Algae Polyphenolic Compounds and Modern Antibacterial Strategies: Current Achievements and Immediate Prospects
Abstract
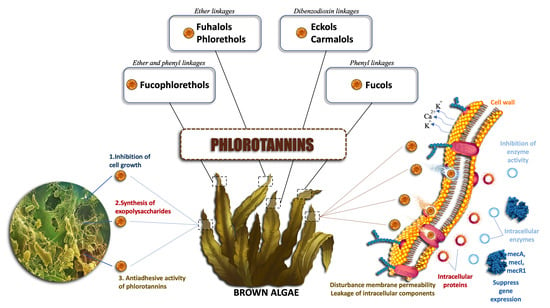
:1. Introduction
2. General Characteristics of Polyphenolic Compounds from Marine Algae
3. Obtaining Polyphenols from Marine Algae
4. Interaction of Algae Polyphenols with Gram-Positive and Gram-Negative Bacteria
5. Interaction of Algal Polyphenols with Methicillin-Resistant S. Aureus
6. Synergism of Marine Algae Phlorotannins and Antibiotics
7. Antibiofilm Effects of Polyphenols from Algae
8. Prospects for the Use of Polyphenols from Marine Algae in Medical Cosmetology
9. Importance of Marine Algal Polyphenols as Food Preservatives
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Infectious Disease Newsletter. Available online: https://www.who.int/topics/infectious_diseases/factsheets/ru/ (accessed on 12 September 2019).
- Amorim, R.D.N.D.S.; Rodrigues, J.A.G.; Holanda, M.; Quinderé, A.L.G.; De Paula, R.C.M.; Melo, V.M.M.; Benevides, N.M.B. Antimicrobial effect of a crude sulfated polysaccharide from the red seaweed Gracilaria ornata. Braz. Arch. Biol. Technol. 2012, 55, 171–181. [Google Scholar] [CrossRef]
- Zhang, W.; Oda, T.; Yu, Q.; Jin, J.-O. Fucoidan from Macrocystis pyrifera Has Powerful Immune-Modulatory Effects Compared to Three Other Fucoidans. Mar. Drugs 2015, 13, 1084–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Blencke, H.-M.; Haug, T.; Jørgensen, Ø.; Stensvåg, K. Expression of antimicrobial peptides in coelomocytes and embryos of the green sea urchin (Strongylocentrotus droebachiensis). Dev. Comp. Immunol. 2014, 43, 106–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Alstyne, K. Comparison of three methods for quantifying brown algal polyphenolic compounds. J. Chem. Ecol. 1995, 21, 45–58. [Google Scholar] [CrossRef]
- Poole, J.; Diop, A.; Rainville, L.-C.; Barnabe, S. Bioextracting Polyphenols from the Brown Seaweed Ascophyllum nodosum from Québec’s North Shore Coastline. Ind. Biotechnol. 2019, 15, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Catarino, M.D.; Silva, A.M.S.; Mateus, N.; Cardoso, S.M. Optimization of Phlorotannins Extraction from Fucus vesiculosus and Evaluation of Their Potential to Prevent Metabolic Disorders. Mar. Drugs 2019, 17, 162. [Google Scholar] [CrossRef] [Green Version]
- Manandhar, B.; Paudel, P.; Seong, S.H.; Jung, H.A.; Choi, J.S. Characterizing Eckol as a Therapeutic Aid: A Systematic Review. Mar. Drugs 2019, 17, 361. [Google Scholar] [CrossRef] [Green Version]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [Green Version]
- Machů, L.; Mišurcová, L.; Ambrozova, J.V.; Orsavová, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic Content and Antioxidant Capacity in Algal Food Products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef] [Green Version]
- Imbs, T.I.; Zvyagintseva, T.N. Phlorotannins are polyphenolic metabolites of brown algae. Russ. J. Mar. Biol. 2018, 44, 217–227. (in Russian). [Google Scholar] [CrossRef]
- Heffernan, N.; Brunton, N.; Fitzgerald, R.J.; Smyth, T.J. Profiling of the Molecular Weight and Structural Isomer Abundance of Macroalgae-Derived Phlorotannins. Mar. Drugs 2015, 13, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.P.; Sidana, J.; Bharate, S.S.; Foley, W.J. Phloroglucinol compounds of natural origin: Synthetic aspects. Nat. Prod. Rep. 2010, 27, 393. [Google Scholar] [CrossRef] [PubMed]
- Clayton, M.N.; Lüder, U.H. Induction of phlorotannins in the brown macroalga Ecklonia radiata (Laminariales, Phaeophyta) in response to simulated herbivory?the first microscopic study. Planta 2004, 218, 928–937. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Sathya, R.; Kanaga, N.; Sankar, P.; Jeeva, S. Antioxidant properties of phlorotannins from brown seaweed Cystoseira trinodis (Forsskål) C. Agardh. Arab. J. Chem. 2017, 10, S2608–S2614. [Google Scholar] [CrossRef] [Green Version]
