Abstract
Objective:
Human foetal development and growth in an environment of maternal obesity associates with high risk of cardiovascular disease and adverse neonatal outcome. We studied whether supraphysiological gestational weight gain results in human fetoplacental endothelial dysfunction and altered fetoplacental vascular reactivity.
Methods:
Primary cultures of human umbilical vein endothelial cells (HUVECs) and umbilical vein rings were obtained from pregnant women (112 total of patients recruited, 7 patients dropped out) exhibiting prepregnancy normal weight that ended with a physiological (pGWG (n=67), total weight gain 11.5–16 kg, rates of weight gain ⩽0.42 kg per week) or supraphysiological (spGWG (n=38), total weight gain >16 kg, rates of weight gain >0.42 kg per week) gestational weight gain (reference values from US Institute of Medicine guidelines). Vascular reactivity to insulin (0.1–1000 nmol l−1, 5 min) in KCl-preconstricted vein rings was measured using a wire myograph. Protein levels of human equilibrative nucleoside transporter 1 (hENT1), total and Ser1177- or Thr495-phosphorylated endothelial nitric oxide synthase (eNOS) were detected by western blot or immunofluorescence, and adenosine transport (0–250 μmol l−1 adenosine, 2 μCi ml−1 [3H]adenosine, 20 s, 25 °C) was measured in the presence or absence of 1 μmol l−1 nitrobenzylthioinosine (hENT1 inhibitor) or 10 μmol l−1 chlorpromazine (CPZ, endocytosis inhibitor) in HUVECs.
Results:
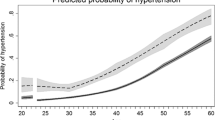
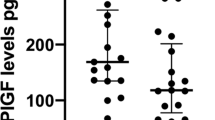
spGWG associates with reduced NOS activity-dependent dilation of vein rings (P=0.001), lower eNOS expression and higher Thr495 (P=0.044), but unaltered Ser1177eNOS phosphorylation. hENT1-adenosine maximal transport activity was reduced (P=0.041), but the expression was increased (P=0.001) in HUVECs from this group. CPZ increased hENT1-adenosine transport (P=0.040) and hENT1 plasma membrane accumulation only in cells from pGWG.
Conclusion:
spGWG in women with a normal prepregnancy weight causes lower fetoplacental vascular reactivity owing to the downregulation of eNOS activity and adenosine transport in HUVECs. Maternal spGWG is a detrimental condition for human fetoplacental endothelial function and reducing these alterations could result in a better neonate outcome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ouchi N, Parker JL, Lugus JJ, Walsh K . Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011; 11: 85–97.
Nigro E, Scudiero O, Monaco ML, Palmieri A, Mazzarella G, Costagliola C et al. New insight into adiponectin role in obesity and obesity-related diseases. Biomed Res Int 2014; 2014: 658913.
World Health Organization (WHO). Obesity and Overweight. Fact Sheet 13. World Health Organization: Geneva, Switzerland, 2014.
Ronnberg A, Ostlund I, Fadl H, Gottvall T, Nilsson K . Intervention during pregnancy to reduce excessive gestational weight gain—a randomised controlled trial. BJOG 2015; 122: 537–544.
Hrolfsdottir L, Rytter D, Olsen SF, Bech BH, Maslova E, Henriksen TB et al. Gestational weight gain in normal weight women and offspring cardio-metabolic risk factors at 20 years of age. Int J Obes (Lond) 2015; 39: 671–676.
Institute of Medicine (IOM) and National Research Council Weight Gain During Pregnancy: Reexamining the Guidelines. The National Academies Press: Washington, DC, USA, 2009.
Poston L . Influence of maternal nutritional status on vascular function in the offspring. Microcirculation 2011; 18: 256–262.
Harper LM, Renth A, Cade WT, Colvin R, Macones GA, Cahill AG . Impact of obesity on maternal and neonatal outcomes in insulin-resistant pregnancy. Am J Perinatol 2014; 31: 383–388.
