J.ophthalmol.(Ukraine).2021;5:3-9.
|
http://doi.org/10.31288/oftalmolzh2021539 Received: 26 August 2021; Published on-line: 23 October 2021 Morphological and ophthalmoscopic features of epiretinal membranes after intravitreal injection of various doses of aflibercept in patients with proliferative diabetic retinopathy Vira S Ponomarchuk, V. V. Vit, M. M. Umanets SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine"; Odesa (Ukraine) E-mail: v.zavodnaya@gmail.com TO CITE THIS ARTICLE: Ponomarchuk Vira S, Vit VV, Umanets MM. Morphological and ophthalmoscopic features of epiretinal membranes after intravitreal injection of various doses of aflibercept in patients with proliferative diabetic retinopathy. J.ophthalmol.(Ukraine).2021;5:3-9. http://doi.org/10.31288/oftalmolzh2021539
Background: Although methods are available to treat proliferative diabetic retinopathy (PDR), 30% of cases progress, which is an indication for vitrectomy. Purpose: To investigate the ophthalmoscopic and morphological features of epiretinal membranes (ERMs) in patients with PDR depending on the dose of preoperative intravitreal aflibercept (PIA). Material and Methods: Seventy-five patients (75 eyes) with PDR and the presence of fibrovascular ERM with a marked proliferative component were involved in the study. Patients were divided into three groups: eyes of group 1 or control group (31 eyes) received vitrectomy without PIA; group 2 (17 eyes), PIA 1.0 mg; and group 3 (27 eyes), PIA 2.0 mg. We performed a histological study on specimens of fibrovascular ERMs surgically obtained from patients to determine the microscopic features of these membranes. Results: There was ophthalmoscopic and microscopic evidence that aflibercept pretreatment in vitrectomy for PDR resulted in fibrosis of the ERM. The extent of fibrosis of the ERM and obliteration of newly formed blood vessels in the ERM depended on the dose of PIA. Complete obliteration of newly formed blood vessels in the ERM was observed as early as day 3 after 2.0-mg intravitreal aflibercept injection compared to day 5 after 1.0-mg intravitreal aflibercept injection. Pretreatment with 1.0-mg intravitreal aflibercept in vitrectomy for PDR reduced the probability of complications associated with ERM contraction, worsening of the tractional component and the development of a retinal break. Keywords: vascular endothelial growth factor, epiretinal membranes, proliferative diabetic retinopathy
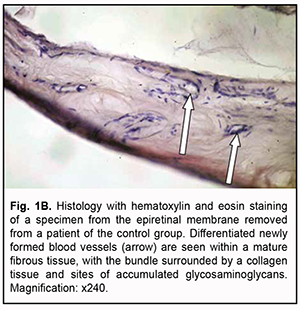
Introduction Although methods are available to treat proliferative diabetic retinopathy (PDR), 30% of cases progress to develop vitreous hemorrhage, epiretinal membranes (ERM), and tractional retinal detachment, which is an indication for vitrectomy. The success rate of vitrectomy has been reported to be 73% to 89%, and vitrectomy success depends on early detection of pathological changes in the fundus and compensation of general patient’s condition [1]. The most common intraoperative and postoperative complication of vitrectomy in PDR is intraocular hemorrhage, with the incidence rate as high as 75% [2]. Massive retinal neovascularization is a major factor that makes surgeon’s task difficult and results in the development of hemorrhagic complications requiring repeat surgery. The source of bleeding is most commonly pathologic new blood vessels of the fibrovascular epiretinal tissue which become damaged during tissue excision [3]. Postoperative resorption of massive vitreous hemorrhage may be prolonged, with a durable toxic effect exerted on the retinal photoreceptor layer and significantly impaired post-surgical functional outcomes. Such techniques as endodiathermy and endolaser photocoagulation can limit the frequency of, but not likely fully prevent these complications [4]. The above indicates that preoperative measures should be taken to prevent the development of intraoperative and postoperative hemorrhagic complications. Anti-vascular endothelial growth factor (VEGF) treatment is currently the most promising preoperative treatment option. Ophthalmologists have gained experience using anti-VEGF agents in the treatment of age-related macular degeneration (AMD), choroidal neovascularization of various origins, diabetic macular edema, and central retinal vein thrombosis [5]. In addition, anti-VEGF agents have been successfully used in combination with vitrectomy in the treatment of patients with PDR [5, 6, 7]. Particularly, studies have shown complete obliteration of neovascularization within 3-7 days of intravitreal bevacizumab injection, which allowed for a fast and efficacious vitrectomy. In some cases, however, ERM contraction caused worsening of the tractional component, thus leading to progression of tractional retinal detachment and even the development of retinal breaks [7]. Others concluded that pretreatment