Abstract
We discuss three areas of antigen presentation and macrophage biology being investigated in the laboratory. Using hen egg-white lysozyme as a protein antigen, all the segments of the molecules selected by the class II histocompatibility molecule I-Ak were identified and characterized. The display of each family of peptides was explained biochemically and quantitated. Conformational isomers of a peptide-major histocompatibility complex (MHC) complex were identified. The relationship between the amounts of peptide-MHC displayed by the antigen-presenting cells and two biologic responses, central thymic selection and T-cell responses after immunization in adjuvant, were examined. The class II MHC molecule of the nonobese diabetic I-Ag7 is being examined for its properties of peptide selection. The objective is to identify the diabetogenic peptides, as well as the repertoire of protein antigens from β-cells that trigger autoantibodies. The I-Ag7 molecule selects peptides that show very distinctive sequence motifs: one or more acidic residues at the carboxy terminus that interact at the P9 pocket of the binding groove. Finally, the investigations in listeriosis examined the early events in immune induction. More important, we found that Listeria causes marked apoptosis of lymphocytes around infective foci resulting from the apoptogenic properties of the poreforming molecule Listeriolysin O.
Similar content being viewed by others
References
Allen PM, Unanue ER: Antigen processing and presentation by macrophages. Am J Anat 1984;170:483–490.
Gammon G, Shastri N, Cogswell J, et al: The choice of T-cell epitopes utilized on a protein antigen depends on multiple factors distant from, as well as at the determinant site. Immunol Rev 1987;98:53–73.
Moudgil KD, Sekiguchi D, Kim SY, Sercarz EE: Immunodominance is indenpendent of structural constraints: each region within hen eggwhite lysozyme is potentially available upon processing of native antigen. J Immunol 1997;159:2574–2579.
Allen PM, Strydom DJ, Unanue ER: Processing of lysozyme by macrophages: identification of the determinant recognized by two T cell hybridomas. Proc Natl Acad Sci USA 1984;81:2489–2495.
Shimonkevitz R, Kappler J, Marrack P, Grey H: Antigen recognition by H-2 restricted T cells: cell-free antigen processing. J Exp Med 1983;158:303–316.
Ziegler K, Unanue ER: Identification of a macrophage antigen-processing event required for I-region-restricted antigen presentation to lymphocytes. J Immunol 1981;127:1869–1877.
Ziegler K, Unanue ER: Decrease in macrophage antigen catabolism by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci USA 1982;78:175–180.
Babbitt BP, Allen PM, Matsueda G, Haber E, Unanue ER: Binding of immunogenic peptides to la histocompatibility molecules. Nature 1985;317:359–362.
Babbitt BP, Matsueda G, Haber E, Unanue ER, Allen PM: Antigenic competition at the level of peptide-la binding. Proc Natl Acad Sci USA 1986;83:4509–4513.
Lindner R, Unanue ER: Distinct antigen MHC class II complexes generated by separate processing pathways. EMBO J 1996;15:6910–6920.
Nelson CA, Roof RW, McCourt DW, Unanue ER: Identification of the naturally processed form of hen egg white lysozyme bound to the murine major histocompatibility complex class II molecule I-Ak. Proc Natl Acad Sci USA 1992;89:7380–7383.
Nelson CA, Viner NJ, Young SP, Petzold SJ, Unanue ER: A negatively charged anchor residue promotes high-affinity binding to the MHC molecule I-Ak. J Immunol 1996;157:755–762.
Gugasyan R, Vidavsky I, Nelson CA, Gross ML, Unanue ER: Isolation and quantitation of a minor determinant of hen egg white lysozyme bound to I-Ak by using peptide-specific immunoaffinity. J Immunol 1998;161:6074–6083.
Gugasyan R, Velazquez C, Vidavsky I, et al: Independent selection by I-Ak molecules of two epitopes found in tandem in an extended polypeptide. J Immunol 2000;165:3206–3213.
Velazquez C, Vidavsky I, van der Drift, K, Gross ML, Unanue ER: Chemical identification of a low abundant lysozyme peptide family bound to I-Ak histocompatibility molecules. J Biol Chem 2002;277:42,514–42,522.
Hunt DF, Michel H, Dickinson TA, et al: Peptides presented to the immune system by the murine class II major histocompatibility complex molecule I-Ad. Science 1992;256:1817–1820.
Chicz RM, Urban RG, Lane WS, et al: Predominant naturally processed peptides bound to HLA-DR1 are derived from MHC-related molecules and are heterogeneous in size. Nature 1992;358:764–768.
