Titanium and Protein Adsorption: An Overview of Mechanisms and Effects of Surface Features
Abstract
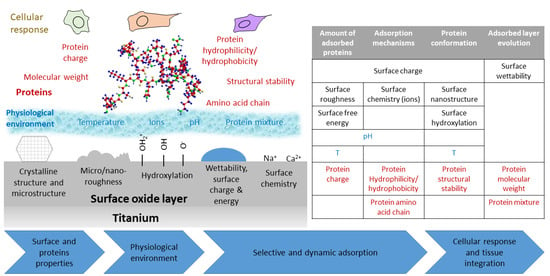
:1. Introduction
2. Driving Forces and Factors Affecting Protein Adsorption
2.1. Driving Forces of Protein Adsorption
2.2. Surface Effect on Protein Adsorption
2.3. Protein Characteristics Affecting Adsorption
2.4. External Parameters Affecting Protein Adsorption
3. How the Characteristics of Titanium Based Biomaterials Influence Protein Adsorption
3.1. General Consideration on Protein Adsorption on Titanium Based Materials
| Hydrogen bonding: |  |
| Proton transfer: |  |
3.2. Effect of Surface Modifications on Titanium: How Topography, Roughness and Surface Chemistry Change Protein Adsorption
3.2.1. Surface Modification by Sand Blasting and Acid Etching (SLA)
3.2.2. Surface Modification by Chemical and Hydrothermal Treatments
3.2.3. Growth of Titania Nanotubes (TNTs)
3.2.4. Other Surface Modification Techniques
3.3. Effect of Alloying Elements and Surface Ion Doping
3.4. Grain Size and Crystalline Phase
3.5. Surface Activation
4. External Parameters Affecting Protein Adsorption on Titanium Surfaces
4.1. Aging and Storage: Contamination of Titanium Surfaces
4.2. Influence of the Solution: pH, Temperature and Ions
4.3. Protein Concentration in Solution
5. Protein Co-Adsorption and Competition for the Surface
6. Methods for Investigating Protein Adsorption on Titanium-Based Materials
| Technique | Output | Substrate | In Situ/Real Time | Advantages | Drawbacks | References | |
|---|---|---|---|---|---|---|---|
| Labeled proteins | 125I-labeling | Quantification | Any | Yes/no | Direct quantification | Change of protein properties, handling issues | [144,145] |
| Fluorescent labeling | Quantification and imaging | Any | Yes/no | Direct quantification, competitive adsorption evaluation | Change of protein properties, expensive reagents | [74,80,100,116] | |
| UV-vis spectroscopy | BCA | Quantification | Any | No/no | Low cost, large range of concentrations | Protein detachment needed | [98,101,114,115] |
| Bradford assay | Quantification | Any | No/no | Low time consume | Protein detachment needed, sensible to surfactant | [88,155,224] | |
| Spectrophotometry (λ = 280 nm) | Quantification | Any | No/no | No reactant needed | Protein detachment needed, inaccurate with complex samples | [81,117] | |
| Labeled antibodies | Quantification, protein recognition and imaging | Any | Yes/no | Targeting of specific proteins | Time consuming, specific reagents | [94,107,131,173] | |
| ELISA | Quantification and protein recognition | Any | Yes/no | High specificity | Time consuming, specific reagents | [104,128,129,152] | |
| Gel electrophoresis | Western blot | Quantification and protein recognition | Any | No/no | No toxic chemicals | Sample preparation, poor band separation | [102,130] |
| SDS-PAGE | Quantification and protein recognition | Any | No/no | High sensitivity, small samples needed | Poor band resolution, toxic chemicals | [109,230] | |
| LC-EIS-MS/MS | Proteomic analysis | Any | No/no | High specificity and sensitivity | High costs | [229,233,234] | |
| XPS | Quantification, protein-surface interaction | Any | Yes/no | High sensitivity, simultaneous evaluation of surface chemistry, depth profiling | No absolute quantification, complex data analysis | [110,114,133,212] | |
| Tof-SIMS | Quantification, protein recognition | Any | Yes/no | High sensitivity, possible orientation and conformation analysis, depth profiling | No absolute quantification, complex data analysis | [50,128,223] | |
| WSD | Quantification | Any | Yes/no | Sensitive to a wide range of protein surface concentration | Thorough calibration needed | [76,147] | |
| AFM | Imaging, adhesion forces, conformation | Flat substrates | Yes/no | High resolution, customizable tip | Low throughput, time consuming | [58,82,110,143] | |
| CLSM | Imaging, relative quantification | Any | Yes/no | High resolution, 3D distribution into surface features | Expensive reagents | [95,100] | |
| TEM | Imaging, thickness measurement | Any | Yes/no | Direct visualization of protein layer | Complex sample preparation | [180] | |
| Zeta potential | Adsorption evaluation, protein conformation | Powder or planar samples | Yes/no | Simple measurement | No protein recognition, preliminary information needed | [54,78,228] | |
| QCM | Quantification, viscoelastic properties of layer, changes in conformation | Sputtered sensors | Yes/Yes | High sensitivity, real time measurement, possibility to change the uptake solution | Co-adsorbed solvent weighted. Mass calculation affected by energy dissipation | [67,112,226,227,238] | |
| FTIR (ATR) | Secondary structure, relative quantification | Planar samples | Yes/no | Very specific protein band | Not highly sensitive, data deconvolution needed | [113,114,216] | |
| Raman spectroscopy | Secondary structure, relative quantification | Any | Yes/no | Very specific protein band | Not highly sensitive, complex data interpretation | [71] | |
| SE | Layer thickness measurement | Flat surfaces | Yes/yes | High sensitivity, low cost, fast measurement | Difficult optical modeling of rough and structured surfaces | [86,208,209] | |
| SFS | Protein conformation | Any | Yes/no | Sensitive, high selectivity towards specific amino acids | Possible instrument artifacts | [113,114,115] | |
| EIS | Layer evolution, protein-surface interactions | Planar samples | Yes/yes | High sensitivity, possible to study adsorption in different condition | Complex modelling and data interpretation | [57,61,169,212] | |
| CD | Protein conformation | Planar samples | Yes/no | Specific bands for secondary structures | Band deconvolution needed | [187] | |
7. Key Concepts
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, J. Metallic biomaterials: State of the art and new challenges. In Fundamental Biomaterials: Metals; Balakrishnan, P., Sreekala, M.S., Sabu, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–33. ISBN 978-0-08-102206-1. [Google Scholar]
- Oldani, C.; Dominguez, A. Titanium as a Biomaterial for Implants. In Recent Advances in Arthroplasty; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Buser, D.; Janner, S.F.M.; Wittneben, J.G.; Brägger, U.; Ramseier, C.A.; Salvi, G.E. 10-Year Survival and Success Rates of 511 Titanium Implants with a Sandblasted and Acid-Etched Surface: A Retrospective Study in 303 Partially Edentulous Patients. Clin. Implant Dent. Relat. Res. 2012, 14, 839–851. [Google Scholar] [CrossRef]
- Trindade, R.; Albrektsson, T.; Tengvall, P.; Wennerberg, A. Foreign Body Reaction to Biomaterials: On Mechanisms for Buildup and Breakdown of Osseointegration. Clin. Implant Dent. Relat. Res. 2016, 18, 192–203. [Google Scholar] [CrossRef]
- Anderson, J.M.; Jiang, S. Implications of the Acute and Chronic Inflammatory Response and the Foreign Body Reaction to the Immune Response of Implanted Biomaterials. In The Immune Response to Implanted Materials and Devices; Corradetti, B., Ed.; Springer: Cham, Switzerland, 2016; ISBN 9783319454337. [Google Scholar]
- Pegueroles, M.; Aguirre, A.; Engel, E.; Pavon, G.; Gil, F.J.; Planell, J.A.; Migonney, V.; Aparicio, C. Effect of blasting treatment and Fn coating on MG63 adhesion and differentiation on titanium: A gene expression study using real-time RT-PCR. J. Mater. Sci. Mater. Med. 2011, 22, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.S.; Chang, J.H.; Huang, H.H. Enhancing the biological response of titanium surface through the immobilization of bone morphogenetic protein-2 using the natural cross-linker genipin. Surf. Coat. Technol. 2016, 303, 289–297. [Google Scholar] [CrossRef]
- Spriano, S.; Yamaguchi, S.; Baino, F.; Ferraris, S. A critical review of multifunctional titanium surfaces: New frontiers for improving osseointegration and host response, avoiding bacteria contamination. Acta Biomater. 2018, 79, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Norde, W. My voyage of discovery to proteins in flatland...and beyond. Colloids Surf. B Biointerfaces 2008, 61, 1–9. [Google Scholar] [CrossRef]
- Yano, Y.F. Kinetics of protein unfolding at interfaces. J. Phys. Condens. Matter 2012, 24, 503101. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.J. The interaction of proteins with solid surfaces. Curr. Opin. Struct. Biol. 2004, 14, 110–115. [Google Scholar] [CrossRef]
- Rabe, M.; Verdes, D.; Seeger, S. Understanding protein adsorption phenomena at solid surfaces. Adv. Colloid Interface Sci. 2011, 162, 87–106. [Google Scholar] [CrossRef] [Green Version]