with intravitreal injection of ranibizumab is an effective adjunct to vitrectomy in reducing intraoperative and early postvitrectomy bleeding in PDR patients, but a reduction in the number of newly formed vessels in the ERM was observed 5 days after intravitreal injection of ranibizumab [8]. Intravitreal aflibercept 2.0 mg is also used to prevent intraoperative hemorrhagic complications in vitrectomy in PDR [9], but, to the best of our knowledge, there have been no reports on preoperative use of intravitreal aflibercept 1.0 mg in eyes with PDR undergoing vitrectomy. Therefore, the purpose of the study was to investigate the ophthalmoscopic and morphological features of epiretinal membranes in patients with proliferative diabetic retinopathy depending on the dose of preoperative intravitreal aflibercept. Material and Methods Before treatment, informed consent was signed by all patients, and they were educated about the possible consequences and complications of the surgical procedure. The study protocol was approved by the Bioethics Committee of the Institute of Eye Disease and Tissue Therapy, NAMS of Ukraine (Protocol no. 1 issued 15.10.2018). This was a pilot open prospective interventional study of 75 patients (75 eyes) aged 18 to 58 years with a neovascular glial form of PDR and the presence of fibrovascular epiretinal membrane with a marked proliferative component [10]. A threat to the macula from tractional retinal detachment (TRD) was noted in 26 eyes; TRD with involvement of the macula, in 23 eyes, and combined tractional and rhegmatogenous retinal detachment (TRRD), in 6 eyes. In addition, no retinal detachment was seen in 20 eyes. Patients underwent an eye examination which included visual acuity assessment, refractometry, tonometry, static automated perimetry, biomicroscopy, gonioscopy and ophthalmoscopy. Patients with a history of vitrectomy, prior anti-VEGF injections, or the presence of uveitis (or intraocular inflammation), iris rubeosis, elevated intraocular pressure (IOP), total vitreous hemorrhage, central retinal vein or branch occlusion, central retinal artery occlusion or ERM with a moderate proliferative component were excluded. Baseline visual acuity ranged from 0.01 to 0.25, and baseline intraocular pressure (IOP), from 18.0 mm Hg to 23.0 mmHg. In addition, initial complicated cataract was observed in 41 eyes and 34 eyes had an intraocular lens (IOL) implanted. Patients were divided into three groups: eyes of group 1 or control group (31 eyes) received vitrectomy without intravitreal aflibercept; group 2, intravitreal aflibercept 1.0 mg (17 eyes; 8 eyes, 3 days before vitrectomy and 9 eyes, 5 days before vitrectomy); and group 3, intravitreal aflibercept 2.0 mg (27 eyes; 13 eyes, 3 days before vitrectomy and 14 eyes, 5 days before vitrectomy). Aflibercept was injected into the vitreous by inserting a 29-gauge needle through the pars plana ciliaris. A color fundus photograph was taken in each patient before intravitreal aflibercept injection and before vitrectomy. Worsening of the tractional component was characterized by a contracted epiretinal membrane and progression after intravitreal aflibercept injection and was determined ophthalmoscopically at day 3 or photographically at day 5. Each patient underwent a 25G three-port vitrectomy using an Alcon Constellation 25-G vitrectomy machine (Alcon Laboratories, Inc., Fort Worth, TX, USA) and OMS-800 OFFISS microscope (Topcon, Tokyo, Japan) on day 3 to day 5 after intravitreal aflibercept injection. Core and peripheral vitrectomy and posterior hyaloid peeling and excision over 3600 were performed using a wide angle viewing system BIOM system (Oculus, Wetzlar, Germany). Epiretinal membranes were removed completely. Fifteen 1 x 1 mm specimens (particularly, three, six and six specimens from eyes of group 1, group 2 and group 3, respectively) were taken from fibrovascular ERMs for histological microscopy studies. The sections were prepared and then routinely stained with hematoxylin and eosin and examined under a light microscope Jenamed II (Carl Zeiss, Gottingen, Germany). Results All patients of the control group showed ophthalmoscopic evidence of the fibrovascular ERM with a marked proliferative component; the membrane featured newly formed blood vessels of mostly small diameter, with active neovascularization along membrane margin. There was microscopic evidence of numerous well-differentiated blood vessels inside mature fibrous tissue. These vessels varied in diameters and appeared non-uniformly arranged. In one control specimen, the vascular bundle was surrounded by a collagen tissue and sites of accumulated glycosaminoglycans, and this fibrous tissue had almost no cells on microscopy. Therefore, this tissue can be classified as a mature, edematous fibrovascular tissue (Fig. 1a, 1b).