Rudensky AY, Preston-Hurlburt P, Al-Ramadi BK, Rothbard J, Janeway CA Jr. Predominant naturally processed peptides bound to HLA-DR1 are derived from MHC-related molecules and are heterogeneous in size. Nature 1992;359:429–431.
Velazquez C, DiPaolo R, Unanue ER: Quantitation of lysozyme peptides bound to class II MHC molecules indicates very large differences in levels of presentation. J Immunol 2001;166:5488–5494.
Dadaglio G, Nelson CA, Deck MB, Petzold SJ, Unanue ER: Characterization and quantitation of peptide-MHC complexes produced from hen egg lysozyme using a monoclonal antibody. Immunity 1997;6:727–738.
Zhong, G, Romagnoli P, Germain RN: Related leucine-based cytoplasmic targeting signals in invariant chain and major histocompatibility complex class II molecules control endocytic presentation of distinct determinants in a single protein. J Exp Med 1997;185:429–438.
Baldwin KK, Reay PA, Wu L, Farr A, Davis MM: A T cell receptor-specific blockade of positive selection. J Exp Med 1999;189:13–24.
Dongre AR, Kovats S, deRoos P, et al.: in vivo MHC class II presentation of cytosolic spectrometry and functional analyses. Eur J Immunol 2001;31:1485–1494.
Vignali DA, Urban RG, Chicz RM, Strominger JL: Minute quantities of a single dominant foreign epitope are presented as large nested sets by major histocompability complex class II molecules. Eur J Immunol 1993;23:1602–1613.
Vignali DA, Strominger JL: Amino acid residues that flank core peptide epitopes and the extracellular domains of CD4 modulate differential signaling through the T cell receptor. J Exp Med 1994;179: 1945–1956.
Nelson CA, Fremont DH: Structural principles of MHC class II antigen presentation. Rev Immunogenet 1999;1:47–59.
Fremont DH, Crawford F, Marrack P, Hendrickson WA, Kappler J: Crystal structure of mouse H2-M. Immunity 1998;9:385–393.
Fremont DH, Hendrickson WA, Marrack P, Kappler J: Structures of an MHC class II molecule with covalently bound single peptides. Science 1996;272:1001–1004.
Corper AL, Stratmann T, Apostolopoulos V, et al.: A structural framework for deciphering the link between I-Ag7 and autoimmune diabetes. Science 2000;288: 505–511.
Scott CA, Peterson PA, Teyton L, Wilson IA: Crystal structures of two I-Ad-peptide complexes reveal that high affinity can be achieved without large anchor residues. Immunity 1998;8:319–329.
Brown JH, Jardetzky TS, Gorga JC, et al.: Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature 1993;364:33–39.
Dessen A, Lawrence CM, Cupo S, Zaller DM, Wiley DC: X-ray crystal structure of HLA-DR4 (DRA*0101, DRB1*0401) complexed with a peptide from human collagen II. Immunity 1997;7:473–481.
Jardetzky TS, Brown JH, Gorga JC, et al.: Three-dimensional structure of a human class II histocompatibility molecule complexed with superantigen. Nature 1994;368:711–718.
Latek RR, Suri A, Petzold SJ, Nelson CA, Kanagawa O, Unanue ER, Fremont DH: Structural basis of peptide binding and presentation by the type 1 diabetes-associated MHC class II molecule of NOD mice. Immunity 2000;12:699–710.
Jardetzky TS, Gorga JC, Busch R, Rothbard J, Strominger JL, Wiley DC: Peptide binding to HLA-DR1: a peptide with most residues substituted to alanine retains MHC binding. EMBO J 1990;9:1797–1803.
Latek RR, Petzold S, Unanue ER: Hindering auxiliary anchors are potent modulators of peptide binding and selection by I-Ak class II molecules. Proc Natl Acad Sci USA 2000;97:11,460–11,465.
Pu Z, Carrero JA, Unanue ER: Distinct recognition by two subsets of T cells of an MHC class II-peptide complex. Proc Natl Acad Sci USA 2002;99:8844–8849.
Allen PM, Matsueda GR, Evans RJ, Dunbar JB Jr, Marshall G, Unanue ER: Identification of the T-cell and Ia contact residues of a T-cell antigenic epitope. Nature 1987;327:713–715.
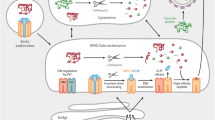
Harding CV, Collins DS, Slot JW, Geuze HJ, Unanue ER: Liposome-capsulated antigens are processed in lysosomes, recycled, and presented to T cells. Cell 1991;64:393–401.
