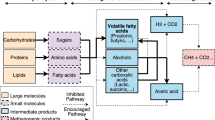
Abstract
Currently, the disposal of sugarcane vinasse is one of the greatest issues of sugarcane biorefineries in Brazil because of the large volumes produced. To contribute with an alternative energy recovery process from this by-product, this study proposed a physicochemical pretreatment and adjustment of operating conditions to improve the performance of the acidogenic stage of the anaerobic digestion of sugarcane vinasse. Therefore, this study evaluated the influence of hydraulic retention time (decreasing from 8 to 6, 4, 2, and 1 h) on the bioconversion of pretreated sugarcane vinasse (5000 mg COD L−1) to hydrogen and value-added products. Two anaerobic fluidized bed reactors were operated under mesophilic (AFBR-M, 30 °C) and thermophilic (AFBR-T, 55 °C) conditions. Despite the low similarity between the bacterial populations of AFBR-M and AFBR-T (40% similarity), the maximum hydrogen production rates (0.27 ± 0.07 and 6.42 ± 1.46 L H2 day−1 L−1) and hydrogen yields (0.27 ± 0.07 and 1.06 ± 0.15 mmol H2 g COD−1) occurred at the hydraulic retention time of 2 h by reducing the values from 8 to 2 h. The highest COD/SO42− ratios of 17.4 and 25.1 were also observed in the effluents of the AFBR-M and AFBR-T, respectively, at the hydraulic retention time of 2 h. Under both mesophilic and thermophilic conditions, a similar metabolic distribution was observed at the HRT of 2 h (acetic, propionic, and butyric acids, for AFBR-M and acetic, propionic, butyric acids, and ethanol for AFBR-T). This finding indicates the functional similarity between bacterial populations in both reactors.






Similar content being viewed by others
References
Conab (2020) 2019/2020 crop surveys. National Company About Sugarcane Supply. www.conab.gov.br/info-agro/safras/cana. Accessed 02 Apr 2020 (In Portuguese)
Godoi LAG, Camiloti PR, Bernardes AN, Sanchez BLS, Torres APR, Gomes AC, Botta LS (2019) Seasonal variation of the organic and inorganic composition of sugarcane vinasse: main implications for its environmental uses. Environ Sci Pollut Res 26:29267–29282. https://doi.org/10.1007/s11356-019-06019-8
Moraes BS, Zaiat M, Bonomi A (2015) Anaerobic digestion of vinasse from sugarcane ethanol production in Brazil: challenges and perspectives. Renew Sust Energ Rev 44:888–903. https://doi.org/10.1016/j.rser.2015.01.023
Christofoletti CA, Escher JP, Correia JE, Marinho JFU, Fontanetti CS (2013) Sugarcane vinasse: environmental implications of its use. Waste Manag 33:2752–2761. https://doi.org/10.1016/j.wasman.2013.09.005
Volpini V, Lovato G, Albanez R, Ratusznei SM, Rodrigues JAD (2018) Biomethane generation in an AnSBBR treating effluent from the biohydrogen production from vinasse: optimization, metabolic pathways modeling and scale-up estimation. Renew Energy 116:288–298. https://doi.org/10.1016/j.renene.2017.09.004
Santos SC, Rosa PRF, Sakamoto IK, Varesche MBA, Silva EL (2014) Continuous thermophilic hydrogen production and microbial community analysis from anaerobic digestion of diluted sugar cane stillage. Int J Hydrog Energy 39:9000–9011. https://doi.org/10.1016/j.ijhydene.2014.03.241
Santos SC, Rosa PRF, Sakamoto IK, Varesche MBA, Silva EL (2014) Hydrogen production from diluted and raw sugarcane vinasse under thermophilic anaerobic conditions. Int J Hydrog Energy 39:9599–9610. https://doi.org/10.1016/j.ijhydene.2014.04.104
Santos SC, Rosa PRF, Sakamoto IK, Varesche MBA, Silva EL (2014) Organic loading rate impact on biohydrogen production and microbial communities at anaerobic fluidized thermophilic bed reactors treating sugarcane stillage. Bioresour Technol 159:55–63. https://doi.org/10.1016/j.biortech.2014.02.051