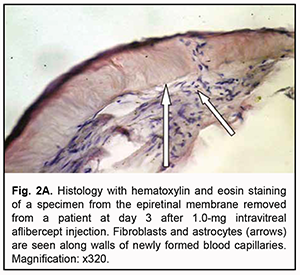
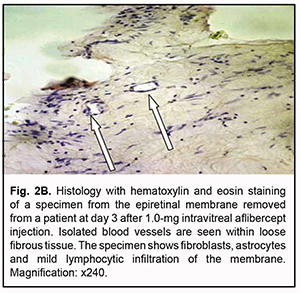
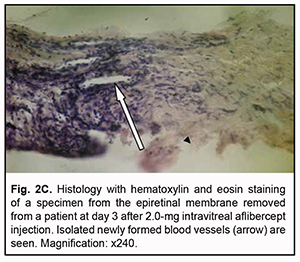
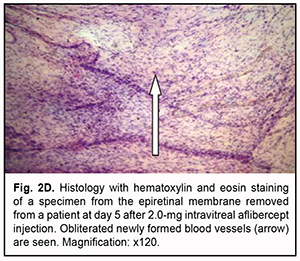
At day 3 after a 1.0 mg intravitreal aflibercept injection, there was ophthalmoscopic evidence of complete obliteration of newly formed blood vessels in epiretinal membranes in 50% of patients (4 eyes). At day 5 after a 1.0 mg intravitreal aflibercept injection, examination showed complete obliteration of newly formed blood vessels in epiretinal membranes in all cases (9 eyes). In addition, no change in visual acuity was noted in any patient following 1.0 mg intravitreal aflibercept injection. Of the 27 eyes that received 1.0 mg intravitreal aflibercept injection, 3 (17.6%) showed evidence of worsening of the tractional component due to ERM contraction. Heterogeneous morphology at various sites of the membrane was a morphological feature of epiretinal membranes following 1.0 mg intravitreal aflibercept injection. Of special note that a number of blood vessels in the membranes were less than for the patients who did not receive aflibercept therapy, and these vessels were mainly the capillaries and not completely differentiated. Vessel formation sites alternated with sites of bundles of differently oriented collagen fibers. Another essential feature was the presence of numerous cells of the types (particularly, fibroblasts and astrocytes) that were morphologically different from each other. Both types of cells are spindle-shaped and have rod-like nuclei, and differ only in size. Fibroblasts are larger and arranged along walls of capillaries (Fig. 2A). In addition, one specimen showed mild lymphocytic infiltration of the membrane, indicating the presence of inflammation (Fig. 2B). At day 3 after 1.0 mg intravitreal aflibercept injection, non-obliterated newly formed vessels were seen on some histological specimens of removed ERMs (Fig. 2C). At day 5 after 1.0 mg intravitreal aflibercept injection, histological specimens of removed ERMs showed a dense and fibrous connective tissue composed of spindle-shaped cells and obliterated newly formed vessels (Fig. 2D).