Collins DS, Unanue ER, Harding CV: Reduction of disulphide bonds with lysosomes is a key step in antigen processing. J Immunol 1991;147:4054–4059.
Pu Z, Lovitch SB, Bikoff EK, Unanue ER: T cells distinguish MHC-peptide complexes formed in separate vesicles and edited by H2-DM. Immunity 2004;20: 467–476.
Jensen PE: Reduction of disulfide bonds during antigen processing: evidence from a thiol-dependent insulin determinant. J Exp Med 1991;174:1121–1130.
Arunachalam B, Phan UT, Dong C, et al.: Defective antigen processing in GILT-free mice. Science 2001;294:1361–1365.
Donermeyer DL, Allen PM: Binding to Ia protects an immunogenic peptide from proteolytic degradation. J Immunol 1989;142:1063–1068.
Werdelin O, Mouritsen S, Peterson BL, Sette A, Buus S: Facts on the fragmentation of antigens in presenting cells, on the association of antigen fragments with MHC molecules in cell-free systems, and speculation on the cell biology of antigen processing. Immunol Rev 1988;106:181–193.
Carrasco-Marin E, Petzold S, Unanue ER: Two structural states of peptide-class II MHC complexes revealed by photoaffinity labeled peptides. J Biol Chem 1999;274:31,333–31,340.
Nelson CA, Vidavsky I, Viner NJ, Gross ML, Unanue ER: Amino-terminal trimming of peptides for presentation on major histocompatibility complex class II molecules. Proc Natl Acad Sci USA 1997;94: 628–633.
Rammensee HG, Friede T, Stevanoviic S: MHC ligands and peptide motifs: first listing. Immunogenetics 1995;41:178–228.
Sercarz EE, Lehmann PV, Ametani A, Benichou G, Miller A, Mougdil K: Dominance and crypticity of T cell antigenic determinants. Annu Rev Immunol 1993;11:729–766.
Viner NJ, Nelson CA, Unanue ER: Identification of a major I-Ek-restricted determinant of hen egg lysozyme: limitations of lymph node proliferation studies in defining immunodominance and crypticity. Proc Natl Acad Sci USA 1995;92:2214–2218.
Viner NJ, Nelson CA, Deck B, Unanue ER: Complexes generated by the binding of free peptides to class II MHC molecules are antigenically diverse compared with those generated by intracellular processing. J Immunol 1996;156:2365–2368.
Peterson DA, DiPaolo RJ, Kanagawa O, Unanue ER: Quantitative analysis of the T cell repertoire that escapes negative selection. Immunity 1999;11: 453–462.
Cirrito TP, Pu Z, Deck MB, Unanue EF: Deamidation of asparagine in a major histocompatibility complex-bound peptide affects T cell recognition but does not explain type B reactivity. J Exp Med 2001;194:1165–1170.
Sloan VS, Cameron P, Porter G, et al.: Mediation by HLA-DM of dissociation of peptides from HLA-DR. Nature 1995;375:802–806.
Kropshofer H, Vogt AB, Moldenhauer G, Hammer J, Blum JS, Hammerling GJ: Editing of the HLA-DR-peptide repertoire by HLA-DM. EMBO J 1996;15: 6144–6154.
van Ham SM, Gruneberg U, Malcherek G, Broker I, Melms A, Trowsdale J: Human histocompatibility leukocyte antigen (HLA)-DM edits peptides presented by HLA-DR according to their ligand binding motifs. J Exp Med 1996;184:2019–2024.
Lovitch SB, Petzold SJ, Unanue ER: Cutting Edge: H-2DM is responsible for the large differences in presentation among peptides selected by I-Ak during antigen processing. J Immunol 2003;171:2183–2186.
Doebele RC, Busch R, Scott HM, Pashine A, Mellins ED: Determination of the HLA-DM interaction, site on HLA-DR molecules. Immunity 2000;13:517–527.
Marrack P, Ignatowicz L, Kappler JW, Boymel J, Freed JH: Comparison of peptides bound to spleen and thymus class II. J Exp Med 1993;178:2173–2183.
Lovitch SB, Walters JJ, Gross ML, Unanue ER: APCs present Ak-derived peptides that are autoantigenic to type B T cells. J Immunol 2003;170:4155–4160.
Santambrogio JL, Stern LJ: Extracellular antigen processing and presentation by immature dendritic cells. Proc Natl Acad Sci USA 1999;96:15,056–15,061.
