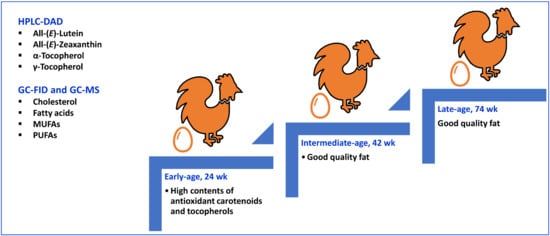
Age of Laying Hens Significantly Influences the Content of Nutritionally Vital Lipophilic Compounds in Eggs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Animals, Diets, Sampling, and Sample Preparation
2.3. Extraction of Major Lipophilic Compounds
2.4. HPLC-DAD Analysis of Carotenoids and Tocols
2.5. Analysis of Sterols and Fatty Acid Methyl Esters (FAMEs)
2.6. Calculation of Fat Quality Indices
2.7. Statistical Analysis and Quality Control
3. Results and Discussion
3.1. Carotenoid Composition
3.2. Sterol and Tocol Composition
3.3. Fatty Acid Composition and Fat Quality Indices
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Committee, D.G.A. Dietary guidelines for Americans 2015–2020; Government Printing Office: Washington, DC, USA, 2015.
- Zaheer, K. Hen egg carotenoids (lutein and zeaxanthin) and nutritional impacts on human health: A review. CyTA-J. Food 2017, 15, 474–487. [Google Scholar] [CrossRef] [Green Version]
- Van Ruth, S.M.; Alewijn, M.; Rogers, K.; Newton-Smith, E.; Tena, N.; Bollen, M.; Koot, A. Authentication of organic and conventional eggs by carotenoid profiling. Food Chem. 2011, 126, 1299–1305. [Google Scholar] [CrossRef]
- Nimalaratne, C.; Wu, J. Hen Egg as an Antioxidant Food Commodity: A Review. Nutrients 2015, 7, 8274–8293. [Google Scholar] [CrossRef] [Green Version]
- Islam, K.; Khalil, M.; Männer, K.; Raila, J.; Rawel, H.; Zentek, J.; Schweigert, F.J. Effect of dietary α-tocopherol on the bioavailability of lutein in laying hen. J. Anim. Physiol. Anim. Nutr. 2016, 100, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.-S. Tocopherols and tocotrienols in plants and their products: A review on methods of extraction, chromatographic separation, and detection. Food Res. Int. 2016, 82, 59–70. [Google Scholar] [CrossRef]
- Özcan, M.M.; Al-Juhaimi, F.; Uslu, N.; Ghafoor, K.; E Babiker, E.; Ahmed, I.A.M.; Alsawmahi, O.N. Effect of boiling on fatty acid composition and tocopherol content of hen, duck, and quail egg oils. J. Food Process. Preserv. 2019, 43, e13986. [Google Scholar] [CrossRef]
- Nolan, J.M.; Meagher, K.A.; Howard, A.N.; Moran, R.; Thurnham, D.I.; Beatty, S. Lutein, zeaxanthin and meso-zeaxanthin content of eggs laid by hens supplemented with free and esterified xanthophylls. J. Nutr. Sci. 2016, 5, e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attia, Y.A.; Al-Harthi, M.A.; Korish, M.A.; Shiboob, M.M. Fatty acid and cholesterol profiles and hypocholesterolemic, atherogenic, and thrombogenic indices of table eggs in the retail market. Lipids Health Dis. 2015, 14, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance — A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Carson, J.A.S.; Lichtenstein, A.H.; Anderson, C.A.; Appel, L.J.; Kris-Etherton, P.M.; Meyer, K.A.; Petersen, K.; Polonsky, T.; Van Horn, L. Dietary Cholesterol and Cardiovascular Risk: A Science Advisory From the American Heart Association. Circulation 2020, 141, e39–e53. [Google Scholar] [CrossRef] [Green Version]