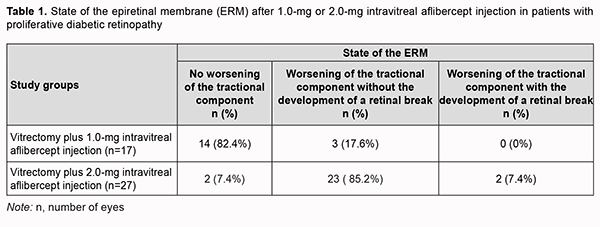
At day 3 after 2.0 mg intravitreal aflibercept injection, there was ophthalmoscopic evidence of complete obliteration of newly formed blood vessels in epiretinal membranes in 84.6% of patients (11 of 13 eyes). At day 5 after 2.0 mg intravitreal aflibercept injection, examination showed complete obliteration of newly formed blood vessels in epiretinal membranes in all cases (14 eyes). Of the 27 eyes that received 2.0 mg intravitreal aflibercept injection, 23 (85.2%) showed evidence of worsening of the tractional component due to ERM contraction, which resulted in the development of a retinal break in 2 eyes (Table 1).
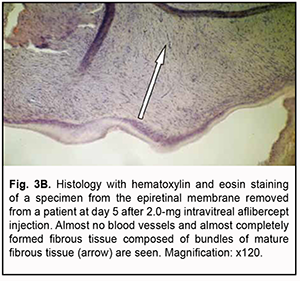
The presence of almost no cells as early as day 3 as well as obliteration was a structural morphological feature of epiretinal membranes following 2.0 mg intravitreal aflibercept injection (Fig. 3A). Epiretinal membranes are accumulations of glycosaminoglycans with initial formation of a low number of collagen fibrils. In addition, they have almost no cells and no lymphocytes. One specimen showed an almost completely formed fibrous tissue composed of bundles of mature fibrous tissue at day 5 after 2.0 mg intravitreal aflibercept injection (Fig. 3B).
Such complications as endophthalmitis and/or fibrous inflammatory reaction were not observed in any case. Transitory increased IOP was noted in all patients following 1.0-mg or 2.0-mg aflibercept injection, and did not require hypotensive medications. Discussion Preoperative intravitreal anti-VEGF therapy for prevention of hemorrhagic complications has been widely used since the first years of the 21st century. Current developments of anti-VEGF therapy are targeted at the synthesis of medications capable of inhibiting an increasing number of angiogenic factors. One of the first anti-VEGF medications was pegaptanib, a pegylated oligonucleotide that selectively binds VEGF165 [11,12]. However, it has been, not registered in most countries because it is less potent than other anti-VEGF agents. Bevacizumab (Avastin; Genentech, Inc., South San Francisco, CA) is a full-length monoclonal humanized antibody, whereas ranibizumab (Lucentis, Genentech, Inc., South San Francisco, CA) is a fragment of the above antibody. Both of them bind all isoforms of VEGF-A, but the use of bevacizumab remains off-label in ophthalmology [12, 13, 14, 15]. Aflibercept is a recombinant fusion protein that consists of portions of VEGF receptor (R) 1 and VEGFR2 extracellular domains fused to the Fc portion of human immunoglobulin G1 (Ig1). Unlike other anti-VEGF agents, it binds not only to all isoforms of VEGF-A, but also to VEGF-B and placental growth factor [16]. Experience has been gained with the use of anti-VEGF agents in vitreoretinal surgery for PDR. Various authors reported that complete obliteration of neovascularization was achieved within 3-5 days after intravitreal bevacizumab injection, which allowed for a fast and efficacious vitrectomy for PDR. In some cases, however, the contraction of the ERM caused worsening of the tractional component and the development of retinal breaks. These complications usually developed 10-14 days after injection [17]. In addition, because bevacizumab has been used off-label in ophthalmology, it has been not approved for use in ophthalmology in many countries, including Ukraine. Ranibizumab is another drug developed for intravitreal use. Various studies have demonstrated that intravitreal ranibizumab pretreatment in vitrectomy for PDR was effective. Obliteration of newly formed blood vessels in the epiretinal membrane was observed seven days after intravitreal ranibizumab (Lucentis, 0.5 mg in 0.05 ml solution for injection, Novartis Pharmaceuticals UK Ltd) injection, somewhat