- Norde, W. Driving forces for protein adsorption at solid surfaces. Macromol. Symp. 1996, 103, 5–18. [Google Scholar] [CrossRef]
- Vogler, E.A. Protein adsorption in three dimensions. Biomaterials 2012, 33, 1201–1237. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Kapp, M.; Boccaccini, A.R. Protein interactions with bioactive glass surfaces: A review. Appl. Mater. Today 2019, 15, 350–371. [Google Scholar] [CrossRef]
- Lee, W.H.; Loo, C.Y.; Rohanizadeh, R. A review of chemical surface modification of bioceramics: Effects on protein adsorption and cellular response. Colloids Surf. B Biointerfaces 2014, 122, 823–834. [Google Scholar] [CrossRef]
- Hedberg, Y.; Wang, X.; Hedberg, J.; Lundin, M.; Blomberg, E.; Odnevall Wallinder, I. Surface-protein interactions on different stainless steel grades: Effects of protein adsorption, surface changes and metal release. J. Mater. Sci. Mater. Med. 2013, 24, 1015–1033. [Google Scholar] [CrossRef] [Green Version]
- Höhn, S.; Virtanen, S.; Boccaccini, A.R. Protein adsorption on magnesium and its alloys: A review. Appl. Surf. Sci. 2019, 464, 212–219. [Google Scholar] [CrossRef]
- Bhakta, S.A.; Evans, E.; Benavidez, T.E.; Garcia, C.D. Protein adsorption onto nanomaterials for the development of biosensors and analytical devices: A review. Anal. Chim. Acta 2015, 872, 7–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsapikouni, T.S.; Missirlis, Y.F. Protein–material interactions: From micro-to-nano scale. Mater. Sci. Eng. B 2008, 152, 2–7. [Google Scholar] [CrossRef]
- Godbey, W.T. Proteins. In An Introduction to Biotechnology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 9–33. ISBN 9781907568282. [Google Scholar]
- Czeslik, C. Factors Ruling Protein Adsorption. Z. Phys. Chem. 2004, 218, 771–801. [Google Scholar] [CrossRef]
- Metwally, S.; Stachewicz, U. Surface potential and charges impact on cell responses on biomaterials interfaces for medical applications. Mater. Sci. Eng. C 2019, 104, 109883. [Google Scholar] [CrossRef] [PubMed]
- Adair, J.H.; Suvaci, E.; Sindel, J. Surface and Colloid Chemistry. In Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2001; Volume 3, pp. 1–10. [Google Scholar]
- Firkowska-Boden, I.; Zhang, X.; Jandt, K.D. Controlling Protein Adsorption through Nanostructured Polymeric Surfaces. Adv. Healthc. Mater. 2018, 7, 1–19. [Google Scholar] [CrossRef]
- Felgueiras, H.P.; Antunes, J.C.; Martins, M.C.L.; Barbosa, M.A. Fundamentals of Protein and Cell Interactions in Biomaterials; Elsevier: Amstredam, The Netherlands, 2018; ISBN 9780081008522. [Google Scholar]
- Vogler, E.A. Structure and reactivity of water at biomaterial surfaces. Adv. Colloid Interface Sci. 1998, 74, 69–117. [Google Scholar] [CrossRef]
- Xu, L.C.; Siedlecki, C.A. Effects of surface wettability and contact time on protein adhesion to biomaterial surfaces. Biomaterials 2007, 28, 3273–3283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michael, K.E.; Vernekar, V.N.; Keselowsky, B.G.; Meredith, J.C.; Latour, R.A.; García, A.J. Adsorption-induced conformational changes in fibronectin due to interactions with well-defined surface chemistries. Langmuir 2003, 19, 8033–8040. [Google Scholar] [CrossRef]
- Roach, P.; Farrar, D.; Perry, C.C. Interpretation of protein adsorption: Surface-induced conformational changes. J. Am. Chem. Soc. 2005, 127, 8168–8173. [Google Scholar] [CrossRef]
- Isoshima, K.; Ueno, T.; Arai, Y.; Saito, H.; Chen, P.; Tsutsumi, Y.; Hanawa, T.; Wakabayashi, N. The change of surface charge by lithium ion coating enhances protein adsorption on titanium. J. Mech. Behav. Biomed. Mater. 2019, 100, 103393. [Google Scholar] [CrossRef]
- Kubiak-Ossowska, K.; Jachimska, B.; Al Qaraghuli, M.; Mulheran, P.A. Protein interactions with negatively charged inorganic surfaces. Curr. Opin. Colloid Interface Sci. 2019, 41, 104–117. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.G.; Li, X.X.; Lv, G.J.; Xie, W.Y.; Zhu, J.; Luxbacher, T.; Ma, R.; Ma, X.J. Effect of surface wettability and charge on protein adsorption onto implantable alginate-chitosan-alginate microcapsule surfaces. J. Biomed. Mater. Res. Part A 2010, 92, 1357–1365. [Google Scholar] [CrossRef]
- Bousnaki, M.; Koidis, P. Advances on Biomedical Titanium Surface Interactions. J. Biomim. Biomater. Tissue Eng. 2014, 19, 43–64. [Google Scholar] [CrossRef]
- Rechendorff, K.; Hovgaard, M.B.; Foss, M.; Zhdanov, V.P.; Besenbacher, F. Enhancement of protein adsorption induced by surface roughness. Langmuir 2006, 22, 10885–10888. [Google Scholar] [CrossRef]
- Fernández-Montes Moraleda, B.; Román, J.S.; Rodríguez-Lorenzo, L.M. Influence of surface features of hydroxyapatite on the adsorption of proteins relevant to bone regeneration. J. Biomed. Mater. Res. Part A 2013, 101A, 2332–2339. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Zhang, L.; Sun, Y. Protein behavior at surfaces: Orientation, conformational transitions and transport. J. Chromatogr. A 2015, 1382, 118–134. [Google Scholar] [CrossRef]
- Zhao, A.; Wang, Z.; Zhou, S.; Xue, G.; Wang, Y.; Ye, C.; Huang, N. Titanium oxide films with vacuum thermal treatment for enhanced hemocompatibility. Surf. Eng. 2015, 31, 898–903. [Google Scholar] [CrossRef]
- Lee, E.-J.; Kwon, J.-S.; Om, J.-Y.; Moon, S.-K.; Uhm, S.-H.; Choi, E.H.; Kim, K.-N. The enhanced integrin-mediated cell attachment and osteogenic gene expression on atmospheric pressure plasma jet treated micro-structured titanium surfaces. Curr. Appl. Phys. 2014, 14, S167–S171. [Google Scholar] [CrossRef]
- Blanco, A.; Blanco, G. Proteins. In Medical Biochemistry; Elsevier: Amsterdam, The Netherlands, 2017; pp. 21–71. ISBN 978-0-12-803550-4. [Google Scholar]
- Bharti, B. Adsorption, Aggregation and Structure Formation in Systems of Charged Particles; Springer Theses; Springer International Publishing: Cham, Switzerland, 2014; ISBN 978-3-319-07736-9. [Google Scholar]
- Li, R.; Wu, Z.; Wangb, Y.; Ding, L.; Wang, Y. Role of pH-induced structural change in protein aggregation in foam fractionation of bovine serum albumin. Biotechnol. Rep. 2016, 9, 46–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnthip, N.; Parhi, P.; Golas, A.; Vogler, E.A. Volumetric interpretation of protein adsorption: Kinetics of protein-adsorption competition from binary solution. Biomaterials 2009, 30, 6495–6513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopac, T.; Bozgeyik, K.; Yener, J. Effect of pH and temperature on the adsorption of bovine serum albumin onto titanium dioxide. Colloids Surf. A Physicochem. Eng. Asp. 2008, 322, 19–28. [Google Scholar] [CrossRef]
- Valero Vidal, C.; Olmo Juan, A.; Igual Muñoz, A. Adsorption of bovine serum albumin on CoCrMo surface: Effect of temperature and protein concentration. Colloids Surf. B Biointerfaces 2010, 80, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kubiak-Ossowska, K.; Cwieka, M.; Kaczynska, A.; Jachimska, B.; Mulheran, P.A. Lysozyme adsorption at a silica surface using simulation and experiment: Effects of pH on protein layer structure. Phys. Chem. Chem. Phys. 2015, 17, 24070–24077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tercinier, L.; Ye, A.; Singh, A.; Anema, S.G.; Singh, H. Effects of Ionic Strength, pH and Milk Serum Composition on Adsorption of Milk Proteins on to Hydroxyapatite Particles. Food Biophys. 2014, 9, 341–348. [Google Scholar] [CrossRef]
- Wei, T.; Kaewtathip, S.; Shing, K. Buffer Effect on Protein Adsorption at Liquid/Solid Interface. J. Phys. Chem. C 2009, 113, 2053–2062. [Google Scholar] [CrossRef]
- Gondim, D.R.; Cecilia, J.A.; Santos, S.O.; Rodrigues, T.N.B.; Aguiar, J.E.; Vilarrasa-García, E.; Rodríguez-Castellón, E.; Azevedo, D.C.S.; Silva, I.J. Influence of buffer solutions in the adsorption of human serum proteins onto layered double hydroxide. Int. J. Biol. Macromol. 2018, 106, 396–409. [Google Scholar] [CrossRef]
- Wilhelmi, M.; Müller, C.; Ziegler, C.; Kopnarski, M. BSA adsorption on titanium: ToF-SIMS investigation of the surface coverage as a function of protein concentration and pH-value. Anal. Bioanal. Chem. 2011, 400, 697–701. [Google Scholar] [CrossRef]
- Hanawa, T. Titanium-tissue interface reaction and its control with surface treatment. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunette, D.M.; Tengvall, P.; Textor, M.; Thomsen, P. (Eds.) Titanium in Medicine, 1st ed; Engineering Materials; Springer: Berlin/Heidelberg, Germany, 2001; ISBN 978-3-642-63119-1. [Google Scholar]