Barlow AK, He X, Janeway CA Jr: Exogenously provide peptides of a self-antigen can be processed into forms that are recognitzed by self-T cells. J Exp Med 1998;187:1403–1415.
Viret C, He X, Janeway CA Jr: Paradoxical intrathymic positive selection in mice with only a covalently presented agonist peptide. Proc Natl Acad Sci USA 2001;98:9243–9248.
Viret C, He X, Janeway CA Jr: Altered positive selection due to corecognition of floppy peptide/MHC II conformers supports an integrative model of thymic selection. Proc Natl Acad Sci USA 2003;100: 5354–5359.
Huang JC, Han M, Minguela A, Pastor S, Qadri A, Ward ES: T cell recognition of distinct peptide: I-Au conformers in murine experimental autoimmune encephalomyelitis. J Immunol 2003;171:2467–2477.
Hulsmeyer M, Fiorillo M, Fiorillo MT, et al.: Dual, HLA-B27 subtype-dependent conformation of a self-peptide. J Exp Med 2004;199:271–281.
Beeson C, Anderson TG, Lee C, McConnell HM: Isomeric complexes of peptides with class II proteins of the major histocompatibility complex. J Am Chem Soc 1995;117:10,429–10,433.
Peterson DA, DiPaolo RJ, Kanagawa O, Unanue ER: Cutting edge: negative selection of immature thymocytes by a few peptide-MHC complexes: differential sensitivity of immature and mature T cells. J Immunol 1999;162:3117–3120.
Reay PA, Matsui K, Haase K, Wulfing C, Chien YH, Davis MM: Determination of the relationship between T cell responsiveness and the number of MHC-peptide complexes using specific monoclonal antibodies. J Immunol 2000;164:5626–5634.
Pape KA, Khoruts A, Mondino A, Jenkins MK: Inflammatory cytokines enhance the in vivo clonal expansion and differentiation of antigen-activated CD4+ T cells. J Immunol 1997;159:591–598.
Ho WY, Cooke MP, Goodnow CC, Davis MM: Resting and anergic B cells are defective in CD28-dependent costimulation of naive CD4+ T cells. J Exp Med 1994;179:1539–1549.
Akkaraju S, Ho WY, Leong D, Canaan K, Davis MM, Goodnow CC: A range of CD4 T cell tolerance: partial inactivation to organ-specific antigen allows nondestructive thyroiditis or insulitis. Immunity 1997: 7:255–271.
DiPaolo RJ, Unanue ER: The level of peptide-MHC complex determines the susceptibility to autoimmune diabetes: studies in HEL transgenic mice. Eur J Immunol 2001;31:3453–3459.
Anderson MS, Venanzi ES, Lein L, et al.: Projection of an immunological self shadow within the thymus by the aire protein. Science 2002;298:1395–1400.
Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC: Aire regulates negaive selection of organ-specific T cells. Nat Immunol 2003;4:350–354.
Venanzi ES, Benoist C, Mathis M: Good riddance: thymocyte clonal deletion prevents autoimmunity. Curr Opin Immunol 2004;16:197–214.
Kanagawa O, Martin SM, Vaupel BA, Carrasco-Marin E, Unanue ER: Autoreactivity of T cells from nonobese diabetic mice: an I-Ag7-dependent reaction. Proc Natl Acad Sci USA 1998;95:1721–1724.
DiPaolo RJ, Unanue ER: Cutting edge: chemical dominance does not relate to immunodominance: studies of the CD4+ T cell response to a model antigen. J Immunol 2002;169:1–4.
Castigli E, Alt FW, Davidson L, et al.: CD40-deficient mice generated by recombination-activating gene-2-deficient blastocyst complementation. Proc Natl Acad Sci USA 1994;91:12,135–12,141.
Borriello F, Sethna MP, Boyd SD, et al.: B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity 1997;6:303–311.
Luhder F, Hoglund P, Allison JP, Benoist C, Mathis D: Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) regulates the unfolding of autoimmune diabetes. J Exp Med 1998;187:427–432.
DiPaolo RJ, Unanue ER: Cutting edge: the relative distribution of T cells responding to chemically dominant or minor epitopes of lysozyme is not affected by CD40-CD40 ligand and B7-CD28-CTLA-4 costimulatory pathways. J Immunol 2002;169:2832–2836.
Byersdorfer C, DiPaolo R, Petzold S, Unanue E: Following immunization antigen becomes concentrated in a limited number of antigen presenting cells including B cells. J Immunol 2004;173:6627–6634.