- Adabi, S.G.; Ahbab, M.; Fani, A.R.; Hajbabaei, A.; Ceylan, N.; Cooper, R.G. Egg yolk fatty acid profile of avian species - influence on human nutrition. J. Anim. Physiol. Anim. Nutr. 2011, 97, 27–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinanoglou, V.J.; Strati, I.F.; Miniadis-Meimaroglou, S. Lipid, fatty acid and carotenoid content of edible egg yolks from avian species: A comparative study. Food Chem. 2011, 124, 971–977. [Google Scholar] [CrossRef]
- Antova, G.; Gerzilov, V.T.; Petkova, Z.; Boncheva, V.N.; Bozhichkova, I.N.; Penkov, D.; Petrov, P.B. Comparative analysis of nutrient content and energy of eggs from different chicken genotypes. J. Sci. Food Agric. 2019, 99, 5890–5898. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.G.H.; Sieo, C.C.; Kalavathy, R.; Saad, W.Z.; Yong, S.T.; Wong, H.K.; Ho, Y.W. Chemical Compositions of Egg Yolks and Egg Quality of Laying Hens Fed Prebiotic, Probiotic, and Synbiotic Diets. J. Food Sci. 2015, 80, C1686–C1695. [Google Scholar] [CrossRef]
- Cruz, R.; Casal, S.; Mendes, E.; Costa, A.; Santos, C.; Morais, S. Validation of a Single-Extraction Procedure for Sequential Analysis of Vitamin E, Cholesterol, Fatty Acids, and Total Fat in Seafood. Food Anal. Methods 2012, 6, 1196–1204. [Google Scholar] [CrossRef] [Green Version]
- Sachindra, N.; Bhaskar, N.; Mahendrakar, N. Recovery of carotenoids from shrimp waste in organic solvents. Waste Manag. 2006, 26, 1092–1098. [Google Scholar] [CrossRef]
- Gulzar, S.; Benjakul, S. Ultrasound Waves Increase the Yield and Carotenoid Content of Lipid Extracted from Cephalothorax of Pacific White Shrimp (Litopenaeus vannamei). Eur. J. Lipid Sci. Technol. 2018, 120, 11. [Google Scholar] [CrossRef]
- Saini, R.K.; Song, M.-H.; Rengasamy, K.R.; Ko, E.-Y.; Keum, Y.-S. Red Shrimp Are a Rich Source of Nutritionally Vital Lipophilic Compounds: A Comparative Study among Edible Flesh and Processing Waste. Foods 2020, 9, 1179. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef]
- Kim, D.-E.; Shang, X.; Assefa, A.D.; Keum, Y.-S.; Saini, R.K. Metabolite profiling of green, green/red, and red lettuce cultivars: Variation in health beneficial compounds and antioxidant potential. Food Res. Int. 2018, 105, 361–370. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S.; Rengasamy, K.R. Profiling of nutritionally important metabolites in green/red and green perilla (Perilla frutescens Britt.) cultivars: A comparative study. Ind. Crops Prod. 2020, 151, 112441. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Wołoszyn, J.; Haraf, G.; Okruszek, A.; Wereńska, M.; Goluch, Z.; Teleszko, M. Fatty acid profiles and health lipid indices in the breast muscles of local Polish goose varieties. Poult. Sci. 2020, 99, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Santossilva, J.; Bessa, R. Effect of genotype, feeding system and slaughter weight on the quality of light lambs. Livest. Prod. Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