- Textor, M.; Sittig, C.; Frauchiger, V.; Tosatti, S.; Brunette, D. Properties and Biological Significance of Natural Oxide Films on Titanium and Its Alloys. In Titanium in Medicine; Brunette, D.M., Tengvall, P., Textor, M., Thomsen, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Ferraris, S.; Cazzola, M.; Peretti, V.; Stella, B.; Spriano, S. Zeta potential measurements on solid surfaces for in Vitro biomaterials testing: Surface charge, reactivity upon contact with fluids and protein absorption. Front. Bioeng. Biotechnol. 2018, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Okawa, S.; Kanatani, M.; Homma, K. Surface analysis of commercially pure titanium implant retrieved from rat bone. part 1: Initial biological response of sandblasted surface. Dent. Mater. J. 2009, 28, 178–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ionita, D.; Popescu, R.; Tite, T.; Demetrescu, I. The Behaviour of Pure Titanium in Albumin Solution. Mol. Cryst. Liq. Cryst. 2008, 486, 166–174. [Google Scholar] [CrossRef]
- Cámara, O.R.; Avalle, L.B.; Oliva, F.Y. Protein adsorption on titanium dioxide: Effects on double layer and semiconductor space charge region studied by EIS. Electrochim. Acta 2010, 55, 4519–4528. [Google Scholar] [CrossRef]
- Van De Keere, I.; Willaert, R.; Hubin, A.; Vereecken, J. Interaction of human plasma fibrinogen with commercially pure titanium as studied with atomic force microscopy and X-ray photoelectron spectroscopy. Langmuir 2008, 24, 1844–1852. [Google Scholar] [CrossRef]
- Kohavi, D.; Badihi Hauslich, L.; Rosen, G.; Steinberg, D.; Sela, M.N. Wettability versus electrostatic forces in fibronectin and albumin adsorption to titanium surfaces. Clin. Oral Implant. Res. 2013, 24, 1002–1008. [Google Scholar] [CrossRef]
- Imamura, K.; Shimomura, M.; Nagai, S.; Akamatsu, M.; Nakanishi, K. Adsorption characteristics of various proteins to a titanium surface. J. Biosci. Bioeng. 2008, 106, 273–278. [Google Scholar] [CrossRef]
- Oliva, F.Y.; Cámara, O.R.; Avalle, L.B. Adsorption of human serum albumin on electrochemical titanium dioxide electrodes: Protein–oxide surface interaction effects studied by electrochemical techniques. J. Electroanal. Chem. 2009, 633, 19–34. [Google Scholar] [CrossRef]
- Kang, Y.; Li, X.; Tu, Y.; Wang, Q.; Ågren, H. On the Mechanism of Protein Adsorption onto Hydroxylated and Nonhydroxylated TiO 2 Surfaces. J. Phys. Chem. C 2010, 114, 14496–14502. [Google Scholar] [CrossRef]
- Mao, C.M.; Sampath, J.; Sprenger, K.G.; Drobny, G.; Pfaendtner, J. Molecular Driving Forces in Peptide Adsorption to Metal Oxide Surfaces. Langmuir 2019, 35, 5911–5920. [Google Scholar] [CrossRef]
- Sun, T.; Han, G.; Lindgren, M.; Shen, Z.; Laaksonen, A. Adhesion of lactoferrin and bone morphogenetic protein-2 to a rutile surface: Dependence on the surface hydrophobicity. Biomater. Sci. 2014, 2, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Utesch, T.; Daminelli, G.; Mroginski, M.A. Molecular dynamics simulations of the adsorption of bone morphogenetic protein-2 on surfaces with medical relevance. Langmuir 2011, 27, 13144–13153. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, Y.; Liu, M.; Ye, M.; Zhang, Y.; Yao, S. Study of fibrinogen adsorption on hydroxyapatite and TiO2 surfaces by electrochemical piezoelectric quartz crystal impedance and FTIR–ATR spectroscopy. Anal. Chim. Acta 2007, 597, 58–66. [Google Scholar] [CrossRef]
- Zhao, A.; Wang, Z.; Zhu, X.; Maitz, M.F.; Huang, N. Real-Time Characterization of Fibrinogen Interaction with Modified Titanium Dioxide Film by Quartz Crystal Microbalance with Dissipation. Chin. J. Chem. Phys. 2014, 27, 355–360. [Google Scholar] [CrossRef]
- Moulton, S.E.; Barisci, J.N.; Bath, A.; Stella, R.; Wallace, G.G. Investigation of Ig.G adsorption and the effect on electrochemical responses at titanium dioxide electrode. Langmuir 2005, 21, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Bouhekka, A.; Bürgi, T. In situ ATR-IR spectroscopy study of adsorbed protein: Visible light denaturation of bovine serum albumin on TiO 2. Appl. Surf. Sci. 2012, 261, 369–374. [Google Scholar] [CrossRef]
- Van De Keere, I.; Willaert, R.; Tourwé, E.; Hubin, A.; Vereecken, J. The interaction of human serum albumin with titanium studied by means of atomic force microscopy. Surf. Interface Anal. 2008, 40, 157–161. [Google Scholar] [CrossRef]
- Wesełucha-Birczyńska, A.; Stodolak-Zych, E.; Piś, W.; Długoń, E.; Benko, A.; Błażewicz, M. A model of adsorption of albumin on the implant surface titanium and titanium modified carbon coatings (MWCNT-EPD). 2D correlation analysis. J. Mol. Struct. 2016, 1124, 61–70. [Google Scholar] [CrossRef]
- Zhang, H.P.; Lu, X.; Fang, L.M.; Weng, J.; Huang, N.; Leng, Y. Molecular dynamics simulation of RGD peptide adsorption on titanium oxide surfaces. J. Mater. Sci. Mater. Med. 2008, 19, 3437–3441. [Google Scholar] [CrossRef]
- Deligianni, D.D.; Katsala, N.; Ladas, S.; Sotiropoulou, D.; Amedee, J.; Missirlis, Y.F. Effect of surface roughness of the titanium alloy Ti-6Al-4V on human bone marrow cell response and on protein adsorption. Biomaterials 2001, 22, 1241–1251. [Google Scholar] [CrossRef]
- Kopf, B.S.; Ruch, S.; Berner, S.; Spencer, N.D.; Maniura-Weber, K. The role of nanostructures and hydrophilicity in osseointegration: In-vitro protein-adsorption and blood-interaction studies. J. Biomed. Mater. Res. Part A 2015, 103, 2661–2672. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Bossert, J.; Jandt, K.D. Does the nanometre scale topography of titanium influence protein adsorption and cell proliferation? Colloids Surf. B Biointerfaces 2006, 49, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Rockwell, G.P.; Lohstreter, L.B.; Dahn, J.R. Fibrinogen and albumin adsorption on titanium nanoroughness gradients. Colloids Surfaces B Biointerfaces 2012, 91, 90–96. [Google Scholar] [CrossRef]
- Lu, J.; Yao, C.; Yang, L.; Webster, T.J. Decreased Platelet Adhesion and Enhanced Endothelial Cell Functions on Nano and Submicron-Rough Titanium Stents. Tissue Eng. Part A 2012, 18, 1389–1398. [Google Scholar] [CrossRef]
- Kopac, T.; Bozgeyik, K. Effect of surface area enhancement on the adsorption of bovine serum albumin onto titanium dioxide. Colloids Surf. B Biointerfaces 2010, 76, 265–271. [Google Scholar] [CrossRef]
- Liu, L.; Bhatia, R.; Webster, T. Atomic layer deposition of nano-TiO2 thin films with enhanced biocompatibility and antimicrobial activity for orthopedic implants. Int. J. Nanomed. 2017, 12, 8711–8723. [Google Scholar] [CrossRef] [Green Version]
- Scopelliti, P.E.; Borgonovo, A.; Indrieri, M.; Giorgetti, L.; Bongiorno, G.; Carbone, R.; Podestà, A.; Milani, P. The Effect of Surface Nanometre-Scale Morphology on Protein Adsorption. PLoS ONE 2010, 5, e11862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Guo, Y.; Hong, Q.; Rao, C.; Zhang, H.; Dong, Y.; Huang, L.; Lu, X.; Bao, N. Bovine Serum Albumin Adsorption in Mesoporous Titanium Dioxide: Pore Size and Pore Chemistry Effect. Langmuir 2016, 32, 3995–4003. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Zhuang, W.; Yang, Z.; Lu, X.; Zhu, J.; Wang, Y.; Dong, Y.; Wu, N. Protein adsorptive behavior on mesoporous titanium dioxide determined by geometrical topography. Chem. Eng. Sci. 2014, 117, 146–155. [Google Scholar] [CrossRef]
- Singh, A.V.; Vyas, V.; Patil, R.; Sharma, V.; Scopelliti, P.E.; Bongiorno, G.; Podestà, A.; Lenardi, C.; Gade, W.N.; Milani, P. Quantitative Characterization of the Influence of the Nanoscale Morphology of Nanostructured Surfaces on Bacterial Adhesion and Biofilm Formation. PLoS ONE 2011, 6, e25029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvakumaran, J.; Keddie, J.L.; Ewins, D.J.; Hughes, M.P. Protein adsorption on materials for recording sites on implantable microelectrodes. J. Mater. Sci. Mater. Med. 2008, 19, 143–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva-Bermudez, P.; Rodil, S.E.; Muhl, S. Albumin adsorption on oxide thin films studied by spectroscopic ellipsometry. Appl. Surf. Sci. 2011, 258, 1711–1718. [Google Scholar] [CrossRef]
- Silva-Bermudez, P.; Muhl, S.; Rodil, S.E. A comparative study of fibrinogen adsorption onto metal oxide thin films. Appl. Surf. Sci. 2013, 282, 351–362. [Google Scholar] [CrossRef]
- Arvidsson, S.; Askendal, A.; Tengvall, P. Blood plasma contact activation on silicon, titanium and aluminium. Biomaterials 2007, 28, 1346–1354. [Google Scholar] [CrossRef]
- Song, L.; Yang, K.; Jiang, W.; Du, P.; Xing, B. Adsorption of bovine serum albumin on nano and bulk oxide particles in deionized water. Colloids Surf. B Biointerfaces 2012, 94, 341–346. [Google Scholar] [CrossRef]