- Pérez, M.J.; Falque, E.; Domínguez, H. Antimicrobial Action of Compounds from Marine Seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef] [Green Version]
- Jegan, S.; Raj, A.; Chandrasekaran, M.; Vencatesalu, V. Anti-MRSA activity of Padina tetrastromatica, Padina gymnospora from guft of mannar biosphere. World Sci. News 2019, 115, 15–26. [Google Scholar]
- Farvin, K.S.; Jacobsen, C. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef]
- Kim, S.M.; Kang, S.W.; Jeon, J.S.; Jung, Y.J.; Chul, W.R.K.; Kim, Y.; Um, B.H. Determination of major phlorotannins in E. bicyclis using hydrophilic interaction chromatography: Seasonal variation and extraction characteristics. Food Chem. 2013, 138, 2399–2406. [Google Scholar] [CrossRef]
- Moon, C.; Kim, S.-H.; Kim, J.-C.; Hyun, J.W.; Lee, N.H.; Park, J.W.; Shin, T. Protective effect of phlorotannin components phloroglucinol and eckol on radiation-induced intestinal injury in mice. Phytother. Res. 2008, 22, 238–242. [Google Scholar] [CrossRef]
- Suleria, H.A.R.; Osborne, S.A.; Masci, P.P.; Gobe, G.C. Marine-Based Nutraceuticals: An Innovative Trend in the Food and Supplement Industries. Mar. Drugs 2015, 13, 6336–6351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koivikko, R. Brown Algal Phlorotannins: Improving and Applying Chemical Methods. Ph.D. Thesis, University of Turku, Turku, Finland, 2008; 61p. [Google Scholar]
- Melanson, J.E.; MacKinnon, S.L. Characterization of Phlorotannins from Brown Algae by LC-HRMS. Adv. Struct. Saf. Stud. 2015, 1308, 253–266. [Google Scholar] [CrossRef]
- Montero, L.; Sánchez-Camargo, A.P.; Garcia-Cañas, V.; Tanniou, A.; Stiger-Pouvreau, V.; Russo, M.; Rastrelli, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E.; et al. Anti-proliferative activity and chemical characterization by comprehensive two-dimensional liquid chromatography coupled to mass spectrometry of phlorotannins from the brown macroalga Sargassum muticum collected on North-Atlantic coasts. J. Chromatogr. A 2016, 1428, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N. Diet-Derived Phenols in Plasma and Tissues and their Implications for Health. Planta Med. 2004, 70, 1103–1114. [Google Scholar] [CrossRef] [Green Version]
- Lewandowska, U.; Szewczyk, K.; Hrabec, E.; Janecka, A.; Gorlach, S. Overview of Metabolism and Bioavailability Enhancement of Polyphenols. J. Agric. Food Chem. 2013, 61, 12183–12199. [Google Scholar] [CrossRef]
- Keyrouz, R.; Abasq, M.; Le Bourvellec, C.; Blanc, N.; Audibert, L.; Argall, E.; Hauchard, D. Total phenolic contents, radical scavenging and cyclic voltammetry of seaweeds from Brittany. Food Chem. 2011, 126, 831–836. [Google Scholar] [CrossRef] [Green Version]
- Galleano, M.; Pechanova, O.; Fraga, C.G. Hypertension, nitric oxide, oxidants, and dietary plant polyphenols. Curr. Pharm. Biotechnol. 2010, 11, 837–848. [Google Scholar] [CrossRef]
- Schoenwaelder, M.E.A.; Clayton, M.N. The presence of phenolic compounds in isolated cell walls of brown algae. Phycologia 1999, 38, 161–166. [Google Scholar] [CrossRef]
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernardo, J.; Mouga, T.; Valentão, P. Can Phlorotannins Purified Extracts Constitute a Novel Pharmacological Alternative for Microbial Infections with Associated Inflammatory Conditions? PLoS ONE 2012, 7, e31145. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Recent developments in the application of seaweeds or seaweed extracts as a means for enhancing the safety and quality attributes of foods. Innov. Food Sci. Emerg. Technol. 2011, 12, 600–609. [Google Scholar] [CrossRef]
