated with the human ether-related gene (hERG) were used to test it. The whole-cell patch-clamp present review and approval approach was used to examine the in vitro consequences of the curcumin liposomal preparation of potassium-selective IKr currents produced in normoxia in stably transfected HEK 293 cells. | [227] |
| 6 | US9283185B2 | Liposomal curcumin for treatment of cancer | In human patients, curcumin analogues and curcumin enclosed as liposomal preparations were revealed to treat pancreatic cancer, breast cancer, and melanoma. | [228] |
| 7 | WO 2013132457 | Nanocrystalline solid dispersion compositions and process of preparation thereof | A curcumin–stearic acid mixture was produced and nebulized by spray-drying to create a dry powder to produce nanocrystalline solid dispersion. Oral dosing of spray-dried curcumin and curcumin–stearic acid nanocrystalline solid dispersion in rats resulted in a 15-fold increase in curcumin oral bioavailability with nanocrystalline solid dispersion compared to the control. | [229] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tagde, P.; Tagde, P.; Islam, F.; Tagde, S.; Shah, M.; Hussain, Z.D.; Rahman, M.H.; Najda, A.; Alanazi, I.S.; Germoush, M.O.; et al. The Multifaceted Role of Curcumin in Advanced Nanocurcumin Form in the Treatment and Management of Chronic Disorders. Molecules 2021, 26, 7109. https://doi.org/10.3390/molecules26237109
Tagde P, Tagde P, Islam F, Tagde S, Shah M, Hussain ZD, Rahman MH, Najda A, Alanazi IS, Germoush MO, et al. The Multifaceted Role of Curcumin in Advanced Nanocurcumin Form in the Treatment and Management of Chronic Disorders. Molecules. 2021; 26(23):7109. https://doi.org/10.3390/molecules26237109
Chicago/Turabian StyleTagde, Priti, Pooja Tagde, Fahadul Islam, Sandeep Tagde, Muddaser Shah, Zareen Delawar Hussain, Md. Habibur Rahman, Agnieszka Najda, Ibtesam S. Alanazi, Mousa O. Germoush, and et al. 2021. "The Multifaceted Role of Curcumin in Advanced Nanocurcumin Form in the Treatment and Management of Chronic Disorders" Molecules 26, no. 23: 7109. https://doi.org/10.3390/molecules26237109