- Yoshida, E.; Hayakawa, T. Adsorption Analysis of Lactoferrin to Titanium, Stainless Steel, Zirconia, and Polymethyl Methacrylate Using the Quartz Crystal Microbalance Method. BioMed Res. Int. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Abdallah, M.-N.; Abughanam, G.; Tran, S.D.; Sheikh, Z.; Mezour, M.A.; Basiri, T.; Xiao, Y.; Cerruti, M.; Siqueira, W.L.; Tamimi, F. Comparative adsorption profiles of basal lamina proteome and gingival cells onto dental and titanium surfaces. Acta Biomater. 2018, 73, 547–558. [Google Scholar] [CrossRef]
- Schweikl, H.; Hiller, K.-A.; Carl, U.; Schweiger, R.; Eidt, A.; Ruhl, S.; Müller, R.; Schmalz, G. Salivary protein adsorption and Streptococccus gordonii adhesion to dental material surfaces. Dent. Mater. 2013, 29, 1080–1089. [Google Scholar] [CrossRef]
- Miyake, A.; Komasa, S.; Hashimoto, Y.; Komasa, Y.; Okazaki, J. Adsorption of Saliva Related Protein on Denture Materials: An X-Ray Photoelectron Spectroscopy and Quartz Crystal Microbalance Study. Adv. Mater. Sci. Eng. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Müller, C.; Lüders, A.; Hoth-Hannig, W.; Hannig, M.; Ziegler, C. Initial bioadhesion on dental materials as a function of contact time, pH, surface wettability, and isoelectric point. Langmuir 2010, 26, 4136–4141. [Google Scholar] [CrossRef]
- Kohavi, D.; Badihi, L.; Rosen, G.; Steinberg, D.; Sela, M.N. An in vivo method for measuring the adsorption of plasma proteins to titanium in humans. Biofouling 2013, 29, 1215–1224. [Google Scholar] [CrossRef]
- Sela, M.N.; Badihi, L.; Rosen, G.; Steinberg, D.; Kohavi, D. Adsorption of human plasma proteins to modified titanium surfaces. Clin. Oral Implant. Res. 2007, 18, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Parisi, L.; Toffoli, A.; Cutrera, M.; Bianchi, M.G.; Lumetti, S.; Bussolati, O.; Macaluso, G.M. Plasma Proteins at the Interface of Dental Implants Modulate Osteoblasts Focal Adhesions Expression and Cytoskeleton Organization. Nanomaterials 2019, 9, 1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Zha, G.; Luo, Q.; Zhang, J.; Zhang, F.; Li, X.; Zhao, S.; Zhu, W.; Li, X. The construction of hierarchical structure on Ti substrate with superior osteogenic activity and intrinsic antibacterial capability. Sci. Rep. 2015, 4, 6172. [Google Scholar] [CrossRef] [Green Version]
- Ferraris, S.; Bobbio, A.; Miola, M.; Spriano, S. Micro- and nano-textured, hydrophilic and bioactive titanium dental implants. Surf. Coat. Technol. 2015, 276, 374–383. [Google Scholar] [CrossRef]
- Lu, X.; Xiong, S.; Chen, Y.; Zhao, F.; Hu, Y.; Guo, Y.; Wu, B.; Huang, P.; Yang, B. Effects of statherin on the biological properties of titanium metals subjected to different surface modification. Colloids Surf. B Biointerfaces 2020, 188, 110783. [Google Scholar] [CrossRef]
- Pegueroles, M.; Aparicio, C.; Bosio, M.; Engel, E.; Gil, F.J.; Planell, J.A.; Altankov, G. Spatial organization of osteoblast fibronectin matrix on titanium surfaces: Effects of roughness, chemical heterogeneity and surface energy. Acta Biomater. 2010, 6, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Mussano, F.; Genova, T.; Laurenti, M.; Gaglioti, D.; Scarpellino, G.; Rivolo, P.; Faga, M.G.; Fiorio, P.A.; Munaron, L.; Mandracci, P.; et al. Beta1-integrin and TRPV4 are involved in osteoblast adhesion to different titanium surface topographies. Appl. Surf. Sci. 2020, 507, 145112. [Google Scholar] [CrossRef]
- Toffoli, A.; Parisi, L.; Bianchi, M.G.; Lumetti, S.; Bussolati, O.; Macaluso, G.M. Thermal treatment to increase titanium wettability induces selective proteins adsorption from blood serum thus affecting osteoblasts adhesion. Mater. Sci. Eng. C 2020, 107, 110250. [Google Scholar] [CrossRef] [PubMed]
- Tugulu, S.; Löwe, K.; Scharnweber, D.; Schlottig, F. Preparation of superhydrophilic microrough titanium implant surfaces by alkali treatment. J. Mater. Sci. Mater. Med. 2010, 21, 2751–2763. [Google Scholar] [CrossRef]
- Martínez-Hernández, M.; García-Pérez, V.I.; Almaguer-Flores, A. Potential of salivary proteins to reduce oral bacterial colonization on titanium implant surfaces. Mater. Lett. 2019, 252, 120–122. [Google Scholar] [CrossRef]
- Richert, L.; Variola, F.; Rosei, F.; Wuest, J.D.; Nanci, A. Adsorption of proteins on nanoporous Ti surfaces. Surf. Sci. 2010, 604, 1445–1451. [Google Scholar] [CrossRef]
- Lee, M.H.; Oh, N.; Lee, S.W.; Leesungbok, R.; Kim, S.E.; Yun, Y.P.; Kang, J.H. Factors influencing osteoblast maturation on microgrooved titanium substrata. Biomaterials 2010, 31, 3804–3815. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Ayukawa, Y.; Furuhashi, A.; Kamo, M.; Ikeda, J.; Atsuta, I.; Haraguchi, T.; Koyano, K. Effect of hydrothermal treatment with distilled water on titanium alloy for epithelial cellular attachment. Materials 2019, 12, 2748. [Google Scholar] [CrossRef] [Green Version]
- Yoneyama, Y.; Matsuno, T.; Hashimoto, Y.; Satoh, T. In vitro evaluation of H2O2 hydrothermal treatment of aged titanium surface to enhance biofunctional activity. Dent. Mater. J. 2013, 32, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Wang, J.; Yuan, T.; Zhu, X.D.; Xiang, Z.; Fan, Y.J.; Zhang, X.D. The enhanced effect of surface microstructured porous titanium on adhesion and osteoblastic differentiation of mesenchymal stem cells. J. Mater. Sci. Mater. Med. 2013, 24, 2235–2246. [Google Scholar] [CrossRef]
- Sousa, S.R.; Brás, M.M.; Moradas-Ferreira, P.; Barbosa, M.A. Dynamics of Fibronectin Adsorption on TiO 2 Surfaces. Langmuir 2007, 23, 7046–7054. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.R.; Lamghari, M.; Sampaio, P.; Moradas-Ferreira, P.; Barbosa, M.A. Osteoblast adhesion and morphology on TiO2 depends on the competitive preadsorption of albumin and fibronectin. J. Biomed. Matter. Res. A. 2008, 84, 281–290. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, Y.; Chen, L.; Yin, D.; Zhang, H.; Tashiro, Y.; Inui, S.; Kusumoto, T.; Nishizaki, H.; Sekino, T.; et al. Optimized Surface Characteristics and Enhanced in Vivo Osseointegration of Alkali-Treated Titanium with Nanonetwork Structures. Int. J. Mol. Sci. 2019, 20, 1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, M.; Biao, M.; Chen, Y.; Xie, M.; Yang, B. Regulating the osteogenic function of rhBMP 2 by different titanium surface properties. J. Biomed. Mater. Res. Part A 2016, 104, 1882–1893. [Google Scholar] [CrossRef]
- Hu, X.N.; Yang, B.C. Conformation change of bovine serum albumin induced by bioactive titanium metals and its effects on cell behaviors. J. Biomed. Mater. Res. Part A 2014, 102, 1053–1062. [Google Scholar] [CrossRef]
- Biao, M.N.; Chen, Y.M.; Xiong, S.B.; Wu, B.Y.; Yang, B.C. Synergistic effects of fibronectin and bone morphogenetic protein on the bioactivity of titanium metal. J. Biomed. Mater. Res. Part A 2017, 105, 2485–2498. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Zhang, Y.; Ma, T.; Chen, H.; Lin, Y. Enhanced hydrophilicity and protein adsorption of titanium surface by sodium bicarbonate solution. J. Nanomater. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Feng, B.; Zhu, Y.; Weng, J.; Wang, J.; Lu, X. Preparation and characterization of a novel porous titanium scaffold with 3D hierarchical porous structures. J. Mater. Sci. Mater. Med. 2011, 22, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Liu, S.; Swain, M.V.; Zhang, X.; Zhao, K.; Jian, Y. Effects of acid-alkali treatment on bioactivity and osteoinduction of porous titanium: An in vitro study. Mater. Sci. Eng. C 2019, 94, 200–210. [Google Scholar] [CrossRef]
- Yu, P.; Zhu, X.; Wang, X.; Wang, S.; Li, W.; Tan, G.; Zhang, Y.; Ning, C. Periodic Nanoneedle and Buffer Zones Constructed on a Titanium Surface Promote Osteogenic Differentiation and Bone Calcification In Vivo. Adv. Healthc. Mater. 2016, 5, 364–372. [Google Scholar] [CrossRef]
- Rani, V.V.D.; Manzoor, K.; Menon, D.; Selvamurugan, N.; Nair, S.V. The design of novel nanostructures on titanium by solution chemistry for an improved osteoblast response. Nanotechnology 2009, 20, 195101. [Google Scholar] [CrossRef]
- Manivasagam, V.K.; Popat, K.C. In Vitro Investigation of Hemocompatibility of Hydrothermally Treated Titanium and Titanium Alloy Surfaces. ACS Omega 2020, 5, 8108–8120. [Google Scholar] [CrossRef]
- Qian, L.; Yu, P.; Zeng, J.; Shi, Z.; Wang, Q.; Tan, G.; Ning, C. Large-scale functionalization of biomedical porous titanium scaffolds surface with TiO2 nanostructures. Sci. China Mater. 2018, 61, 557–564. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Feng, B.; Lu, X.; Weng, J. Adsorption of bovine serum albumin onto titanium dioxide nanotube arrays. Int. J. Mater. Res. 2012, 103, 889–896. [Google Scholar] [CrossRef]