Creusot RJ, Thomsen LL, Tite JP, Chain BM: Local cooperation dominates over competition between CD4+ T cells of different antigen/MHC specificity. J Immunol 2003;171:240–246.
Kedl RM, Rees WA, Hildeman DA, Schaefer B, Mitchell T, Kappler J, Marrack P: T cells compete for access to antigen-bearing antigen-presenting cells. J Exp Med 2000;192:1105–1113.
Todd JA, Bell JI, McDevitt HO: HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature 1987;329:599–604.
Lee KH, Wucherpfennig KW, Wiley DC: Structure of a human insulin peptide-HLA-DQ8 complex and susceptibility to type 1 diabetes. Nat Immunol 2001;2: 501–507.
Gregori S, Bono E, Gallazzi F, Hammer J, Harrison LC, Adorini L: The motif for peptide binding to the insulin-dependent diabetes mellitus-associated class II MHC molecule I-A(g7) validated by phage display library [in process citation]. Int Immunol 2000;12:493–503.
Carrasco-Marin E, Shimizu J, Kanagawa O, Unanue ER: The class II MHC I-Ag7 molecules from nonobese diabetic mice are poor peptide binders. J Immunol 1996;156:450–458.
Carrasco-Marin E, Kanagawa O, Unanue ER: The lack of consensus for I-A(g7)-peptide binding motifs: is there a requirement for anchor amino acid side chains? Proc Natl Acad Sci USA 1999;96:8621–8626.
Bhatnagar A, Milburn PJ, Lobigs M, Blanden RV, Gautam AM: Nonobese diabetic mice display elevated levels of class II-associated invariant chain peptide associated with I-Ag7 on the cell surface. J Immunol 2001;166:4490–4497.
Suri A, Vidavsky I, van der Drift K, Kanagawa O, Gross ML, Unanue ER: In APCs, the autologous peptides selected by the diabetogenic I-Ag7 molecule are unique and determined by the amino acid changes in the P9 pocket. J Immunol 2002;168:1235–1243.
Suri A, Walters JJ, Kanagawa O, Gross ML, Unanue ER: Specificity of peptide selection by antigen-presenting cells homozygous or heterozygous for expression of class II MHC molecules: the lack of competition. Proc Natl Acad Sci USA 2003;100: 5330–5335.
Quartey-Papafio R, Lund T, Chandler P, et al.: Aspartate at position 57 of nonobese diabetic I-Ag7 beta-chain diminishes the spontaneous incidence of insulin-dependent diabetes mellitus. J Immunol 1995;154:5567–5575.
Singer SM, Tisch R, Yang XD, McDevitt HO: An Abd transgene prevents diabetes in nonobese diabetic mice by inducing regulatory T cells. Proc Natl Acad Sci USA 1993;90:9566–9570.
Deng H, Apple R, Clare-Salzler M, et al.: Determinant capture as a possible mechanism of protection afforded by major histocompatibility complex class II molecules in autoimmune disease. J Exp Med 1993;178: 1675–1680.
McDevitt HO: The role of MHC class II molecules in susceptibility and resistance to autoimmunity. Curr Opin Immunol 1998;10:677–681.
Chao CC, McDevitt HO: Identification of immunogenic epitopes of GAD 65 presented by Ag7 in nonobese diabetic mice. Immunogenetics 1997;46:29–34.
Bockova J, Elias D, Cohen IR: Treatment of NOD diabetes with a novel peptide of the hsp60 molecule induces Th2-type antibodies. J Autoimmun 1997;10:323–329.
Reizis B, Eisenstein M, Bockova J, et al.: Molecular characterization of the diabetes-associated mouse MHC class II protein, I-Ag7. Int Immunol 1997;9:43–51.
Bottazzo GF, Florin-Christensen A, Doniach D: Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet 1974;2:1279–1283.
Baekkeskov S, Aanstoot HJ, Christgau S, et al.: Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature 1990;347:151–156.
Rabin DU, Pleasic SM, Shapiro JA, et al.: Islet cell antigen 512 is a diabetess-specific islet autoantigen related to protein tyrosine phosphatases. J Immunol 1994; 152:3183–3188.
Castano L, Eisenbarth GS: Type-I diabetes: a chronic autoimmune disease of human, mouse, and rat. Annu Rev Immunol 1990;8:647–679.
Levisetti MG, Suri S, Vidavsky I, Gross ML, Kanagawa O, Unanue ER: Antibodies and CD4 T cells target a beta cell retroviral envelope protein in non-obese diabetic mice. Int Immunol 2003;15:1473–1483.