- Chojnacka, K.; Kim, S.-K. Introduction of Marine Algae Extracts. Mar. Algae Extr. Process. Prod. Appl. 2015, 1–14. [Google Scholar] [CrossRef]
- Pádua, D.; Rocha, E.; Gargiulo, D.; Ramos, A.A. Bioactive compounds from brown seaweeds: Phloroglucinol, fucoxanthin and fucoidan as promising therapeutic agents against breast cancer. Phytochem. Lett. 2015, 14, 91–98. [Google Scholar] [CrossRef]
- Kim, A.-R.; Shin, T.-S.; Lee, M.-S.; Park, J.-Y.; Park, K.-E.; Yoon, N.-Y.; Kim, J.-S.; Choi, J.S.; Jang, B.-C.; Byun, D.-S.; et al. Isolation and Identification of Phlorotannins fromEcklonia stoloniferawith Antioxidant and Anti-inflammatory Properties. J. Agric. Food Chem. 2009, 57, 3483–3489. [Google Scholar] [CrossRef]
- Li, Y.; Lee, S.-H.; Le, Q.-T.; Kim, M.-M.; Kim, S.-K. Anti-allergic Effects of Phlorotannins on Histamine Release via Binding Inhibition between IgE and Fc epsilonRI. J. Agric. Food Chem. 2008, 56, 12073–12080. [Google Scholar] [CrossRef]
- Ahn, M.-J.; Yoon, K.D.; Min, S.-Y.; Lee, J.S.; Kim, J.H.; Kim, T.G.; Kim, S.H.; Kim, N.-G.; Huh, H.; Kim, J. Inhibition of HIV-1 reverse transcriptase and protease by phlorotannins from the brown alga Ecklonia cava. Biol. Pharm. Bull. 2004, 27, 544–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parys, S.; Kehraus, S.; Krick, A.; Glombitza, K.-W.; Carmeli, S.; Klimo, K.; Gerhauser, C.; König, G.M. In vitro chemopreventive potential of fucophlorethols from the brown alga Fucus vesiculosus L. by anti-oxidant activity and inhibition of selected cytochrome P450 enzymes. Phytochemistry 2010, 71, 221–229. [Google Scholar] [CrossRef]
- Wijesekara, I.; Yoon, N.Y.; Kim, S.K. Phlorotannins from Ecklonia cava (Phaeophyceae): Biological activities and potential health benefits. Biofactors. 2010, 6, 408–414. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.; Liu, H.; Gu, L.; Kristinsson, H.G.; Raghavan, S.; Olafsdottir, G. Antioxidant Capacities of Phlorotannins Extracted from the Brown Algae Fucus vesiculosus. J. Agric. Food Chem. 2012, 60, 5874–5883. [Google Scholar] [CrossRef]
- Audibert, L.; Fauchon, M.; Blanc, N.; Hauchard, D.; Gall, E.A. Phenolic compounds in the brown seaweed Ascophyllum nodosum: Distribution and radical-scavenging activities. Phytochem. Anal. 2010, 21, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Ishimaru, K.; Kawaguchi, S.; Yoshikawa, H.; Hama, Y. Antioxidant activities of phlorotannins isolated from Japanese Laminariaceae. Environ. Biol. Fishes 2007, 20, 705–711. [Google Scholar] [CrossRef]
- Aminina, N.M.; Vishnevskaya, T.I.; Karaulova, E.P.; Epur, N.V.; Yakush, E.V. Prospects for the Use of Commercial and Potentially Commercial Brown Algae of the Far Eastern Seas as a Source of Polyphenols. Russ. J. Mar. Biol. 2020, 46, 34–41. [Google Scholar] [CrossRef]
- Aminina, N.M.; Vishnevskaya, T.I.; Karaulova, E.P.; Yakush, E.V. Content of polyphenols and antioxidant activity of extracts from certain species of seaweeds. TINRO News 2017, 189, 184–191. (in Russian). [Google Scholar]
- Moizer, E.B.; Skerget, M.; Knez, S.; Epur, N.V.; Yakush, E.V. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticancerogenic effects. Molecules 2016, 21, 901–939. [Google Scholar]
- Puspita, M.; Déniel, M.; Widowati, I.; Radjasa, O.K.; Douzenel, P.; Marty, C.; Vandanjon, L.; Bedoux, G.; Bourgougnon, N. Total phenolic content and biological activities of enzymatic extracts from Sargassum muticum (Yendo) Fensholt. Environ. Biol. Fishes 2017, 29, 2521–2537. [Google Scholar] [CrossRef] [PubMed]
- Rajbhar, K.; Dawda, H.; Mucundan, U. Polyphenols: Methods of extraction. Sci. Rev. Chem. Commun. 2015, 5, 1–6. [Google Scholar]