- Zhao, D.P.; Tang, J.C.; Nie, H.M.; Zhang, Y.; Chen, Y.K.; Zhang, X.; Li, H.X.; Yan, M. Macro-micron-nano-featured surface topography of Ti-6Al-4V alloy for biomedical applications. Rare Met. 2018, 37, 1055–1063. [Google Scholar] [CrossRef]
- Yang, W.; Xi, X.; Shen, X.; Liu, P.; Hu, Y.; Cai, K. Titania nanotubes dimensions-dependent protein adsorption and its effect on the growth of osteoblasts. J. Biomed. Mater. Res. Part A 2014, 102, 3598–3608. [Google Scholar] [CrossRef]
- Jia, E.; Zhao, X.; Lin, Y.; Su, Z. Protein adsorption on titanium substrates and its effects on platelet adhesion. Appl. Surf. Sci. 2020, 529, 146986. [Google Scholar] [CrossRef]
- Yang, W.; Xi, X.; Ran, Q.; Liu, P.; Hu, Y.; Cai, K. Influence of the titania nanotubes dimensions on adsorption of collagen: An experimental and computational study. Mater. Sci. Eng. C 2014, 34, 410–416. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Park, J.; Gongadze, E.; Killian, M.S.; Kralj, S.; von der Mark, K.; Iglič, A.; Schmuki, P. Protein interactions with layers of TiO2 nanotube and nanopore arrays: Morphology and surface charge influence. Acta Biomater. 2016, 45, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Sabino, R.M.; Kauk, K.; Movafaghi, S.; Kota, A.; Popat, K.C. Interaction of blood plasma proteins with superhemophobic titania nanotube surfaces. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102046. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, D.; Bai, J.; Zheng, H.; Deng, J.; Gou, Z.; Gao, C. Adsorption of serum proteins on titania nanotubes and its role on regulating adhesion and migration of mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2020, 108, 2305–2318. [Google Scholar] [CrossRef]
- Lu, R.; Wang, C.; Wang, X.; Wang, Y.; Wang, N.; Chou, J.; Li, T.; Zhang, Z.; Ling, Y.; Chen, S. Effects of hydrogenated TiO2 nanotube arrays on protein adsorption and compatibility with osteoblast-like cells. Int. J. Nanomed. 2018, 13, 2037–2049. [Google Scholar] [CrossRef] [Green Version]
- Teng, F.; Li, J.; Wu, Y.; Chen, H.; Zhang, Q.; Wang, H.; Ou, G. Fabrication and bioactivity evaluation of porous anodised Tio2 films in vitro. Biosci. Trends 2014, 8, 260–265. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.-E.; Huang, H.-H. Multiform TiO2 nano-network enhances biological response to titanium surface for dental implant applications. Appl. Surf. Sci. 2019, 471, 1041–1052. [Google Scholar] [CrossRef]
- Ning, C.; Wang, S.; Zhu, Y.; Zhong, M.; Lin, X.; Zhang, Y.; Tan, G.; Li, M.; Yin, Z.; Yu, P.; et al. Ti nanorod arrays with a medium density significantly promote osteogenesis and osteointegration. Sci. Rep. 2016, 6, 19047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayrak, Ö.; Ghahramanzadeh Asl, H.; Ak, A. Protein adsorption, cell viability and corrosion properties of Ti6Al4V alloy treated by plasma oxidation and anodic oxidation. Int. J. Miner. Metall. Mater. 2020, 27, 1269–1280. [Google Scholar] [CrossRef]
- Lin, D.J.; Fuh, L.J.; Chen, W.C. Nano-morphology, crystallinity and surface potential of anatase on micro-arc oxidized titanium affect its protein adsorption, cell proliferation and cell differentiation. Mater. Sci. Eng. C 2020, 107, 110204. [Google Scholar] [CrossRef] [PubMed]
- Fadlallah, S.A.; Amin, M.A.; Alosaimi, G.S. Construction of Nanophase Novel Coatings-Based Titanium for the Enhancement of Protein Adsorption. Acta Metall. Sin. Eng. Let. 2016, 29, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Kuczyńska, D.; Kwaśniak, P.; Pisarek, M.; Borowicz, P.; Garbacz, H. Influence of surface pattern on the biological properties of Ti grade 2. Mater. Charact. 2018, 135, 337–347. [Google Scholar] [CrossRef]
- Dumas, V.; Guignandon, A.; Vico, L.; Mauclair, C.; Zapata, X.; Linossier, M.T.; Bouleftour, W.; Granier, J.; Peyroche, S.; Dumas, J.C.; et al. Femtosecond laser nano/micro patterning of titanium influences mesenchymal stem cell adhesion and commitment. Biomed. Mater. 2015, 10, 055002. [Google Scholar] [CrossRef]
- Mukherjee, S.; Dhara, S.; Saha, P. Enhancing the biocompatibility of Ti6Al4V implants by laser surface microtexturing: An in vitro study. Int. J. Adv. Manuf. Technol. 2015, 76, 5–15. [Google Scholar] [CrossRef]
- Hao, L.; Lawrence, J. Wettability modification and the subsequent manipulation of protein adsorption on a Ti6Al4V alloy by means of CO2 laser surface treatment. J. Mater. Sci. Mater. Med. 2007, 18, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Parmar, V.; Kumar, A.; Mani Sankar, M.; Datta, S.; Vijaya Prakash, G.; Mohanty, S.; Kalyanasundaram, D. Oxidation facilitated antimicrobial ability of laser micro-textured titanium alloy against gram-positive Staphylococcus aureus for biomedical applications. J. Laser Appl. 2018, 30, 032001. [Google Scholar] [CrossRef]
- Kuczyńska, D.; Kwaśniak, P.; Marczak, J.; Bonarski, J.; Smolik, J.; Garbacz, H. Laser surface treatment and the resultant hierarchical topography of Ti grade 2 for biomedical application. Appl. Surf. Sci. 2016, 390, 560–569. [Google Scholar] [CrossRef]
- Clarke, B.; Kingshott, P.; Hou, X.; Rochev, Y.; Gorelov, A.; Carroll, W. Effect of nitinol wire surface properties on albumin adsorption. Acta Biomater. 2007, 3, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Michiardi, A.; Aparicio, C.; Ratner, B.D.; Planell, J.A.; Gil, J. The influence of surface energy on competitive protein adsorption on oxidized NiTi surfaces. Biomaterials 2007, 28, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Filiaggi, M.J.; Sanderson, R.J.; Lohstreter, L.B.; McArthur, M.A.; Dahn, J.R. Surface characteristics and protein adsorption on combinatorial binary Ti-M (Cr, Al, Ni) and Al-M (Ta, Zr) library films. J. Biomed. Mater. Res. Part A 2010, 92, 521–532. [Google Scholar] [CrossRef]
- Bai, Z.; Filiaggi, M.J.; Dahn, J.R. Fibrinogen adsorption onto 316L stainless steel, Nitinol and titanium. Surf. Sci. 2009, 603, 839–846. [Google Scholar] [CrossRef]
- Lefaix, H.; Galtayries, A.; Prima, F.; Marcus, P. Nano-size protein at the surface of a Ti–Zr–Ni quasi-crystalline alloy: Fibronectin adsorption on metallic nano-composites. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 207–214. [Google Scholar] [CrossRef]
- Chen, L.; Wei, K.; Qu, Y.; Li, T.; Chang, B.; Liao, B.; Xue, W. Characterization of plasma electrolytic oxidation film on biomedical high niobium-containing β-titanium alloy. Surf. Coat. Technol. 2018, 352, 295–301. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Beline, T.; Ribeiro, A.L.R.; Rangel, E.C.; da Cruz, N.C.; Landers, R.; Faverani, L.P.; Vaz, L.G.; Fais, L.M.G.; Vicente, F.B.; et al. Development of binary and ternary titanium alloys for dental implants. Dent. Mater. 2017, 33, 1244–1257. [Google Scholar] [CrossRef] [Green Version]
- Majumdar, P.; Singh, S.B.; Dhara, S.; Chakraborty, M. Influence of boron addition to Ti–13Zr–13Nb alloy on MG63 osteoblast cell viability and protein adsorption. Mater. Sci. Eng. C 2015, 46, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Blanquer, A.; Musilkova, J.; Barrios, L.; Ibáñez, E.; Vandrovcova, M.; Pellicer, E.; Sort, J.; Bacakova, L.; Nogués, C. Cytocompatibility assessment of Ti-Zr-Pd-Si-(Nb) alloys with low Young’s modulus, increased hardness, and enhanced osteoblast differentiation for biomedical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 834–842. [Google Scholar] [CrossRef]
- Herranz-Diez, C.; Gil, F.; Guillem-Marti, J.; Manero, J. Mechanical and physicochemical characterization along with biological interactions of a new Ti25Nb21Hf alloy for bone tissue engineering. J. Biomater. Appl. 2015, 30, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Ren, N.; Li, J.; Qiu, J.; Sang, Y.; Jiang, H.; Boughton, R.I.; Huang, L.; Huang, W.; Liu, H. Nanostructured titanate with different metal ions on the surface of metallic titanium: A facile approach for regulation of rBMSCs fate on titanium implants. Small 2014, 10, 3169–3180. [Google Scholar] [CrossRef]
- Haraguchi, T.; Ayukawa, Y.; Shibata, Y.; Takeshita, T.; Atsuta, I.; Ogino, Y.; Yasunami, N.; Yamashita, Y.; Koyano, K. Effect of Calcium Chloride Hydrothermal Treatment of Titanium on Protein, Cellular, and Bacterial Adhesion Properties. J. Clin. Med. 2020, 9, 2627. [Google Scholar] [CrossRef]
- Shi, X.; Nakagawa, M.; Kawachi, G.; Xu, L.; Ishikawa, K. Surface modification of titanium by hydrothermal treatment in Mg-containing solution and early osteoblast responses. J. Mater. Sci. Mater. Med. 2012, 23, 1281–1290. [Google Scholar] [CrossRef]
