Changes of Bacterial Communities in an Anaerobic Digestion and a Bio-Electrochemical Anaerobic Digestion Reactors According to Organic Load
Abstract
:1. Introduction
2. Materials and Methods
2.1. Set-Up and Operation Conditions
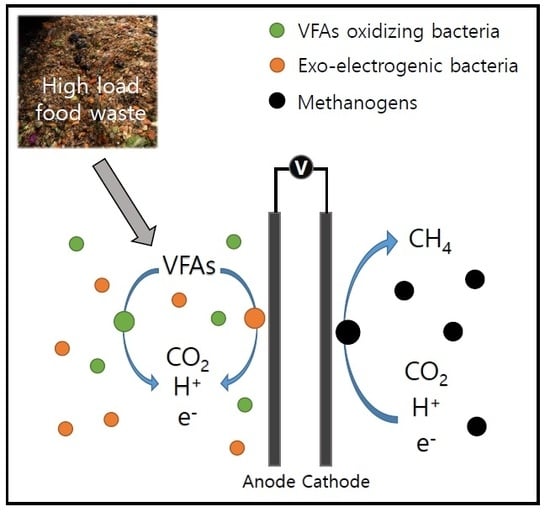
2.1.1. Reactors and Electrodes Set-Up
2.1.2. Operational Conditions
2.2. Bacterial Communities Analysis
2.2.1. Sampling of Sludge and Extraction of DNA
2.2.2. Polymer Chain Reaction (PCR) Amplification and Illumina Sequencing
2.2.3. Method of MiSeq Pipeline
2.3. Measurements
3. Results and Discussion
3.1. pH and Alkalinity
3.2. COD Removal, Methane Production, and VFAs Concentration
3.3. Bacterial Community Analysis
3.3.1. Bacterial Communities at S1 Stage
3.3.2. Bacterial Communities at S2 Stage
3.3.3. Bacterial Communities at S3 Stage
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Butti, S.K.; Velvizhi, G.; Sulonen, M.L.K.; Haavisto, J.M.; Koroglu, E.O.; Cetinkaya, A.Y.; Singh, S.; Arya, D.; Modestra, J.A.; Krishna, K.V.; et al. Microbial electrochemical technologies with the perspective of harnessing bioenergy: Maneuvering towards upscaling. Renew. Sust. Energy Rev. 2016, 53, 462–476. [Google Scholar] [CrossRef]
- Haralambopoulos, D.A.; Polatidis, H. Renewable energy projects: Structuring a multi-criteria group decision-making framework. Renew. Energy 2003, 28, 961–973. [Google Scholar] [CrossRef]
- Lee, B.; Park, J.G.; Shin, W.B.; Tian, D.J.; Jun, H.B. Microbial communities change in an anaerobic digestion after application of microbial electrolysis cells. Bioresour. Technol. 2017, 234, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Mohan, S.V. Effect of food to vegetable waste ratio on acidogenesis and methanogenesis during two-stage integration. Bioresour. Technol. 2018, 254, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Capson-Tojo, G.; Moscoviz, R.; Ruiz, D.; Santa-Catalina, G.; Trably, E.; Rouez, M.; Crest, M.; Steyer, J.P.; Bernet, N.; Delgenès, J.P.; et al. Addition of granular activated carbon and trace elements to favor volatile fatty acid consumption during anaerobic digestion of food waste. Bioresour. Technol. 2018, 260, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Asztalos, J.R.; Kim, Y. Enhanced digestion of waste activated sludge using microbial electrolysis cells at ambient temperature. Water Res. 2015, 87, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Themelis, N.J.; Ulloa, P.A. Methane generation in landfills. Renew. Energy 2017, 32, 1243–1257. [Google Scholar] [CrossRef]
- Liu, H.; Logan, B.E. Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ. Sci. Technol. 2004, 38, 4040–4046. [Google Scholar] [CrossRef]
- Logan, B.E.; Call, D.; Cheng, S.; Hamelers, H.V.; Sleutels, T.H.; Jeremiasse, A.W.; Rozendal, R.A. Microbial electrolysis cells for high yield hydrogen gas production from organic matter. Environ. Sci. Technol. 2008, 42, 8630–8640. [Google Scholar] [CrossRef]
- Song, Y.C.; Feng, Q.; Ahn, Y. Performance of the bio-electrochemical anaerobic digestion of sewage sludge at different hydraulic retention times. Energy Fuel 2016, 30, 352–359. [Google Scholar] [CrossRef]
- Ahring, B.K.; Sandberg, M.; Angelidaki, I. Volatile fatty acids as indicators of process imbalance in anaerobic digestors. Appl. Microbiol. Biotechnol. 1995, 43, 559–565. [Google Scholar] [CrossRef]