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.; Gao, X. Extraction and identification of phlorotannins from the brown alga Sargassum fusiforme (Harwey) Setchell. Mar. Drugs 2017, 15, 49. [Google Scholar] [CrossRef] [Green Version]
- Ummat, V.; Tiwari, B.; Jaiswal, A.; Condon, K.; García-Vaquero, M.; O’Doherty, J.V.; O’Donnell, C.; Rajauria, G. Optimisation of Ultrasound Frequency, Extraction Time and Solvent for the Recovery of Polyphenols, Phlorotannins and Associated Antioxidant Activity from Brown Seaweeds. Mar. Drugs 2020, 18, 250. [Google Scholar] [CrossRef]
- Stern, J.L.; Hagerman, A.E.; Steinberg, P.D.; Mason, P.K. Phlorotannin-protein interactions. J. Chem. Ecol. 1996, 22, 1877–1899. [Google Scholar] [CrossRef]
- Moubayed, N.M.; Al Houri, H.J.; Al Khulaifi, M.M.; Al Farraj, D.A. Antimicrobial, antioxidant properties and chemical composition of seaweeds collected from Saudi Arabia (Red Sea and Arabian Gulf). Saudi J. Biol. Sci. 2016, 24, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Venkatesan, J.; Keekan, K.K.; Anil, S.; Bhatnagar, I.; Kim, S.-K. Phlorotannins. Encycl. Food Chem. 2019, 515–527. [Google Scholar] [CrossRef]
- Heldt, H.-W.; Piechulla, B. Plant Biochemistry; Academic Press: San Diego, CA, USA, 2010. [Google Scholar]
- Wang, Y.; Xu, Z.; Bach, S.J.; McAllister, T.A. Sensitivity of Escherichia coli to Seaweed (Ascophyllum nodosum) Phlorotannins and Terrestrial Tannins. Asian-Australasian J. Anim. Sci. 2009, 22, 238–245. [Google Scholar] [CrossRef]
- Golan, D.E.; Tashjian, A.H.; Armstrong, E.J. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011. [Google Scholar]
- Wei, Y.; Liu, Q.; Xu, C.; Yu, J.; Zhao, L.; Guo, Q. Damage to the Membrane Permeability and Cell Death of Vibrio parahaemolyticus Caused by Phlorotannins With Low Molecular Weight from Sargassum thunbergii. J. Aquat. Food Prod. Technol. 2015, 25, 323–333. [Google Scholar] [CrossRef]
- McDonnell, G.E.; Russell, A.D. Antiseptics and Disinfectants: Activity, Action, and Resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonnell, G.E. Chemical Disinfection. In Antisepsis, Disinfection, and Sterilization: Types, Action, and Resistance; ASM Press: Washington, DC, USA, 2007; pp. 96–148. [Google Scholar]
- Chibane, L.B.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2018, 99, 1457–1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hierholtzer, A.; Chatellard, L.; Kierans, M.; Akunna, J.; Collier, P. The impact and mode of action of phenolic compounds extracted from brown seaweed on mixed anaerobic microbial cultures. J. Appl. Microbiol. 2013, 114, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Vissers, A.M.; Caligiani, A.; Sforza, S.; Vincken, J.-P.; Gruppen, H. Phlorotannin Composition of Laminaria digitata. Phytochem. Anal. 2017, 28, 487–495. [Google Scholar] [CrossRef]
- Nagayama, K.; Iwamura, Y.; Shibata, T.; Hirayama, I.; Nakamura, T. Bactericidal activity of phlorotannins from the brown alga Ecklonia kurome. J. Antimicrob. Chemother. 2002, 50, 889–893. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.S.; Song, J.S.; Lee, H.J.; Choe, P.G.; Park, K.H.; Cho, J.H.; Park, W.B.; Kim, S.-H.; Bang, J.H.; Kim, C.-M.; et al. A survey of community-associated methicillin-resistant Staphylococcus aureus in Korea. J. Antimicrob. Chemother. 2007, 60, 1108–1114. [Google Scholar] [CrossRef] [Green Version]
- Kamei, Y.; Isnansetyo, A. Lysis of methicillin-resistant Staphylococcus aureus by 2,4-diacetylphloroglucinol produced by Pseudomonas sp. AMSN isolated from a marine alga. Int. J. Antimicrob. Agents 2003, 21, 71–74. [Google Scholar] [CrossRef]
- Brumfitt, W.; Hamilton-Miller, J.M.T. The worldwide problem of methicillin-resistant Staphylococcus aureus. Drugs Exp. Clin. Res. 1999, 16, 205–214. [Google Scholar]