Lane FC, Unanue ER: Requirement of T (thymus) lymphocytes for resistance to listerosis. J Exp Med 1972;135:1104–1112.
Beller DI, Kiely J-M, Unanue ER: Regulation of macrophage populations. I. Preferential induction of Ia-rich peritoneal exudates by immunological stimuli. J Immunol 1980;124:1426–1432.
Farr AG, Dorf ME, Unanue ER: Secretion of mediators following T lymphocyte-macrophage interaction is regulated by the major histocompatibility complex. Proc Natl Acad Sci USA 1977;74:3542–3546.
Bancroff GJ, Bosma MJ, Bosma GC, Unanue ER: Regulation of macrophage Ia expression in mice with severe combined immunodeficiency: induction of Ia expression by a T cell independent mechanism. J Immunol 1986;137:4–9.
Unanue ER: Studies in Listeriosis show the strong symbiosis between the innate cellular system and the T cell response. Immunol Rev 1997;158:11–25.
Edelson BT, Unanue ER: Immunity to Listeria infection. Curr Opin Immunol 2000;12:425–431.
Portnoy DA, Tweten RK, Kehoe M, Bielecki J: Capacity of listeriolysin O, streptolysin O, and perfringolysin O to mediate growth of Bacillus subtilis within mammalian cells. Infect Immun 1992;60:2710–2717.
Bielecki J, Youngman P, Connelly P, Portnoy DA: Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature 1990;345:175, 176.
Busch DH, Pilip IM, Vijh S, Pamer EG: Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity 1998;8:353–362.
Harty JT, Len LL, Bevan MJ: Primary and secondary immune responses to Listeria monocytogenes. Curr Opin Immunol 1996;8:526–530.
Bouwer HG, Gibbins BL, Jones S, Hinrichs DJ: Antilisterial immunity includes specificity to listeriolysin O (LLO) and non-LLO-derived determinants. Infect Immun 1994;62:1039–1045.
Bhardwaj V, Kanagawa O, Swanson PE, Unanue ER: Chronic Listeria infection in SCID mice: requirements for the carrier state and the dual role of T cells in transferring protection or suppression. J Immunol 1998;160:376–384.
Merrick JC, Edelson BT, Bhardwaj V, Swanson PE, Unanue ER: Lymphocyte apoptosis during early phase of Listeria infection in mice. Am J Pathol 1997; 151:785–792.
Rogers HW, Callery MP, Deck B, Unanue ER: Listeria monocytogenes induces apoptosis of infected hepatocytes. J Immunol 1996;156:679–684.
Edelson BT, Cossart P, Unanue ER: Cutting edge: paradigm revisited: antibody provides resistance to Listeria infection. J Immunol 1999;163:4087–4090.
Edelson BT, Unanue ER: Intracellular antibody neutralizes Listeria growth. Immunity 2001;14:503–512.
Edelson BT, Unanue ER: MyD88-dependent but TLR 2-independent innate immunity to Listeria: no role for either in macrophage listericidal activity. J Immunol 2002;169:3869–3875.
Mackaness GB: Cellular resistance to infection. J Exp Med 1962;116:381–406.
Carrero JA, Calderon B, Unanue ER: Listeriolysin O from Listeria monocytogenes is a lymphocyte apoptogenic molecule. J Immunol 2004;172:4866–4874.
Carrero JA, Calderon B, Unanue ER: Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection and apoptosis. J Exp Med 2004;200:535–540.
Jiang J, Lau LL, Shen H: Selective depletion of nonspecific T cells during the early stage of immune responses to infection. J Immunol 2003;171:4352–4358.
O’Connell RM, Saha SK, Vaidya SA, et al: Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J Exp Med 2004;200:437–445.
Auerbuch V, Brockstedt DG, Meyer-Morse N, O’Riordan M, Portnoy DA: Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J Exp Med 2004;200:527–533.
Ochsenbein AF, Fehr T, Lutz C, et al: Control of early viral and bacterial distribution and disease by natural antibodies. Science 1999;286:2156–2159.
Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM: Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 1998;101:890–898.
Valerie A, Fadok DL, Bratton DM, et al: A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature 2000;405:85–90.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Unanue, E., Byersdorfer, C., Carrero, J. et al. Antigen presentation. Immunol Res 32, 267–292 (2005). https://doi.org/10.1385/IR:32:1-3:267
Issue Date:
DOI: https://doi.org/10.1385/IR:32:1-3:267