- Jiang, N.; Guo, Z.; Sun, D.; Li, Y.; Yang, Y.; Chen, C.; Zhang, L.; Zhu, S. Promoting osseointegration of Ti implants through micro/nanoscaled hierarchical Ti phosphate/Ti oxide hybrid coating. ACS Nano 2018, 12, 7883–7891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lingli, X.; Xingling, S.; Chun, O.; Wen, L. In vitro Apatite Formation, Protein Adsorption and Initial Osteoblast Responses on Titanium Surface Enriched with Magnesium. Rare Met. Mater. Eng. 2017, 46, 1512–1517. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Tsuru, K.; Xu, L.; Kawachi, G.; Ishikawa, K. Effects of solution pH on the structure and biocompatibility of Mg-containing TiO 2 layer fabricated on titanium by hydrothermal treatment. Appl. Surf. Sci. 2013, 270, 445–451. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Q.; Zhang, X.; Qiu, J.; Qian, S.; Liu, X. Co-implantation of magnesium and zinc ions into titanium regulates the behaviors of human gingival fibroblasts. Bioact. Mater. 2021, 6, 64–74. [Google Scholar] [CrossRef]
- Yuan, Z.; Liu, P.; Liang, Y.; Tao, B.; He, Y.; Hao, Y.; Yang, W.; Hu, Y.; Cai, K. Investigation of osteogenic responses of Fe-incorporated micro/nano-hierarchical structures on titanium surfaces. J. Mater. Chem. B 2018, 6, 1359–1372. [Google Scholar] [CrossRef] [PubMed]
- Anbazhagan, E.; Rajendran, A.; Natarajan, D.; Kiran, M.S.; Pattanayak, D.K. Divalent ion encapsulated nano titania on Ti metal as a bioactive surface with enhanced protein adsorption. Colloids Surf. B Biointerfaces 2016, 143, 213–223. [Google Scholar] [CrossRef]
- Soares, P.; Dias-Netipanyj, M.F.; Elifio-Esposito, S.; Leszczak, V.; Popat, K. Effects of calcium and phosphorus incorporation on the properties and bioactivity of TiO 2 nanotubes. J. Biomater. Appl. 2018, 33, 410–421. [Google Scholar] [CrossRef]
- Mei, S.; Zhao, L.; Wang, W.; Ma, Q.; Zhang, Y. Biomimetic titanium alloy with sparsely distributed nanotubes could enhance osteoblast functions. Adv. Eng. Mater. 2012, 14, 166–174. [Google Scholar] [CrossRef]
- Cai, K.Y. Surface modification of titanium films with sodium ion implantation: Surface properties and protein adsorption. Acta Metall. Sin. Eng. Lett. 2007, 20, 148–156. [Google Scholar] [CrossRef]
- Misra, R.D.K.; Nune, C.; Pesacreta, T.C.; Somani, M.C.; Karjalainen, L.P. Interplay between grain structure and protein adsorption on functional response of osteoblasts: Ultrafine-grained versus coarse-grained substrates. J. Biomed. Mater. Res. Part A 2013, 101A, 1–12. [Google Scholar] [CrossRef]
- Yin, F.; Xu, R.; Hu, S.; Zhao, K.; Yang, S.; Kuang, S.; Li, Q.; Han, Q. Enhanced Mechanical and Biological Performance of an Extremely Fine Nanograined 316L Stainless Steel Cell-Substrate Interface Fabricated by Ultrasonic Shot Peening. ACS Biomater. Sci. Eng. 2018, 4, 1609–1621. [Google Scholar] [CrossRef] [PubMed]
- Bahl, S.; Aleti, B.T.; Suwas, S.; Chatterjee, K. Surface nanostructuring of titanium imparts multifunctional properties for orthopedic and cardiovascular applications. Mater. Des. 2018, 144, 169–181. [Google Scholar] [CrossRef]
- Kubacka, D.; Yamamoto, A.; Wieciński, P.; Garbacz, H. Biological behavior of titanium processed by severe plastic deformation. Appl. Surf. Sci. 2019, 472, 54–63. [Google Scholar] [CrossRef]
- Huo, W.T.; Zhao, L.Z.; Zhang, W.; Lu, J.W.; Zhao, Y.Q.; Zhang, Y.S. In vitro corrosion behavior and biocompatibility of nanostructured Ti6Al4V. Mater. Sci. Eng. C 2018, 92, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhang, L.; Huang, L.; Zhu, J. Enhanced in-vitro osteoblastic functions on β-type titanium alloy using surface mechanical attrition treatment. Mater. Sci. Eng. C 2019, 97, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Awang Shri, D.N.; Tsuchiya, K.; Yamamoto, A. Effect of high-pressure torsion deformation on surface properties and biocompatibility of Ti-50.9 mol. %Ni alloys. Biointerphases 2014, 9, 029007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talha, M.; Ma, Y.; Kumar, P.; Lin, Y.; Singh, A. Role of protein adsorption in the bio corrosion of metallic implants—A review. Colloids Surfaces B Biointerfaces 2019, 176, 494–506. [Google Scholar] [CrossRef]
- Dias-Netipanyj, M.F.; Cowden, K.; Sopchenski, L.; Cogo, S.C.; Elifio-Esposito, S.; Popat, K.C.; Soares, P. Effect of crystalline phases of titania nanotube arrays on adipose derived stem cell adhesion and proliferation. Mater. Sci. Eng. C 2019, 103, 109850. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Hu, Y.; Gao, F.; Quan, L.; Liu, T.; Gong, T.; Pan, C. Effects of diameters and crystals of titanium dioxide nanotube arrays on blood compatibility and endothelial cell behaviors. Colloids Surf. B Biointerfaces 2019, 184, 110521. [Google Scholar] [CrossRef]
- Li, Y.; Dong, Y.; Zhang, Y.; Yang, Y.; Hu, R.; Mu, P.; Liu, X.; Lin, C.; Huang, Q. Synergistic effect of crystalline phase on protein adsorption and cell behaviors on TiO2 nanotubes. Appl. Nanosci. 2020, 10, 3245–3257. [Google Scholar] [CrossRef]
- Hong, Y.; Yu, M.; Lin, J.; Cheng, K.; Weng, W.; Wang, H. Surface hydroxyl groups direct cellular response on amorphous and anatase TiO2 nanodots. Colloids Surf. B Biointerfaces 2014, 123, 68–74. [Google Scholar] [CrossRef]
- Raffaini, G.; Ganazzoli, F. Molecular modelling of protein adsorption on the surface of titanium dioxide polymorphs. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2012, 370, 1444–1462. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Cheng, K.; Weng, W.; Yu, M.; Lin, J.; Wang, H.; Du, P.; Han, G. Influence of rod-surface structure on biological interactions between TiO2 nanorod films and proteins/cells. Thin Solid Films 2013, 544, 285–290. [Google Scholar] [CrossRef]
- Yang, C.; Peng, C.; Zhao, D.; Liao, C.; Zhou, J.; Lu, X. Molecular simulations of myoglobin adsorbed on rutile (110) and (001) surfaces. Fluid Phase Equilib. 2014, 362, 349–354. [Google Scholar] [CrossRef]
- Keller, T.F.; Reichert, J.; Thanh, T.P.; Adjiski, R.; Spiess, L.; Berzina-Cimdina, L.; Jandt, K.D.; Bossert, J. Facets of protein assembly on nanostructured titanium oxide surfaces. Acta Biomater. 2013, 9, 5810–5820. [Google Scholar] [CrossRef] [PubMed]
- Sugita, Y.; Saruta, J.; Taniyama, T.; Kitajima, H.; Hirota, M.; Ikeda, T.; Ogawa, T. UV-pre-treated and protein-adsorbed titanium implants exhibit enhanced osteoconductivity. Int. J. Mol. Sci. 2020, 21, 4194. [Google Scholar] [CrossRef]
- Jeong, W.S.; Kwon, J.S.; Choi, E.H.; Kim, K.M. The Effects of Non-Thermal Atmospheric Pressure Plasma treated Titanium Surface on Behaviors of Oral Soft Tissue Cells. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kamo, M.; Kyomoto, M.; Miyaji, F. Time course of surface characteristics of alkali- and heat-treated titanium dental implants during vacuum storage. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 105, 1453–1460. [Google Scholar] [CrossRef]
- Aita, H.; Hori, N.; Takeuchi, M.; Suzuki, T.; Yamada, M.; Anpo, M.; Ogawa, T. The effect of ultraviolet functionalization of titanium on integration with bone. Biomaterials 2009, 30, 1015–1025. [Google Scholar] [CrossRef]
- Yu, M.; Gong, J.; Zhou, Y.; Dong, L.; Lin, Y.; Ma, L.; Weng, W.; Cheng, K.; Wang, H. Surface hydroxyl groups regulate the osteogenic differentiation of mesenchymal stem cells on titanium and tantalum metals. J. Mater. Chem. B 2017, 5, 3955–3963. [Google Scholar] [CrossRef]
- Hori, N.; Ueno, T.; Minamikawa, H.; Iwasa, F.; Yoshino, F.; Kimoto, K.; Il Lee, M.C.; Ogawa, T. Electrostatic control of protein adsorption on UV-photofunctionalized titanium. Acta Biomater. 2010, 6, 4175–4180. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, L.; Ding, X.; Gao, Y.; Liu, X. Biological Effect of Ultraviolet Photocatalysis on Nanoscale Titanium with a Focus on Physicochemical Mechanism. Langmuir 2015, 31, 10037–10046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Komasa, S.; Mashimo, C.; Sekino, T.; Okazaki, J. Effect of ultraviolet treatment on bacterial attachment and osteogenic activity to alkali-treated titanium with nanonetwork structures. Int. J. Nanomed. 2017, 12, 4633–4646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dini, C.; Nagay, B.E.; Cordeiro, J.M.; da Cruz, N.C.; Rangel, E.C.; Ricomini-Filho, A.P.; de Avila, E.D.; Barão, V.A.R. UV-photofunctionalization of a biomimetic coating for dental implants application. Mater. Sci. Eng. C 2020, 110, 110657. [Google Scholar] [CrossRef]
- Han, I.; Vagaska, B.; Seo, H.J.; Kang, J.K.; Kwon, B.-J.; Lee, M.H.; Park, J.-C. Promoted cell and material interaction on atmospheric pressure plasma treated titanium. Appl. Surf. Sci. 2012, 258, 4718–4723. [Google Scholar] [CrossRef]