Hayward CE, Cowley EJ, Mills TA, Sibley CP, Wareing M . Maternal obesity impairs specific regulatory pathways in human myometrial arteries. Biol Reprod 2014; 90: 65.
Josefson JL, Hoffmann JA, Metzger BE . Excessive weight gain in women with a normal pre-pregnancy BMI is associated with increased neonatal adiposity. Pediatr Obes 2013; 8: e33–e36.
Tesauro M, Cardillo C . Obesity, blood vessels and metabolic syndrome. Acta Physiol (Oxf) 2011; 203: 279–286.
Pardo F, Arroyo P, Salomón C, Westermeier F, Salsoso R, Sáez T et al. Role of equilibrative adenosine transporters and adenosine receptors as modulators of the human placental endothelium in gestational diabetes mellitus. Placenta 2013; 34: 1121–1127.
Pardo F, Silva L, Salsoso R, Sáez T, Farías M, Villalobos R et al. Fetoplacental endothelial dysfunction in maternal hypercholesterolemia and obesity in pregnancy. Physiol Mini Rev 2014; 7: 60–66.
Fox SB, Khong TY . Lack of innervation of human umbilical cord. An immunohistological and histochemical study. Placenta 1990; 11: 59–62.
Sobrevia L, Salsoso R, Sáez T, Sanhueza C, Pardo F, Leiva A . Insulin therapy and fetoplacental vascular function in gestational diabetes mellitus. Exp Physiol 2015; 100: 231–238.
Burnstock G . Purinergic signalling: from discovery to current developments. Exp Physiol 2014; 99: 16–34.
Fredholm B . Adenosine—a physiological or pathophysiological agent? J Mol Med 2014; 92: 201–206.
Guzmán-Gutiérrez E, Westermeier F, Salomón C, González M, Pardo F, Leiva A et al. Insulin-increased l-arginine transport requires A2A adenosine receptors activation in human umbilical vein endothelium. PLoS One 2012; 7: e41705.
Westermeier F, Salomón C, Farías M, Arroyo P, Fuenzalida B, Sáez T et al. Insulin requires normal expression and signaling of insulin receptor A to reverse gestational diabetes-reduced adenosine transport in human umbilical vein endothelium. FASEB J 2015; 29: 37–49.
Guzmán-Gutiérrez E, Arroyo P, Salsoso R, Fuenzalida B, Sáez T, Leiva A et al. Role of insulin and adenosine in the human placenta microvascular and macrovascular endothelial cell dysfunction in gestational diabetes mellitus. Microcirculation 2014; 21: 26–37.
Georgescu A, Popov D, Constantin A, Nemecz M, Alexandru N, Cochior D et al. Dysfunction of human subcutaneous fat arterioles in obesity alone or obesity associated with Type 2 diabetes. Clin Sci 2011; 120: 463–472.
Iantorno M, Campia U, di Daniele N, Nisticò S, Forleo GB, Cardillo C et al. Obesity, inflammation and endothelial dysfunction. J Biol Regul Homeost Agents 2014; 28: 169–176.
Roberts KA, Riley SC, Reynolds RM, Barr S, Evans M, Statham A et al. Placental structure and inflammation in pregnancies associated with obesity. Placenta 2011; 32: 247–254.
Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta 2008; 29: 274–281.
Contreras R, Fuentes O, Mann GE, Sobrevia L . Diabetes and insulin-induced stimulation of l-arginine transport and nitric oxide synthesis in rabbit isolated gastric glands. J Physiol 1997; 498: 787–796.
Sobrevia L, Yudilevich DL, Mann GE . Elevated d-glucose induces insulin insensitivity in human umbilical endothelial cells isolated from gestational diabetic pregnancies. J Physiol 1998; 506: 219–230.
Wu G, Meininger CJ . Analysis of citrulline, arginine, and methylarginines using high-performance liquid chromatography. Methods Enzymol 2008; 440: 177–189.