- Eom, S.-H.; Kim, D.-H.; Lee, S.-H.; Yoon, N.-Y.; Kim, J.H.; Kim, T.H.; Chung, Y.-H.; Kim, S.-B.; Kim, Y.-M.; Kim, H.-W.; et al. In VitroAntibacterial Activity and Synergistic Antibiotic Effects of Phlorotannins Isolated fromEisenia bicyclisAgainst Methicillin-ResistantStaphylococcus aureus. Phytother. Res. 2012, 27, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Graber, C.J.; Karr, M.; Diep, B.A.; Basuino, L.; Schwartz, B.S.; Enright, M.C.; O’Hanlon, S.; Thomas, J.C.; Perdreau-Remington, F.; et al. A Population-Based Study of the Incidence and Molecular Epidemiology of Methicillin-ResistantStaphylococcus aureusDisease in San Francisco, 2004–2005. Clin. Infect. Dis. 2008, 46, 1637–1646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebrahimzadeh, M.A.; Khalili, M.; Dehpour, A.A. Antioxidant activity of ethyl acetate and methanolic extracts of two marine algae, Nannochloropsis oculata and Gracilaria gracilis—An in vitro assay. Braz. J. Pharm. Sci. 2018, 54. [Google Scholar] [CrossRef] [Green Version]
- Lavoie, S.; Sweeney-Jones, A.M.; Mojib, N.; Dale, B.; Gagaring, K.; McNamara, C.W.; Quave, C.L.; Soapi, K.; Kubanek, J. Antibacterial Oligomeric Polyphenols from the Green Alga Cladophora socialis. J. Org. Chem. 2019, 84, 5035–5045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Worthington, R.J.; Melander, C. Combination approaches to combat multidrug-resistant bacteria. Trends Biotechnol. 2013, 31, 177–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eom, S.-H.; Kang, Y.-M.; Park, J.-H.; Yu, D.-U.; Jeong, E.-T.; Lee, M.-S.; Kim, Y.-M. Enhancement of Polyphenol Content and Antioxidant Activity of Brown Alga Eisenia bicyclis extract by Microbial Fermentation. Fish. Aquat. Sci. 2011, 14, 192–197. [Google Scholar] [CrossRef] [Green Version]
- Eom, S.-H.; Lee, D.-S.; Jung, Y.-J.; Park, J.-H.; Choi, J.-I.; Yim, M.-J.; Jeon, J.-M.; Kim, H.-W.; Son, K.-T.; Je, J.; et al. The mechanism of antibacterial activity of phlorofucofuroeckol-A against methicillin-resistant Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2014, 98, 9795–9804. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.J.; Hopwood, S.; Davies, S.C. Antimicrobial Resistance: A Global Challenge. Sci. Transl. Med. 2014, 6, 236ed10. [Google Scholar] [CrossRef]
- Harrison, J.J.; Ceri, H.; Turner, R.J. Multimetal resistance and tolerance in microbial biofilms. Nat. Rev. Genet. 2007, 5, 928–938. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [Green Version]
- Maisonneuve, E.; Gerdes, K. Molecular Mechanisms Underlying Bacterial Persisters. Cell 2014, 157, 539–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, N.; Nyvad, B. The role of bacteria in the caries process: Ecological perspectives. J. Dent. Res. 2011, 90, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D.; Do, T.; Devine, D. Oral biofilms: Molecular analysis, challenges, and future prospects in dental diagnostics. Clin. Cosmet. Investig. Dent. 2013, 5, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Barranquero, J.A.; Reen, F.J.; McCarthy, R.R.; O’Gara, F. Deciphering the role of coumarin as a novel quorum sensing inhibitor suppressing virulence phenotypes in bacterial pathogens. Appl. Microbiol. Biotechnol. 2015, 99, 3303–3316. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.S.; Lee, J.-H.; Cho, M.H.; Lee, J. Red wines and flavonoids diminish Staphylococcus aureus virulence with anti-biofilm and anti-hemolytic activities. Biofouling 2014, 31, 1–11. [Google Scholar] [CrossRef]
- Shen, X.-F.; Ren, L.-B.; Teng, Y.; Zheng, S.; Yang, X.-L.; Guo, X.-J.; Wang, X.-Y.; Sha, K.-H.; Li, N.; Xu, G.-Y.; et al. Luteolin decreases the attachment, invasion and cytotoxicity of UPEC in bladder epithelial cells and inhibits UPEC biofilm formation. Food Chem. Toxicol. 2014, 72, 204–211. [Google Scholar] [CrossRef]