- Kelly, E.R.; Plat, J.; Haenen, G.R.M.M.; Kijlstra, A.; Berendschot, T.T.J.M. The Effect of Modified Eggs and an Egg-Yolk Based Beverage on Serum Lutein and Zeaxanthin Concentrations and Macular Pigment Optical Density: Results from a Randomized Trial. PLoS ONE 2014, 9, e92659. [Google Scholar] [CrossRef]
- Brulc, L.; Simonovska, B.; Vovk, I.; Glavnik, V. Determination of egg yolk xanthophylls by isocratic high-performance liquid chromatography. J. Chromatogr. A 2013, 1318, 134–141. [Google Scholar] [CrossRef]
- Perry, A.; Rasmussen, H.; Johnson, E.J. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J. Food Compos. Anal. 2009, 22, 9–15. [Google Scholar] [CrossRef]
- Anderson, K.E. Comparison of fatty acid, cholesterol, and vitamin A and E composition in eggs from hens housed in conventional cage and range production facilities. Poult. Sci. 2011, 90, 1600–1608. [Google Scholar] [CrossRef]
- Monsen, E.R. Dietary Reference Intakes for The Antioxidant Nutrients. J. Am. Diet. Assoc. 2000, 100, 637–640. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, Z.-Y. Do We No Longer Need To Worry about Dietary Cholesterol? J. Agric. Food Chem. 2017, 65, 9931–9933. [Google Scholar] [CrossRef]
- Samson, F.P.; Patrick, A.T.; Fabunmi, T.E.; Yahaya, M.F.; Madu, J.; He, W.; Sripathi, S.R.; Tyndall, J.; Raji, H.; Jee, D.; et al. Oleic Acid, Cholesterol, and Linoleic Acid as Angiogenesis Initiators. ACS Omega 2020, 5, 20575–20585. [Google Scholar] [CrossRef] [PubMed]
- Krauss, R.M.; Eckel, R.H.; Howard, B.V.; Appel, L.J.; Daniels, S.R.; Deckelbaum, R.J.; Erdman, J.W.; Krisetherton, P.M.; Goldberg, I.J.; Kotchen, T.A.; et al. AHA Dietary Guidelines. Circulation 2000, 102, 2284–2299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krittanawong, C.; Narasimhan, B.; Wang, Z.; Virk, H.U.H.; Farrell, A.M.; Zhang, H.; Tang, W.H.W. Association Between Egg Consumption and Risk of Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. Am. J. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, H.-B. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef] [PubMed]








| Ingredients | Age Groups | ||
|---|---|---|---|
| Early (24 wk) | Intermediate (42 wk) | Late (74 wk) | |
| Corn Soybean meal Canola meal Corn gluten meal Distiller’s corn Tallow | 54.805 11.000 5.514 4.150 12.000 1.100 | 56.031 10.500 5.301 2.460 13.000 1.050 | 57.281 10.000 5.675 1.000 13.166 1.000 |
| Layer vitamin premix Lysine, 55% Monocalcium phosphate (MCP) Methionine (Met) 90% Limestone Choline-chloride 50% Salt Layer Mineral Mix Phytase | 0.110 0.256 0.636 0.040 9.914 0.005 0.250 0.170 0.050 | 0.105 0.245 0.590 0.058 10.200 - 0.250 0.160 0.050 | 0.100 0.222 0.542 0.073 10.481 - 0.250 0.160 0.050 |
| Total | 100 | 100 | 100 |
| Calculated analysis | |||
| Crude protein, % Calcium (Ca), % Phosphorus (P), % Available P, % Cysteine + Met, % Metabolizable energy, kcal/kg | 17.0 3.9 0.52 0.23 0.69 2800 | 16.0 4.0 0.51 0.22 0.66 2790 | 15.0 4.1 0.50 0.21 0.64 2780 |
| Peak No | Fatty Acid Methyl Ester (FAME) | RT (min) | Early (24 wk) | Intermediate (42 wk) | Late(74 wk) |
|---|---|---|---|---|---|
| 1 | C14:0 (Myristic) | 16.848 | 0.74 ± 0.03 a (0.31) | 0.60 ± 0.06 b (0.30) | 0.62 ± 0.02 b (0.31) |