Kiyuna LSM, Fuess LT, Zaiat M (2017) Unraveling the influence of the COD/sulfate ratio on organic matter removal and methane production from the biodigestion of sugarcane vinasse. Bioresour Technol 232:103–112. https://doi.org/10.1016/j.biortech.2017.02.028
Mizuno O, Li YY, Noike T (1998) The behavior of sulfate-reducing bacteria in acidogenic phase of anaerobic digestion. Water Res 32:1626–1634. https://doi.org/10.1016/S0043-1354(97)00372-2
Barrera EL, Spanjers H, Romero O, Rosa E, Dewulf J (2014) Characterization of the sulfate reduction process in the anaerobic digestion of a very high strength and sulfate rich vinasse. Chem Eng J 248:383–393. https://doi.org/10.1016/j.cej.2014.03.057
Jiménez J, Barrera EL, De Vrieze J, Boon N, DeMeester S, Spanjers H, Romero OR, Dewulf J (2018) Microbial community dynamics reflect reactor stability during the anaerobic digestion of a very high strength and sulfate-rich vinasse. J Chem Technol Biotechnol 93:975–984. https://doi.org/10.1002/jctb.5449
Fuess LT, Zaiat M, do Nascimento CAO (2019) Novel insights on the versatility of biohydrogen production from sugarcane vinasse via thermophilic dark fermentation: impacts of pH-driven operating strategies on acidogenesis metabolite profiles. Bioresour Technol 286:121379. https://doi.org/10.1016/j.biortech.2019.121379
Rodrigues CSD, Neto AR, Duda RM, Oliveira RA, Boaventura RAR, Madeira LM (2017) Combination of chemical coagulation, photo-Fenton oxidation and biodegradation for the treatment of vinasse from sugar cane ethanol distillery. J Clean Prod 142:3634–3644. https://doi.org/10.1016/j.jclepro.2016.10.104
Arreola AR, Tizapa MS, Zurita F, Morán-Lázaro JP, Valderrama RC, Rodríguez-López JL, Carreon-Alvarez A (2020) Treatment of tequila vinasse and elimination of phenol by nanoparticles. Environ Technol 41:1023–1033. https://doi.org/10.1080/09593330.2018.1518994
Ferreira TB, Rego GC, Ramos LR, Soares LA, Sakamoto IK, Oliveira LL, Varesche MBA, Silva EL (2018) Selection of metabolic pathways for continuous hydrogen production under thermophilic and mesophilic temperature conditions in anaerobic fluidized bed reactors. Int J Hydrog Energy 43:18908–18917. https://doi.org/10.1016/j.ijhydene.2018.08.177
Ferreira TB, Rego GC, Ramos LR, Menezes CA, Soares LA, Sakamoto IK, Varesche MBA, Silva EL (2019) HRT control as a strategy to enhance continuous hydrogen production from sugarcane juice under mesophilic and thermophilic conditions in AFBRs. Int J Hydrog Energy 44:19719–19729. https://doi.org/10.1016/j.ijhydene.2019.06.050
Ferraz Júnior ADN, Etchebehere C, Zaiat M (2015) Mesophilic hydrogen production in acidogenic packed-bed reactors (APBR) using raw sugarcane vinasse as substrate: influence of support materials. Anaerobe 34:94–105. https://doi.org/10.1016/j.anaerobe.2015.04.008
Reis CM, Carosia MF, Sakamoto IK, Varesche MBA, Silva EL (2015) Evaluation of hydrogen and methane production from sugarcane vinasse in an anaerobic fluidized bed reactor. Int J Hydrog Energy 40:8498–8509. https://doi.org/10.1016/j.ijhydene.2015.04.136
Magrini FE, de Almeida GM, Soares DM, Fuentes L, Ecthebehere C, Beal LL, da Silveira MM, Paesi S (2020) Effect of different heat treatments of inoculum on the production of hydrogen and volatile fatty acids by dark fermentation of sugarcane vinasse. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-020-00687-0
Ramos LR, Silva EL (2017) Continuous hydrogen production from agricultural wastewaters at thermophilic and hyperthermophilic temperatures. Appl Biochem Biotechnol 182:846–869. https://doi.org/10.1007/s12010-016-2366-3