- Rapuano, B.E.; MacDonald, D.E. Surface oxide net charge of a titanium alloy: Modulation of fibronectin-activated attachment and spreading of osteogenic cells. Colloids Surf. B Biointerfaces 2011, 82, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, H.; Shibata, Y.; Miyazaki, T. Anode Glow Discharge Plasma Treatment of Titanium Plates Facilitates Adsorption of Extracellular Matrix Proteins. J. Dent. Res. 2005, 84, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Genova, T.; Wang, H.-L.; Carossa, S.; Mussano, F. Plasma of Argon Increases Cell Attachment and Bacterial Decontamination on Different Implant Surfaces. Int. J. Oral Maxillofac. Implant. 2017, 32, 1315–1323. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.-H.; Jeong, W.-S.; Cha, J.-Y.; Lee, J.-H.; Yu, H.-S.; Choi, E.-H.; Kim, K.-M.; Hwang, C.-J. Time-dependent effects of ultraviolet and nonthermal atmospheric pressure plasma on the biological activity of titanium. Sci. Rep. 2016, 6, 33421. [Google Scholar] [CrossRef] [PubMed]
- Han, I.; Vagaska, B.; Joo Park, B.; Lee, M.H.; Jin Lee, S.; Park, J.C. Selective fibronectin adsorption against albumin and enhanced stem cell attachment on helium atmospheric pressure glow discharge treated titanium. J. Appl. Phys. 2011, 109, 124701. [Google Scholar] [CrossRef]
- Zhu, W.; Teel, G.; O’Brien, C.M.; Zhuang, T.; Keidar, M.; Zhang, L.G. Enhanced human bone marrow mesenchymal stem cell functions on cathodic arc plasma-treated titanium. Int. J. Nanomed. 2015, 10, 7385–7396. [Google Scholar]
- Santos, O.; Svendsen, I.E.; Lindh, L.; Arnebrant, T. Adsorption of HSA, IgG and laminin-1 on model titania surfaces—Effects of glow discharge treatment on competitively adsorbed film composition. Biofouling 2011, 27, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-H.; Jeong, W.-S.; Cha, J.-Y.; Lee, J.-H.; Lee, K.-J.; Yu, H.-S.; Choi, E.-H.; Kim, K.-M.; Hwang, C.-J. Overcoming the biological aging of titanium using a wet storage method after ultraviolet treatment. Sci. Rep. 2017, 7, 3833. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Hao, P.; He, F.; Yao, Z.; Zhang, X. Molecular dynamics simulations of BSA absorptions on pure and formate-contaminated rutile (1 1 0) surface. Appl. Surf. Sci. 2020, 533, 147574. [Google Scholar] [CrossRef]
- Hori, N.; Att, W.; Ueno, T.; Sato, N.; Yamada, M.; Saruwatari, L.; Suzuki, T.; Ogawa, T. Age-dependent degradation of the protein adsorption capacity of titanium. J. Dent. Res. 2009, 88, 663–667. [Google Scholar] [CrossRef]
- Att, W.; Hori, N.; Takeuchi, M.; Ouyang, J.; Yang, Y.; Anpo, M.; Ogawa, T. Time-dependent degradation of titanium osteoconductivity: An implication of biological aging of implant materials. Biomaterials 2009, 30, 5352–5363. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Matsuno, T.; Hashimoto, Y.; Miyake, A.; Satomi, T. In Vitro and In Vivo Evaluation of Titanium Surface Modification for Biological Aging by Electrolytic Reducing Ionic Water. Appl. Sci. 2019, 9, 713. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Zhou, L.; Wan, L.; Li, S.; Rong, M.; Guo, Z. Effects of storage methods on time-related changes of titanium surface properties and cellular response. Biomed. Mater. 2012, 7, 055002. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-H.; Ryu, J.-H.; Kwon, J.-S.; Kim, J.-E.; Cha, J.-Y.; Lee, K.-J.; Yu, H.-S.; Choi, E.-H.; Kim, K.-M.; Hwang, C.-J. Effect of wet storage on the bioactivity of ultraviolet light- and non-thermal atmospheric pressure plasma-treated titanium and zirconia implant surfaces. Mater. Sci. Eng. C 2019, 105, 110049. [Google Scholar] [CrossRef]
- Kratz, F.; Müller, C.; Körber, N.; Umanskaya, N.; Hannig, M.; Ziegler, C. Characterization of protein films on dental materials: Bicinchoninic acid assay (BCA) studies on loosely and firmly adsorbed protein layers. Phys. Status Solidi Appl. Mater. Sci. 2013, 210, 964–967. [Google Scholar] [CrossRef]
- Wehmeyer, J.L.; Synowicki, R.; Bizios, R.; García, C.D. Dynamic adsorption of albumin on nanostructured TiO2 thin films. Mater. Sci. Eng. C 2010, 30, 277–282. [Google Scholar] [CrossRef] [Green Version]
- Imamura, K.; Oshita, M.; Iwai, M.; Kuroda, T.; Watanabe, I.; Sakiyama, T.; Nakanishi, K. Influences of properties of protein and adsorption surface on removal kinetics of protein adsorbed on metal surface by H2O2-electrolysis treatment. J. Colloid Interface Sci. 2010, 345, 474–480. [Google Scholar] [CrossRef]
- Forov, Y.; Paulus, M.; Dogan, S.; Salmen, P.; Weis, C.; Gahlmann, T.; Behrendt, A.; Albers, C.; Elbers, M.; Schnettger, W.; et al. Adsorption Behavior of Lysozyme at Titanium Oxide-Water Interfaces. Langmuir 2018, 34, 5403–5408. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Grassian, V.H. Bovine serum albumin adsorption on TiO2 nanoparticle surfaces: Effects of ph and coadsorption of phosphate on protein-surface interactions and protein structure. J. Phys. Chem. C 2017, 121, 21763–21771. [Google Scholar] [CrossRef]
- Burgos-Asperilla, L.; García-Alonso, M.C.; Escudero, M.L.; Alonso, C. Study of the interaction of inorganic and organic compounds of cell culture medium with a Ti surface. Acta Biomater. 2010, 6, 652–661. [Google Scholar] [CrossRef] [Green Version]
- Sultan, A.M.; Hughes, Z.E.; Walsh, T.R. Effect of calcium ions on peptide adsorption at the aqueous rutile titania (110) interface. Biointerphases 2018, 13, 06D403. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Kitaoka, S.; Furuya, M.; Kanetaka, H.; Hoshikaya, K.; Yamashita, H.; Abe, M. Enhancement of cell differentiation on a surface potential-controlled nitrogen-doped TiO2 surface. J. Ceram. Soc. Jpn. 2019, 127, 636–641. [Google Scholar] [CrossRef]
- Leeman, M.; Choi, J.; Hansson, S.; Storm, M.U.; Nilsson, L. Proteins and antibodies in serum, plasma, and whole blood—Size characterization using asymmetrical flow field-flow fractionation (AF4). Anal. Bioanal. Chem. 2018, 410, 4867–4873. [Google Scholar] [CrossRef] [Green Version]
- Boix, M.; Eslava, S.; Costa Machado, G.; Gosselin, E.; Ni, N.; Saiz, E.; De Coninck, J. ATR-FTIR measurements of albumin and fibrinogen adsorption: Inert versus calcium phosphate ceramics. J. Biomed. Mater. Res. Part A 2015, 103, 3493–3502. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, J.D.; Scriba, P.C.; Klüter, H. 5 Human Albumin. In Transfusion Medicine and Hemotherapy: Offizielles Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie; Karger Publishers: Basel, Switzerland, 2009; Volume 36, pp. 399–407. [Google Scholar]
- Hemmersam, A.G.; Foss, M.; Chevallier, J.; Besenbacher, F. Adsorption of fibrinogen on tantalum oxide, titanium oxide and gold studied by the QCM-D technique. Colloids Surf. B Biointerfaces 2005, 43, 208–215. [Google Scholar] [CrossRef]
- Vadillo-Rodríguez, V.; Pacha-Olivenza, M.A.; Gõnzalez-Martín, M.L.; Bruque, J.M.; Gallardo-Moreno, A.M.; Gónzalez-Martín, M.L.; Bruque, J.M.; Gallardo-Moreno, A.M. Adsorption behavior of human plasma fibronectin on hydrophobic and hydrophilic Ti6Al4V substrata and its influence on bacterial adhesion and detachment. J. Biomed. Mater. Res. Part A 2013, 101A, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Genova, T.; Tallarico, M.; Gautier, G.; Mussano, F.; Botticelli, D. Plasma of Argon Affects the Earliest Biological Response of Different Implant Surfaces. J. Dent. Res. 2016, 95, 566–573. [Google Scholar] [CrossRef]
- Dodo, C.G.; Senna, P.M.; Custodio, W.; Paes Leme, A.F.; Del Bel Cury, A.A. Proteome analysis of the plasma protein layer adsorbed to a rough titanium surface. Biofouling 2013, 29, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Rösch, C.; Kratz, F.; Hering, T.; Trautmann, S.; Umanskaya, N.; Tippkötter, N.; Müller-Renno, C.; Ulber, R.; Hannig, M.; Ziegler, C. Albumin-lysozyme interactions: Cooperative adsorption on titanium and enzymatic activity. Colloids Surf. B Biointerfaces 2017, 149, 115–121. [Google Scholar] [CrossRef]
- Wald, J.; Müller, C.; Wahl, M.; Hoth-Hannig, W.; Hannig, M.; Kopnarski, M.; Ziegler, C. ToF-SIMS investigations of adsorbed proteins on dental titanium. Phys. Status Solidi 2010, 207, 831–836. [Google Scholar] [CrossRef]
- Parisi, L.; Ghezzi, B.; Bianchi, M.G.; Toffoli, A.; Rossi, F.; Bussolati, O.; Macaluso, G.M. Titanium dental implants hydrophilicity promotes preferential serum fibronectin over albumin competitive adsorption modulating early cell response. Mater. Sci. Eng. C 2020, 117, 111307. [Google Scholar] [CrossRef]