later than following intravitreal bevacizumab injection [18]. Because in the ROTATE trial [19], ranibizumab 0.3 mg (i.e., half of the standard dose) demonstrated improved visual and anatomic outcomes in patients with persistent diabetic macular edema following bevacizumab, we supposed that half of the standard dose will allow to achieve obliteration of newly formed blood vessels in the epiretinal membrane, reduce the frequency of intraoperative and postoperative hemorrhagic complications, and reduce the risk of complications associated with worsening of the tractional component in ERM and the development of retinal breaks in patients with PDR. We found that, in patients with PDR of 1.0-mg aflibercept and 2.0-mg aflibercept groups, obliteration of newly formed blood vessels in the fibrovascular ERM with a marked proliferative component was seen as early as day 5 after aflibercept injection. However, of the 27 eyes that received 2.0 mg intravitreal aflibercept injection, 23 (85.2%) showed ophthalmoscopic evidence of worsening of the tractional component due to ERM contraction, which resulted in the development of a retinal break in 2 eyes (7.4%). In addition, of the 27 eyes that received 1.0 mg intravitreal aflibercept injection, 3 (17.6%) showed evidence of worsening of the tractional component, but no eye developed a retinal break. Hsu and colleagues [20] investigated the clinical, histopathological, and surgical features of the epiretinal membrane (ERM) after diabetic vitrectomy. Histopathological examinations of ERMs in 6 of 16 cases showed abundant collagen fibers with epithelial cells, which is in agreement with our findings. Immunohistochemical staining with CD 34 demonstrated the presence of vascular endothelium in two of the six specimens [20]. In the current study, newly formed blood vessels in the fibrovascular ERM were obliterated at day 3 after 2.0-mg intravitreal aflibercept injection, whereas individual newly formed capillaries were seen in the fibrovascular ERM at day 3 after 1.0-mg intravitreal aflibercept injection. In addition, at day 5, histological specimens of removed ERMs from both aflibercept injection groups showed a dense and fibrous connective tissue and obliterated newly formed blood vessels. We also found that activation of the tractional component was more common after 2.0-mg intravitreal aflibercept injection, and can lead to the development of a retinal break. Therefore, in patients with PDR receiving the standard dose of intravitreal aflibercept, it is reasonable to perform vitrectomy at day 3 after injection, when most newly formed vessels in the ERM are already obliterated, and the risk of worsening of the tractional component is not high. In patients with PDR receiving 1.0-mg intravitreal aflibercept injection, vitrectomy should be performed at day 5 after injection, given the fact of obliteration of newly formed blood vessels in the ERM at this time. Therefore, there was ophthalmoscopic and microscopic evidence that aflibercept pretreatment in vitrectomy for PDR resulted in fibrosis of the epiretinal fibrovascular membrane with a marked proliferative component. The extent of fibrosis of the fibrovascular membrane and obliteration of newly formed blood vessels in the ERM depended on the dose of intravitreal aflibercept. Thus, complete obliteration of newly formed blood vessels in the epiretinal membrane was observed as early as day 3 after 2.0-mg intravitreal aflibercept injection compared to day 5 after 1.0-mg intravitreal aflibercept injection. Pretreatment with 1.0-mg intravitreal aflibercept in vitrectomy for PDR reduces the probability of complications associated with epiretinal membrane contraction, worsening of the tractional component and the development of a retinal break.