- Rendeková, K.; Fialová, S.; Jánošová, L.; Mučaji, P.; Slobodníková, L. The Activity of Cotinus coggygria Scop. Leaves on Staphylococcus aureus Strains in Planktonic and Biofilm Growth Forms. Molelules 2015, 21, 50. [Google Scholar] [CrossRef] [Green Version]
- Asahi, Y.; Noiri, Y.; Miura, J.; Maezono, H.; Yamaguchi, M.; Yamamoto, R.; Azakami, H.; Hayashi, M.; Ebisu, S. Effects of the tea catechin epigallocatechin gallate on Porphyromonas gingivalis biofilms. J. Appl. Microbiol. 2014, 116, 1164–1171. [Google Scholar] [CrossRef]
- Lee, P.; Tan, K.S. Effects of Epigallocatechin gallate against Enterococcus faecalis biofilm and virulence. Arch. Oral Biol. 2015, 60, 393–399. [Google Scholar] [CrossRef]
- Hussain, M.A.; Dawson, C.O. Economic Impact of Food Safety Outbreaks on Food Businesses. Foods 2013, 2, 585–589. [Google Scholar] [CrossRef] [Green Version]
- Mikhail, A.F.W.; Jenkins, C.; Dallman, T.J.; Inns, T.; Douglas, A.; Martín, A.I.C.; Fox, A.; Cleary, P.; Elson, R.; Hawker, J. An outbreak of Shiga Toxin-producing Escherichia coli O157:H7 associated with contaminated salad leaves: Epidemiological, genomic and food trace back investigations. Epidemiol. Infect. 2018, 146, 1879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, D.; Dolan, G.; Aird, H.; Sorrell, S.; Dallman, T.J.; Jenkins, C.; Robertson, L.; Gorton, R. Farm-to-fork investigation of an outbreak of Shiga toxin-producing Escherichia coli O157. Microb. Genom. 2018, 4, e000160. [Google Scholar] [CrossRef] [PubMed]
- Bumunang, E.W.; McAllister, T.A.; Zaheer, R.; Polo, R.O.; Stanford, K.; King, R.; Niu, Y.D.; Ateba, C.N. Characterization of Non-O157 Escherichia coli from Cattle Faecal Samples in the North-West Province of South Africa. Microorganisms 2019, 7, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bumunang, E.W.; Ateba, C.; Stanford, K.; McAllister, T.A.; Niu, J.D. Biofilm formation by South-African Shiga toxigenic non-O157 Escherichia coli on stainless steel coupons. Can. J. Microbiol. 2020, 66, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Biofilms and Meat Safety: A Mini-Review. J. Food Prot. 2018, 82, 120–127. [Google Scholar] [CrossRef]
- Galiè, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 1–18. [Google Scholar] [CrossRef]
- Lu, L.; Hu, W.; Tian, Z.; Yuan, D.; Yi, G.; Zhou, Y.; Cheng, Q.; Zhu, J.; Li, M. Developing natural products as potential anti-biofilm agents. Chin. Med. 2019, 14, 11. [Google Scholar] [CrossRef] [Green Version]
- Moraes, J.O.; Cruz, E.A.; Souza, E.G.; Oliveira, T.C.; Alvarenga, V.O.; Peña, W.E.; Sant’Ana, A.S.; Magnani, M. Predicting adhesion and biofilm formation boundaries on stainless steel surfaces by five Salmonella enterica strains belonging to different serovars as a function of pH, temperature and NaCl concentration. Int. J. Food Microbiol. 2018, 281, 90–100. [Google Scholar] [CrossRef]
- Lee, D.-S.; Kang, M.-S.; Hwang, H.-J.; Eom, S.-H.; Yang, J.-Y.; Lee, M.-S.; Lee, W.-J.; Jeon, Y.-J.; Choi, J.-S.; Kim, Y.-M. Synergistic effect between dieckol from Ecklonia stolonifera and β-lactams against methicillin-resistant Staphylococcus aureus. Biotechnol. Bioprocess Eng. 2008, 13, 758–764. [Google Scholar] [CrossRef]
- Norden, C.W.; Wentzel, H.; Keleti, E. Comparison of Techniques for Measurement of in Vitro Antibiotic Synergism. J. Infect. Dis. 1979, 140, 629–633. [Google Scholar] [CrossRef]
- Oliver, S.P. Foodborne Pathogens and Disease Special Issue on the National and International PulseNet Network. Foodborne Pathog Dis. 2019, 16, 439–440. [Google Scholar] [CrossRef] [PubMed]