- Liu, W.; Cai, W.; Guo, Z.; Wang, L.; Yang, C.; Varrone, C.; Wang, A. Microbial electrolysis contribution to anaerobic digestion of waste activated sludge, leading to accelerated methane production. Renew. Energy 2016, 91, 334–339. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Lee, B.; Tian, D.; Jun, H. Bioelectrochemical enhancement of methane production from highly concentrated food waste in a combined anaerobic digester and microbial electrolysis cell. Bioresour. Technol. 2018, 247, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Song, Y.C.; Ahn, Y. Electroactive microorganisms in bulk solution contribute significantly to methane production in bioelectrochemical anaerobic reactor. Bioresour. Technol. 2018, 259, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, B.; Shin, W.; Jo, S.; Jun, H. Application of a rotating impeller anode in a bioelectrochemical anaerobic digestion reactor for methane production from high-strength food waste. Bioresour. Technol. 2018, 259, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Song, Y.C.; Yoo, K.; Kuppanan, N.; Subudhi, S.; Lal, B. Influence ofneutralization in acidic distillery wastewater on direct interspecies electron transferfor methane production in an upflow anaerobic bioelectrochemical reactor. Int. J. Hydrogen Energy 2017, 42, 27774–27783. [Google Scholar] [CrossRef]
- Kiran, E.U.; Trzcinski, A.P.; Ng, W.J.; Liu, Y. Bioconversion of food waste to energy: A review. Fuel 2014, 134, 389–399. [Google Scholar] [CrossRef]
- Hirano, S.I.; Matsumoto, N. Analysis of a bio-electrochemical reactor containing carbon fiber textiles for the anaerobic digestion of tomato plant residues. Bioresour. Technol. 2018, 249, 809–817. [Google Scholar] [CrossRef]
- Cai, W.; Han, T.; Guo, Z.; Varrone, C.; Wang, A.; Liu, W. Methane production enhancement by an independent cathode in integrated anaerobic reactor with microbial electrolysis. Bioresour. Technol. 2016, 208, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Moreno, R.; San-Martín, M.I.; Escapa, A.; Morán, A. Domestic wastewater treatment in parallel with methane production in a microbial electrolysis cell. Renew. Energy 2016, 93, 442–448. [Google Scholar] [CrossRef]
- Jin, X.; Angelidaki, I.; Zhang, Y. Microbial electrochemical monitoring of volatile fatty acids during anaerobic digestion. Environ. Sci. Technol. 2016, 50, 4422–4429. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yin, J.; Shen, D.; Li, N. Anaerobic digestion of food waste for volatile fatty acids (VFAs) production with different types of inoculum: Effect of pH. Bioresour. Technol. 2014, 161, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, C.; Da Ros, C.; Pavan, P.; Bolzonella, D. Influence of temperature and hydraulic retention on the production of volatile fatty acids during anaerobic fermentation of cow manure and maize silage. Bioresour. Technol. 2017, 223, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Su, H.; Baeyens, J.; Tan, T. Reviewing the anaerobic digestion of food waste for biogas production. Renew. Sust. Energy Rev. 2014, 38, 383–392. [Google Scholar] [CrossRef]