- Felgueiras, H.; Migonney, V.; Sommerfeld, S.; Murthy, N.; Kohn, J. Competitive Adsorption of Albumin, Fibronectin and Collagen Type I on Different Biomaterial Surfaces: A QCM-D Study. In XIII Mediterranean Conference on Medical and Biological Engineering and Computing 2013; IFMBE Proceedings; Roa Romero, L.M., Ed.; Springer International Publishing: Cham, Switzerland, 2014; Volume 41, pp. 1597–1600. [Google Scholar]
- Felgueiras, H.P.; Murthy, N.S.; Sommerfeld, S.D.; Brás, M.M.; Migonney, V.; Kohn, J. Competitive Adsorption of Plasma Proteins Using a Quartz Crystal Microbalance. ACS Appl. Mater. Interfaces 2016, 8, 13207–13217. [Google Scholar] [CrossRef]
- Pegueroles, M.; Tonda-Turo, C.; Planell, J.A.; Gil, F.-J.; Aparicio, C. Adsorption of Fibronectin, Fibrinogen, and Albumin on TiO 2: Time-Resolved Kinetics, Structural Changes, and Competition Study. Biointerphases 2012, 7, 48. [Google Scholar] [CrossRef] [Green Version]
- Lorenzetti, M.; Bernardini, G.; Luxbacher, T.; Santucci, A.; Kobe, S.; Novak, S. Surface properties of nanocrystalline TiO 2 coatings in relation to the in vitro plasma protein adsorption. Biomed. Mater. 2015, 10, 045012. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gavilán, F.; Gomes, N.C.; Ródenas, J.; Sánchez, A.; Azkargorta, M.; Iloro, I.; Elortza, F.; García Arnáez, I.; Gurruchaga, M.; Goñi, I.; et al. Proteome analysis of human serum proteins adsorbed onto different titanium surfaces used in dental implants. Biofouling 2017, 33, 98–111. [Google Scholar] [CrossRef] [Green Version]
- Svendsen, I.E.; Lindh, L. The composition of enamel salivary films is different from the ones formed on dental materials. Biofouling 2009, 25, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Zuanazzi, D.; Xiao, Y.; Siqueira, W.L. Evaluating protein binding specificity of titanium surfaces through mass spectrometry–based proteomics. Clin. Oral Investig. 2020. [Google Scholar] [CrossRef]
- Wei, C.-X.; Burrow, M.F.; Botelho, M.G.; Lam, H.; Leung, W.K. In Vitro Salivary Protein Adsorption Profile on Titanium and Ceramic Surfaces and the Corresponding Putative Immunological Implications. Int. J. Mol. Sci. 2020, 21, 3083. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.G.S.; Bertolini, M.; Costa, R.C.; Lima, C.V.; Barão, V.A.R. Proteomic profile of the saliva and plasma protein layer adsorbed on Ti–Zr alloy: The effect of sandblasted and acid-etched surface treatment. Biofouling 2020, 36, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Jäger, M.; Jennissen, H.P.; Haversath, M.; Busch, A.; Grupp, T.; Sowislok, A.; Herten, M. Intrasurgical Protein Layer on Titanium Arthroplasty Explants: From the Big Twelve to the Implant Proteome. PROTEOMICS Clin. Appl. 2019, 13, 1800168. [Google Scholar] [CrossRef]
- Martins, M.C.L.; Sousa, S.R.; Antunes, J.C.; Barbosa, M.A. Protein adsorption characterization. Methods Mol. Biol. 2012, 811, 141–161. [Google Scholar]
- Migliorini, E.; Weidenhaupt, M.; Picart, C. Practical guide to characterize biomolecule adsorption on solid surfaces (Review). Biointerphases 2018, 13, 06D303. [Google Scholar] [CrossRef] [Green Version]
- Ledesma, A.E.; Chemes, D.M.; de los Angeles Frías, M.; Torres, M.D.P.G. Spectroscopic characterization and docking studies of ZnO nanoparticle modified with BSA. Appl. Surf. Sci. 2017, 412, 177–188. [Google Scholar] [CrossRef]
- Hemmersam, A.G.; Rechendorff, K.; Foss, M.; Sutherland, D.S.; Besenbacher, F. Fibronectin adsorption on gold, Ti-, and Ta-oxide investigated by QCM-D and RSA modelling. J. Colloid Interface Sci. 2008, 320, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Sakiyama, T.; Imamura, K. On the adsorption of proteins on solid surfaces, a common but very complicated phenomenon. J. Biosci. Bioeng. 2001, 91, 233–244. [Google Scholar] [CrossRef]
- Adamczyk, Z. Protein adsorption: A quest for a universal mechanism. Curr. Opin. Colloid Interface Sci. 2019, 41, 50–65. [Google Scholar] [CrossRef]










| Parameters | General Rules of Thumb | |
|---|---|---|
| Surface | Topography/roughness | Higher surface roughness ≥ higher amount of adsorbed proteins |
| Hydrophobicity (non-polar surfaces) Hydrophilicity (polar surfaces, with a net surface charge)- | Higher hydrophobicity ≥ higher amount of adsorbed proteins and denaturation degree; hydrophobic interaction as adsorption mechanism Different mechanisms of adsorption on hydrophilic surfaces: electrostatic, van der Waals, dipole-dipole; adsorbed water must be removed for adsorption | |
| Chemistry (functional groups, metal ions) | Influence on the surface charge | |
| Protein | Amino acid chain | Affects structural stability |
| Hydrophilicity/hydrophobicity | Surface charges and non-polar residues are always present; they can be differently arranged according to the environment; hydrophobic residues interact with hydrophobic surfaces | |
| Charge | Higher amount of adsorbed proteins at IEP | |
| Molecular weight | Small proteins adsorb quicker Large proteins replace the smaller ones and make stronger bonds with the hydrophobic surfaces | |
| Structural stability | Soft proteins change easier configuration and adsorb larger on hydrophilic surfaces; denaturation can enhance or reduce biological activity | |
| Solution | pH | Affects surface charge of both proteins and surfaces |
| Ionic strength | Adsorbed ions reduce repulsive effects among proteins; some ions compete with proteins for adsorption | |
| Protein concentration | Higher protein concentration higher amount of adsorption | |
| Protein mixture(single, binary or more complex) | Vroman effect | |
| Temperature | Higher temperature ≥ faster kinetics of adsorption |
| Surface Characteristic | Impact on Protein Adsorption | Conformation | Mechanism | Examples |
|---|---|---|---|---|
| Microroughness | ↑ | n.r. | Higher interaction area, physical adsorption | SLA surfaces adsorb fourfold more of albumin, fibronectin, fibrinogen and immunoglobulin vs. untreated surface because of roughness. Laser patterning increases adsorption of FIB. |
| Nanoroughness | ≈ | ↑ | Dependent on other characteristics. Aspect ratio of nanofeatures can influence protein conformation. | BSA aggregates into nanopores larger than its hydrodynamic radius with a strong interaction with the surface, while FN is too large. BSA/FIB adsorb as multilayer with stronger protein-protein interaction on nano-rough surfaces |
| Hydroxylation | ↑↑ | ↑↑ | According to the specific adsorbed proteins, OH can promote or hinder interaction with the surface | BSA adorbs through hydrogen bonding and proton transfer with interaction with OH surface groups. FIB adsorbs through positive charged αC domains. Rutile adsorbs more COL, FN and BSA than anatase or amorphous titania due to higher OH density |
| SFE | ↑↑ | n.r. | High surface energy, in particular the polar component, increases adsorption | Ti adsorbs larger amount of plasma proteins vs. other metals with lower SFE, but TiO2 adsorbs less proteins and in a weaker manner than other oxides with higher SFE. Ti adsorbs less basal lamina and salivary proteins than polymers for dentistry. Sandblasting with SiC induces higher SFE and preferential adsorption of FN. Laser patterning induces higher adsorption of FN by increasing the polar component of SFE. Nanograined surfaces have higher volumes of grain boundaries, which increase the SFE and adsorption of FN and VN |
| Charge | ↑↑ | ↑ | Can promote or limit protein adsorption, depending on charge of both surface and proteins | BSA is adsorbed in a lower amount on negatively charged surfaces while it is the opposite for histone that is positively charged. UV-generated positive surface can adsorb more BSA at pH 7, when the protein is negatively charged. |
| Chemistry (alloying metals, ions) | ↑ | n.r. | Increase protein adsorption, divalent ions in particular | TiNi alloys results in lower BSA (dependent on Ni content), FIB, and FN adsorption vs. cp-Ti. Ion-doped Ti has increased surface charge and protein adsorption because of bridging effect of divalent ions or specific chemical bonds (Ag) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barberi, J.; Spriano, S. Titanium and Protein Adsorption: An Overview of Mechanisms and Effects of Surface Features. Materials 2021, 14, 1590. https://doi.org/10.3390/ma14071590
Barberi J, Spriano S. Titanium and Protein Adsorption: An Overview of Mechanisms and Effects of Surface Features. Materials. 2021; 14(7):1590. https://doi.org/10.3390/ma14071590
Chicago/Turabian StyleBarberi, Jacopo, and Silvia Spriano. 2021. "Titanium and Protein Adsorption: An Overview of Mechanisms and Effects of Surface Features" Materials 14, no. 7: 1590. https://doi.org/10.3390/ma14071590