References 1.Shkvorchenko DO, Levina LV. [Retinotomy and retinectomy in the management of proliferative diabetic retinopathy complicated by anterior proliferative vitreoretinopathy]. In: [Proceedings of the National Russian Conference on Modern Technologies for the Treatment of Vitreoretinal Pathology]. Moscow; 2006. p. 205-10. Russian. 2.Hendrick AM, Gibson MV, Kulshresthra A. Diabetic Retinopathy. Prim Care. 2015 Sep;42(3):451-64. doi: 10.1016/j.pop.2015.05.005. 3.Sawa H, Ikeda T, Matsumoto Y. Neovascularization from scleral wound as cause of vitreous rebleeding after vitrectomy for proliferative diabetic retinopathy. Jpn J Ophthalmol. Mar – Apr 2000;44(2):154-60. 4.Blumenkranz MS. New developments in diabetic vitrectomy. The Vitreoretinal Frontier; Dallas, Texas. November 6–7, 1992. 5.Korol A, Kustryn T, Zadorozhnyy O, Pasyechnikova N, Kozak I. Comparison of Efficacy of Intravitreal Ranibizumab and Aflibercept in Eyes with Myopic Choroidal Neovascularization: 24-Month Follow-Up. J Ocul Pharmacol Ther. 2020 Mar;36(2):122-125. 6.Aleman I, Castillo Velazquez J, Rush SW. Ziv-aflibercept versus bevacizumab administration prior to diabetic vitrectomy: a randomised and controlled trial. Br J Ophthalmol. 2019 Dec;103(12):1740-6. 7.Velazquez JC, Aleman I, Rush SW. Bevacizumab before Diabetic Vitrectomy: A Clinical Trial Assessing 3 Dosing Amounts. Ophthalmol Retina. 2018 Oct; 2(10):1010-20. 8.Chen HJ, Wang CG, Dou HL. Effect of intravitreal ranibizumab pretreatment on vitrectomy in young patients with proliferative diabetic retinopathy. Ann Palliat Med. 2020 Jan;9(1):82-9. 9.Umanets N, Korol A, Vit V, et al. Umanets N. Peculiarities of vitrectomy and morphologic changes in the epiretinal membrane after intravitreal aflibercept in patients with severe proliferative diabetic retinopathy. Retin Cases Brief Rep. 2017 spring;11(2):114-8. 10.Ponomarchuk Vira S, Velychko LM, Umanets MM. Vitreous VEGF levels among patients with proliferative diabetic retinopathy depending on the general clinical status and ocular status. J Ophthalmol (Ukraine). 2021;4: 19-25. 11.Bezdetko PA. [Pharmacological therapy for developing diabetic retinopathy: Problems, Questions and Solutions]. Oftalmologiia Vostochnaia Ievropa. 2016; 1 (28):109-23. Russian. 12.Velichko PB, Osmanov EM. [Current methofological approaches to the treatment of diabetic retinopathy]. Vestnik Tambovskogo universiteta. 2013; 6(18):3248–9. Russian. 13.Nicholson BP, Barkmeier AJ. Bevacizumab and diabetic vitrectomy. Int Ophthalmol Clin. 2014 Spring;54(2):111-26. 14.Konenkov VI, Klomontov VV, Chernykh VV. [Anti-VEGF agents in the treatment of diabetic macular edema]. Diabetes mellitus. 2013;16(4):78-84. Russian. 15.Papadopoulos N, Martin J., Ruan Q. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, Ranibizumab and Bevacizumab. Angiogenesis. 2012 Jun; 15 (2): 171–85. 16.Abd Elhamid AH, Mohamed AAEA, Khattab AM. Intravitreal Aflibercept injection with Panretinal photocoagulation versus early Vitrectomy for diabetic vitreous hemorrhage: randomized clinical trial. BMC Ophthalmol. 2020 Apr 6; 20(1):130. 17.Zhao LQ, Zhu H, Zhao PQ. A systematic review and meta-analysis of clinical outcomes of vitrectomy with or without intravitreal bevacizumab pretreatment for severe diabetic retinopathy. J Ophthalmol. 2011 Sep; 95(9):1216-22. 18.Comyn O, Wickham L, Charteris DG. Ranibizumab pretreatment in diabetic vitrectomy: a pilot randomised controlled trial (the RaDiVit study). Eye (Lond). 2017 Sep;31(9):1253-58. 19.Fechter C, Frazier H, Marcus WB. Ranibizumab 0.3 mg for Persistent Diabetic Macular Edema After Recent, Frequent, and Chronic Bevacizumab: The ROTATE Trial. Ophthalmic Surg Lasers Imaging Retina. 2016 Nov 1;47(11):1-18. 20.Hsu Y-R, Yang C-M, Yeh P-T. Clinical and histological features of epiretinal membrane after diabetic vitrectomy. Graefes Arch Clin Exp Ophthalmol Actions. 2014 Mar; 252(3)
Author Contributions: Vira S. Ponomarchuk: conducting intravitreal injections, data collection and analysis, writing the text; V.V. Vit: histological studies, editing; M.M.Umanets: concept and design of the study, surgical interventions, and editing. Acknowledgement: The authors express their gratitude to O.I.Dragomiretska for assistance in statistical data processing. Disclosures: The authors declare no conflict of interest. There are no external sources of funding.
|