| 2 | C16:0 (Palmitic) | 20.508 | 64.6 ± 3.80 a (27.4) | 51.3 ± 3.24 b (25.7) | 52.3 ± 1.04 b (26.3) |
| 3 | C16:1 (Palmitoleic) | 21.774 | 6.00 ± 0.19 a (2.55) | 4.01 ± 0.60 c (2.00) | 5.06 ± 0.35 b (2.55) |
| 4 | C17:0 (Heptadecanoic) | 22.235 | 0.88 ± 0.09 a (0.37) | 0.89 ± 0.09 a (0.45) | 0.70 ± 0.03 b (0.35) |
| 5 | C18:0 (Stearic) | 24.02 | 22.3 ± 1.41 a (9.43) | 19.8 ± 0.06 b (9.91) | 18.58 ± 0.12 b (9.35) |
| 6 | C18:1n9c (Oleic) | 25.204 | 88.3 ± 5.26 a (37.4) | 71.5 ± 1.49 b (35.8) | 73.8 ± 0.83 b (37.2) |
| 7 | C18:2n6c (Linoleic) | 26.789 | 40.5 ± 0.74 a (17.2) | 40.7 ± 1.95 a (20.4) | 37.8 ± 0.25 b (19.1) |
| 8 | C18:3n6 (γ-Linolenic) | 27.964 | 0.47 ± 0.11 a (0.20) | 0.34 ± 0.01 b (0.17) | 0.27 ± 0.02 b (0.14) |
| 9 | C20:1n9 (cis-11-Eicosenoic) | 28.343 | 0.39 ± 0.05 a (0.16) | 0.32 ± 0.02 a (0.16) | 0.37 ± 0.03 a (0.18) |
| 10 | C18:3n3 (α-Linolenic) | 28.606 | 0.68 ± 0.02 a (0.29) | 0.63 ± 0.05 b (0.31) | 0.54 ± 0.05 b (0.27) |
| 11 | C20:2n6 (cis-11,14-Eicosadienoic) | 29.898 | 0.32 ± 0.02 a (0.14) | 0.34 ± 0.03 a (0.17) | 0.34 ± 0.02 a (0.17) |
| 12 | C20:3n6 (cis-8,11,14-Eicosatrienoic) | 31.058 | 0.51 ± 0.04 a (0.22) | 0.49 ± 0.03 a (0.25) | 0.41 ± 0.02 b (0.21) |
| 13 | C20:4n6 (Arachidonic) | 31.985 | 8.11 ± 0.71 a (3.44) | 7.1 ± 0.25 b (3.58) | 6.32 ± 0.21 b (3.18) |
| 14 | C22:6n3 (cis-4,7,10,13,16,19-Docosahexaenoic) | 38.862 | 2.06 ± 0.18 a (0.87) | 1.6 ± 0.18 b (0.80) | 1.48 ± 0.02 b (0.75) |
| Total SFAs | 88.5 ± 5.32 a (37.5) | 72.6 ± 3.36 b (36.4) | 72.16 ± 1.17 b (36.3) | ||
| Total MUFAs | 94.7 ± 5.50 a (40.1) | 75.8 ± 2.11 b (38.0) | 79.27 ± 0.47 b (39.9) | ||
| Total PUFAs | 52.7 ± 1.76 a (22.4) | 51.2 ± 1.65 a (25.7) | 47.20 ± 0.51 b (23.8) | ||
| Crude lipids (%) | 34.7 ± 0.39 a | 34.8 ± 0.32 a | 36.2 ± 0.29 a |
| Early (24 wk) | Intermediate (42 wk) | Late (74 wk) | |
|---|---|---|---|
| PUFAs: SFAs | 0.60 ± 0.02 c | 0.71 ± 0.01 a | 0.65 ± 0.00 b |
| PUFAs: MUFAs | 0.56 ± 0.01 c | 0.68 ± 0.00 a | 0.60 ± 0.01 b |
| n3 PUFA | 2.74 ± 0.15 a | 2.22 ± 0.13 b | 2.03 ± 0.07 b |
| n6 PUFA | 49.9 ± 1.61 a | 49.0 ± 1.78 a | 45.2 ± 0.45 b |
| n6/n3 | 18.2 ± 0.42 c | 22.2 ± 2.10 a | 22.3 ± 0.52 a |
| h/H | 2.16 ± 0.02 b | 2.37 ± 0.09 a | 2.30 ± 0.05 a |
| AI | 0.46 ± 0.00 a | 0.42 ± 0.01 b | 0.43 ± 0.01 b |
| TI | 1.09 ± 0.01 a | 1.04 ± 0.02 b | 1.05 ± 0.01 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, E.-Y.; Saini, R.K.; Keum, Y.-S.; An, B.-K. Age of Laying Hens Significantly Influences the Content of Nutritionally Vital Lipophilic Compounds in Eggs. Foods 2021, 10, 22. https://doi.org/10.3390/foods10010022
Ko E-Y, Saini RK, Keum Y-S, An B-K. Age of Laying Hens Significantly Influences the Content of Nutritionally Vital Lipophilic Compounds in Eggs. Foods. 2021; 10(1):22. https://doi.org/10.3390/foods10010022
Chicago/Turabian StyleKo, Eun-Young, Ramesh Kumar Saini, Young-Soo Keum, and Byoung-Ki An. 2021. "Age of Laying Hens Significantly Influences the Content of Nutritionally Vital Lipophilic Compounds in Eggs" Foods 10, no. 1: 22. https://doi.org/10.3390/foods10010022