- Ariesyady, H.D.; Ito, T.; Okabe, S. Functional bacterial and archaeal community structures of major trophic groups in a full-scale anaerobic sludge digester. Water Res. 2007, 41, 1554–1568. [Google Scholar] [CrossRef]
- Nelson, K.E.; Zinder, S.H.; Hance, I.; Burr, P.; Odongo, D.; Wasawo, D.; Odenyo, A.; Bishop, R. Phylogenetic analysis of the microbial populations in the wild herbivore gastrointestinal tract: Insights into an unexplored niche. Environ. Microbiol. 2003, 5, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Ziganshin, A.M.; Schmidt, T.; Scholwin, F.; Il’inskaya, O.N.; Harms, H.; Kleinsteuber, S. Bacteria and archaea involved in anaerobic digestion of distillers grains with solubles. Appl. Microbiol. Biotechnol. 2011, 89, 2039–2052. [Google Scholar] [CrossRef]
- Hania, W.B.; Godbane, R.; Postec, A.; Hamdi, M.; Ollivier, B.; Fardeau, M.L. Defluviitoga tunisiensis gen. nov., sp. nov., a thermophilic bacterium isolated from a mesothermic and anaerobic whey digester. Int. J. Syst. Evol. Microbiol. 2012, 62, 1377–1382. [Google Scholar] [CrossRef] [Green Version]
- Maus, I.; Cibis, K.G.; Wibberg, D.; Winkler, A.; Stolze, Y.; König, H.; Pühler, A.; Schlüter, A. Complete genome sequence of the strain Defluviitoga tunisiensis L3, isolated from a thermophilic, production-scale biogas plant. J. Biotechnol. 2015, 203, 17–18. [Google Scholar] [CrossRef]
- Hahnke, S.; Maus, I.; Wibberg, D.; Tomazetto, G.; Pühler, A.; Klocke, M.; Schlüter, A. Complete genome sequence of the novel Porphyromonadaceae bacterium strain ING2-E5B isolated from a mesophilic lab-scale biogas reactor. J. Biotechnol. 2015, 193, 34–36. [Google Scholar] [CrossRef]
- Jabari, L.; Gannoun, H.; Cayol, J.L.; Hedi, A.; Sakamoto, M.; Falsen, E.; Ohkuma, M.; Hamdi, M.; Fauque, G.; Ollivier, B.; et al. Macellibacteroides fermentans gen. nov., sp. nov., a member of the family Porphyromonadaceae isolated from an upflow anaerobic filter treating abattoir wastewaters. Int. J. Syst. Evol. Microbiol. 2012, 62, 2522–2527. [Google Scholar] [CrossRef]
- Westerholm, M.; Roos, S.; Schnürer, A. Syntrophaceticus schinkii gen. nov., sp. nov., an anaerobic, syntrophic acetate-oxidizing bacterium isolated from a mesophilic anaerobic filter. FEMS Microbiol. Lett. 2010, 309, 100–104. [Google Scholar] [CrossRef]
- Kong, X.; Yu, S.; Fang, W.; Liu, J.; Li, H. Enhancing syntrophic associations among Clostridium butyricum, Syntrophomonas and two types of methanogen by zero valent iron in an anaerobic assay with a high organic loading. Bioresour. Technol. 2018, 257, 181–191. [Google Scholar] [CrossRef]
- Sarkar, O.; Kumar, A.N.; Dahiya, S.; Krishna, K.V.; Yeruva, D.K.; Mohan, S.V. Regulation of acidogenic metabolism towards enhanced short chain fatty acid biosynthesis from waste: Metagenomic profiling. RSC Adv. 2016, 6, 18641–18653. [Google Scholar] [CrossRef]
- Verbarg, S.; Rheims, H.; Emus, S.; Frühling, A.; Kroppenstedt, R.M.; Stackebrandt, E.; Schumann, P. Erysipelothrix inopinata sp. nov., isolated in the course of sterile filtration of vegetable peptone broth, and description of Erysipelotrichaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2004, 54, 221–225. [Google Scholar] [CrossRef]
- Deng, H.; Xue, H.; Zhong, W. A novel exoelectrogenic bacterium phylogenetically related to Clostridium sporogenes isolated from copper contaminated soil. Electroanalysis 2017, 29, 1294–1300. [Google Scholar] [CrossRef]