- Ford, L.; Stratakos, A.C.; Theodoridou, K.; Dick, J.T.A.; Sheldrake, G.N.; Linton, M.; Corcionivoschi, N.; Walsh, P.J. Polyphenols from Brown Seaweeds as a Potential Antimicrobial Agent in Animal Feeds. ACS Omega 2020, 5, 9093–9103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busetti, A.; Thompson, T.P.; Tegazzini, D.; Megaw, J.; Maggs, C.A.; Gilmore, B.F. Antibiophilm activity of the brown alga Halidrys siliquosa against clinically relevant human pathogens. Mar. Drugs 2015, 13, 3581–3605. [Google Scholar] [CrossRef] [PubMed]
- Tamanai-Shacoori, Z.; Chandal, F.; Rebillard, A.; Cillard, J.; Bonnaure-Mallet, M. Silver-zeolite combided to polyphenol-rich-extracts of Ascophyllum nodosum: Potential active role in prevention of periodontal diseases. PLoS ONE 2014, 9, e105475. [Google Scholar] [CrossRef] [PubMed]
- Jappe, U. Pathological mechanisms of acne with special emphasis on Propionibacterium acnes and related therapy. Acta Derm. Venereol. 2003, 83, 241–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.S.; Baik, J.S.; Oh, T.H.; Yoon, W.J.; Lee, N.H.; Hyun, C.G. Biological activities of corean Citrus obovoides and Citrus natsudaidai essential oils against acne-inducing bacteria. Biosci. Biotechnol. 2008, 72, 2507–2513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.-S.; Bae, H.-J.; Kim, S.-J.; Choi, I.S. In vitro antibacterial and anti-inflammatory properties of seaweed extracts against acne inducing bacteria, Propionibacterium acnes. J. Environ. Biol. 2011, 32, 313–318. [Google Scholar]
- Yu, Y.; Wang, L.; Fu, X.; Fu, X.; Yang, M.; Han, Z.; Mou, H.; Jeon, Y.-J.; Wang, L. Anti-oxidant and anti-inflammatory activities of ultrasonic-assistant extracted polyphenol-rich compounds from Sargassum muticum. J. Oceanol. Limnol. 2019, 37, 836–847. [Google Scholar] [CrossRef]
- Gomez-Guzman, M.; Rodrigues-Nogales, A.; Algieri, F.; Galves, J. Potential role of seaweed polyphenols in cardio-vascular-associated disorders. Mar. Drugs. 2018, 16, 250. [Google Scholar] [CrossRef] [Green Version]
- Dréno, B. What is new in the pathophysiology of acne, an overview. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 8–12. [Google Scholar] [CrossRef]
- Eom, S.-H.; Lee, E.-H.; Park, K.; Kwon, J.-Y.; Kim, P.-H.; Jung, W.-K.; Kim, Y.-M. Eckol fromEisenia bicyclisInhibits Inflammation Through the Akt/NF-κB Signaling inPropionibacterium acnes-Induced Human Keratinocyte Hacat Cells. J. Food Biochem. 2016, 41, e12312. [Google Scholar] [CrossRef]
- Choi, J.-S.; Lee, K.; Lee, B.-B.; Kim, Y.C.; Kim, Y.D.; Hong, Y.K.; Cho, K.K.; Choi, I.S. Antibacterial activity of the phlorotannins dieckol and phlorofucofuroeckol-A from Ecklonia cava against Propionibacterium acnes. Bot. Sci. 2014, 92, 425–431. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, K.B.; Oh, S.M.; Lee, B.H.; Chee, H.Y. Anti-fungal activities of dieckol isolated from the marine brown alga Ecklonia cava against Trichophyton rubrum. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 504–507. [Google Scholar] [CrossRef]
- Lee, J.-H.; Eom, S.-H.; Lee, E.-H.; Jung, Y.-J.; Kim, H.-J.; Jo, M.-R.; Son, K.-T.; Lee, H.-J.; Kim, J.H.; Lee, M.-S.; et al. In vitro antibacterial and synergistic effect of phlorotannins isolated from edible brown seaweed Eisenia bicyclis against acne-related bacteria. Algae 2014, 29, 47–55. [Google Scholar] [CrossRef]
- WHO, N.D. Food Safety and Food Borne Illness. Biochim. Clin. 2002, 26, 39. [Google Scholar]
- Prescott, L.M.; Harley, J.P.; Klein, D.A. Microbiology 5; Mc Graw Hill: New York, NY, USA, 2002; p. 932. [Google Scholar]
- Chen, B.-Y.; Pyla, R.; Kim, T.-J.; Silva, J.L.; Jung, Y.-S. Prevalence and contamination patterns of Listeria monocytogenes in catfish processing environment and fresh fillets. Food Microbiol. 2010, 27, 645–652. [Google Scholar] [CrossRef]