Leiva A, Diez de Medina C, Salsoso R, Sáez T, San Martín S, Abarzúa F et al. Maternal hypercholesterolemia in pregnancy associates with umbilical vein endothelial dysfunction: role of endothelial nitric oxide synthase and arginase II. Arterioscler Thromb Vasc Biol 2013; 33: 2444–2453.
Puebla C, Farías M, González M, Vecchiola A, Aguayo C, Krause B et al. High D-glucose reduces SLC29A1 promoter activity and adenosine transport involving specific protein 1 in human umbilical vein endothelium. J Cell Physiol 2008; 215: 645–656.
Vercauteren D, Vandenbroucke RE, Jones AT, Rejman J, Demeester J, De Smedt SC et al. The use of inhibitors to study endocytic pathways of gene carriers: optimization and pitfalls. Mol Ther 2010; 18: 561–569.
Salsoso R, Guzmán-Gutiérrez E, Sáez T, Bugueño K, Ramírez MA, Farías M et al. Insulin restores l-arginine transport requiring adenosine receptors activation in umbilical vein endothelium from late-onset preeclampsia. Placenta 2015; 36: 287–296.
Gemma C, Sookoian S, Alvariñas J, García SI, Quintana L, Kanevsky D et al. Maternal pregestational BMI is associated with methylation of the PPARGC1A promoter in newborns. Obesity 2009; 17: 1032–1039.
Henriksson P, Eriksson B, Forsum E, Löf M . Gestational weight gain according to Institute of Medicine recommendations in relation to infant size and body composition. Pediatr Obes 2014. e-pub ahead of print 17 December 2014 doi:10.1111/ijpo.276.
Kessler J, Rasmussen S, Godfrey K, Hanson M, Kiserud T . Longitudinal study of umbilical and portal venous blood flow to the fetal liver: low pregnancy weight gain is associated with preferential supply to the fetal left liver lobe. Pediatr Res 2008; 63: 315–320.
Fleming I, Busse R . Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol 2003; 284: R1–R12.
Fleming I . Molecular mechanisms underlying the activation of eNOS. Pflugers Arch 2010; 459: 793–806.
Farias M, Puebla C, Westermeier F, Jo M, Pastor-Anglada M, Casanello P et al. Nitric oxide reduces SLC29A1 promoter activity and adenosine transport involving transcription factor complex hCHOP–C/EBPα in human umbilical vein endothelial cells from gestational diabetes. Cardiovasc Res 2010; 86: 45–54.
Nivillac NM, Bacani J, Coe IR . The life cycle of human equilibrative nucleoside transporter 1: from ER export to degradation. Exp Cell Res 2011; 317: 1567–1579.
Gaillard R, Steegers EA, Franco OH, Hofman A, Jaddoe VW . Maternal weight gain in different periods of pregnancy and childhood cardio-metabolic outcomes. The Generation R Study. Int J Obes (Lond) 2015; 39: 677–685.
Park S, Kim MH, Kim SH . Early gestational weight gains within current recommendations result in increased risk of gestational diabetes mellitus among Korean women. Diabetes Metab Res Rev 2014; 30: 716–725.
Kirchengast S, Hartmann B . Maternal obesity affects newborn somatometrics and vital parameters in a gender typical manner—evidence for the male disadvantage hypothesis? Coll Antropol 2013; 37: 1057–1063.
Acknowledgements
We thank Mrs Amparo Pacheco and Mrs Ninoska Muñoz from CMPL, Pontificia Universidad Católica de Chile (PUC), for excellent technical and secretarial assistance, respectively, and the personnel of Hospital Clínico Universidad Católica labour ward for the supply of placentas. This work was supported by Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT 3130583, 1110977, 11110059, 3140516), Chile. RS and LS hold Faculty of Medicine, PUC-PhD fellowships; TS, RS and LS hold Comisión Nacional de Investigación Científica y Tecnológica (CONICYT, Chile)-PhD fellowships.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Pardo, F., Silva, L., Sáez, T. et al. Human supraphysiological gestational weight gain and fetoplacental vascular dysfunction. Int J Obes 39, 1264–1273 (2015). https://doi.org/10.1038/ijo.2015.57
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2015.57