- De Vrieze, J.; Gildemyn, S.; Arends, J.B.; Vanwonterghem, I.; Verbeken, K.; Boon, N.; Verstraete, W.; Tyson, G.W.; Hennebel, T.; Rabaey, K. Biomass retention on electrodes rather than electrical current enhances stability in anaerobic digestion. Water Res. 2014, 54, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Vanwonterghem, I.; Jensen, P.D.; Dennis, P.G.; Hugenholtz, P.; Rabaey, K.; Tyson, G.W. Deterministic processes guide long-term synchronised population dynamics in replicate anaerobic digesters. ISME J. 2014, 8, 2015–2028. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Quan, X.; Zhang, Y. Improving the co-digestion performance of waste activated sludge and wheat straw through ratio optimization and ferroferric oxide supplementation. Bioresour. Technol. 2018, 267, 591–598. [Google Scholar] [CrossRef]
- Park, J.G.; Lee, B.; Kwon, H.J.; Park, H.R.; Jun, H.B. Effects of a novel auxiliary bio-electrochemical reactor on methane production from highly concentrated food waste in an anaerobic digestion reactor. Chemosphere 2019, 220, 403–411. [Google Scholar] [CrossRef]




| Parameters | S1 | S2 | S3 | |
|---|---|---|---|---|
| OLR (kg-COD/m3·d) | 2 | 4 | 6 | |
| HRT (d) | 20 | |||
| Temperature (℃) | 35–37 | |||
| Operation time (d) | 1–364 | 365–598 | 599–657 | |
| Inoculated sludge | Anaerobic sludge from food waste to energy facility | |||
| Voltage (V) | 0.3 | |||
| Electrodes materials | Anode | GC coated with Ni | ||
| Cathode | GC coated with Ni, Fe, and Cu | |||
| Operation type | SBR | |||
| Items | AD Reactor | BEAD Reactor | ||||
|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S1 | S2 | S3 | |
| COD removal efficiency (%) | 46 ± 17.33 | 69 ± 4.27 | 48 ± 19.18 | 61 ± 7.17 | 71 ± 2.93 | 73 ± 2.95 |
| Methane production (L/day) | 10 ± 6.39 | 31 ± 5.90 | 23 ± 19.12 | 16 ± 4.59 | 35 ± 3.87 | 53 ± 6.32 |
| Items | AD Reactor | BEAD Reactor | ||||
|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S1 | S2 | S3 | |
| VFAs (g/L as CaCO3) | 3.24 ± 1.71 | 3.44 ± 0.46 | 5.85 ± 1.96 | 2.57 ± 0.96 | 3.08 ± 0.23 | 3.87 ± 0.23 |
| Acetic acid (g/L as CaCO3) | 0.66 ± 0.20 | 0.84 ± 0.19 | 0.69 ± 0.14 | 0.59 ± 0.13 | 0.74 ± 0.18 | 1.04 ± 0.12 |
| Propionic acid (g/L as CaCO3) | 0.99 ± 0.50 | 1.17 ± 0.18 | 1.92 ± 0.50 | 0.84 ± 0.36 | 1.08 ± 0.17 | 1.36 ± 0.15 |
| Butyric acid (g/L as CaCO3) | 1.58 ± 1.07 | 1.43 ± 0.29 | 3.24 ± 0.86 | 1.14 ± 0.52 | 1.26 ± 0.17 | 1.48 ± 0.19 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-G.; Shin, W.-B.; Shi, W.-Q.; Jun, H.-B. Changes of Bacterial Communities in an Anaerobic Digestion and a Bio-Electrochemical Anaerobic Digestion Reactors According to Organic Load. Energies 2019, 12, 2958. https://doi.org/10.3390/en12152958
Park J-G, Shin W-B, Shi W-Q, Jun H-B. Changes of Bacterial Communities in an Anaerobic Digestion and a Bio-Electrochemical Anaerobic Digestion Reactors According to Organic Load. Energies. 2019; 12(15):2958. https://doi.org/10.3390/en12152958
Chicago/Turabian StylePark, Jun-Gyu, Won-Beom Shin, Wei-Qi Shi, and Hang-Bae Jun. 2019. "Changes of Bacterial Communities in an Anaerobic Digestion and a Bio-Electrochemical Anaerobic Digestion Reactors According to Organic Load" Energies 12, no. 15: 2958. https://doi.org/10.3390/en12152958