- Eom, S.-H.; Kim, Y.-M.; Kim, S.-K. Antimicrobial effect of phlorotannins from marine brown algae. Food Chem. Toxicol. 2012, 50, 3251–3255. [Google Scholar] [CrossRef]
- Xu, M.; Xue, H.; Li, X.; Zhao, Y.; Lin, L.; Yang, L.; Zheng, G. Chemical composition, antibacterial properties, and mechanism of Smilax china L. polyphenols. Appl. Microbiol. Biotechnol. 2019, 103, 9013–9022. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Ghosh, D.; Sarkar, S.; Karmakar, P.; Koley, H.; Gachhui, R. Antibacterial activity of polyphenolic fraction of Kombucha against Vibrio cholerae: Targeting cell membrane. Lett. Appl. Microbiol. 2018, 66, 145–152. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Pereira, V.; Dias, C.; Vasconcelos, M.; Rosa, E.A.; Saavedra, M.J. Antibacterial activity and synergistic effects between Eucalyptus globulus leaf residues (essential oils and extracts) and antibiotics against several isolates of respiratory tract infections (Pseudomonas aeruginosa). Ind. Crop. Prod. 2014, 52, 1–7. [Google Scholar] [CrossRef]
- Ziani, B.E.; Heleno, S.A.; Bachari, K.; Dias, M.I.; Alves, M.J.; Barros, L.; Ferreira, I.C. Phenolic compounds characterization by LC-DAD- ESI/MSn and bioactive properties of Thymus algeriensis Boiss. & Reut. and Ephedra alata Decne. Food Res. Int. 2019, 116, 312–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasconcelos, N.; Croda, J.; Simionatto, S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef]
- Borchers, A.T.; Keen, C.L.; Gershwin, M.E. Mushrooms, Tumors, and Immunity: An Update. Exp. Biol. Med. 2004, 229, 393–406. [Google Scholar] [CrossRef]
- Devi, K.P.; Suganthy, N.; Kesika, P.; Pandian, S.K. Bioprotective properties of seaweeds: In vitro evaluation of antioxidant activity and antimicrobial activity against food borne bacteria in relation to polyphenolic content. BMC Complement. Altern. Med. 2008, 8, 38. [Google Scholar] [CrossRef] [Green Version]
- Estevinho, L.M.; Pereira, A.; Moreira, L.F.; Dias, L.G.; Pereira, E.L. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem. Toxicol. 2008, 46, 3774–3779. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Dasagrandhi, C.; Eom, S.-H. In Vitro Antibacterial Activity of Phlorotannins from Edible Brown Algae, Eisenia bicyclis Against Streptomycin-Resistant Listeria monocytogenes. Indian J. Microbiol. 2017, 58, 105–108. [Google Scholar] [CrossRef]
- Lee, B.H.; Cole, S.; Mioche, L.; Badel-Berchoux, S. Biofilm Formation of Listeria monocytogenes Strains Under Food Processing Environments and Pan-Genome-Wide Association Study. Front Microbiol. 2019, 2698. [Google Scholar] [CrossRef] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Besednova, N.N.; Andryukov, B.G.; Zaporozhets, T.S.; Kryzhanovsky, S.P.; Kuznetsova, T.A.; Fedyanina, L.N.; Makarenkova, I.D.; Zvyagintseva, T.N. Algae Polyphenolic Compounds and Modern Antibacterial Strategies: Current Achievements and Immediate Prospects. Biomedicines 2020, 8, 342. https://doi.org/10.3390/biomedicines8090342
Besednova NN, Andryukov BG, Zaporozhets TS, Kryzhanovsky SP, Kuznetsova TA, Fedyanina LN, Makarenkova ID, Zvyagintseva TN. Algae Polyphenolic Compounds and Modern Antibacterial Strategies: Current Achievements and Immediate Prospects. Biomedicines. 2020; 8(9):342. https://doi.org/10.3390/biomedicines8090342
Chicago/Turabian StyleBesednova, Natalya N., Boris G. Andryukov, Tatyana S. Zaporozhets, Sergey P. Kryzhanovsky, Tatyana A. Kuznetsova, Ludmila N. Fedyanina, Ilona D. Makarenkova, and Tatyana N. Zvyagintseva. 2020. "Algae Polyphenolic Compounds and Modern Antibacterial Strategies: Current Achievements and Immediate Prospects" Biomedicines 8, no. 9: 342. https://doi.org/10.3390/biomedicines8090342