Kim D-H, Han S-K, Kim S-H, Shin H-S (2006) Effect of gas sparging on continuous fermentative hydrogen production. Int J Hydrog Energy 31:2158–2169. https://doi.org/10.1016/j.ijhydene.2006.02.012
APHA – American Public Health Association (2012) Standard methods for the examination of water and wastewater, 22nd edn. APHA/AWWA/WEF, Washington
DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Penteado ED, Lazaro CZ, Sakamoto IK, Zaiat M Influence of seed sludge and pretreatment method on hydrogen production in packed-bed anaerobic reactors. Int J Hydrog Energy 38:6137–6145. https://doi.org/10.1016/j.ijhydene.2013.01.067
Walker M, Zhang Y, Heaven S, Banks C (2009) Potential errors in the quantitative evaluation of biogas production in anaerobic digestion processes. Bioresour Technol 100:6339–6346. https://doi.org/10.1016/j.biortech.2009.07.018
Griffiths RI, Whiteley AS, O’Donnell AG, Bailey MJ (2000) Rapid method for Coextraction of DNA and RNA from natural environments for analysis of ribosomal DNA- and rRNA-based microbial community composition. Appl Environ Microbiol 66:5488–5491.https://doi.org/10.1128/aem.66.12.5488-5491
Nübel U, Engelen B, Felske A, Snaidr J, Wieshuber A, Amann RI, Ludwig W, Backhaus H (1996) Sequence heterogeneities of genes encoding 16S rRNAs in Paenibacillus polymyxa detected by temperature gradient gel electrophoresis. J Bacteriol 178:5636–5643. https://doi.org/10.1128/jb.178.19.5636-5643.1996
Muyzer G, de Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700
Ferreira TB, Rego GC, Ramos LR, Menezes CA, Silva EL (2020) Improved dark fermentation of cane molasses in mesophilic and thermophilic anaerobic fluidized bed reactors by selecting operational conditions. Int J Energy Res 2020:1–11. https://doi.org/10.1002/er.5673
Ferraz Júnior ADN, Wenzel J, Etchebehere C, Zaiat M (2014) Effect of organic loading rate on hydrogen production from sugarcane vinasse in thermophilic acidogenic packed bed reactors. Int J Hydrog Energy 39:16852–16862. https://doi.org/10.1016/j.ijhydene.2014.08.017
Ferraz Júnior ADN, Etchebehere C, Zaiat M (2015) High organic loading rate on thermophilic hydrogen production and metagenomic study at an anaerobic packed-bed reactor treating a residual liquid stream of a Brazilian biorefinery. Bioresour Technol 186:81–88. https://doi.org/10.1016/j.biortech.2015.03.035
Ramos LR, Silva EL (2018) Continuous hydrogen production from cofermentation of sugarcane vinasse and cheese whey in a thermophilic anaerobic fluidized bed reactor. Int J Hydrog Energy 43:13081–13089. https://doi.org/10.1016/j.ijhydene.2018.05.070
Elbeshbishy E, Dhar BR, Nakhla G, Lee H-S (2017) A critical review on inhibition of dark biohydrogen fermentation. Renew Sust Energ Rev 79:656–668. https://doi.org/10.1016/j.rser.2017.05.075
Intanoo P, Suttikul T, Leethochawalit M, Gulari E, Chavadej S (2014) Hydrogen production from alcohol wastewater with added fermentation residue by an anaerobic sequencing batch reactor (ASBR) under thermophilic operation. Int J Hydrog Energy 39:9611–9620. https://doi.org/10.1016/j.ijhydene.2014.04.105
Albanez R, Lovato G, Zaiat M, Ratusznei SM, Rodrigues JAD (2016) Optimization, metabolic pathways modeling and scale-up estimative of an AnSBBR applied to biohydrogen production by co-digestion of vinasse and molasses. Int J Hydrog Energy 41:20473–20484. https://doi.org/10.1016/j.ijhydene.2016.08.145
Lazaro CZ, Perna V, Etchebehere C, Varesche MBA (2014) Sugarcane vinasse as substrate for fermentative hydrogen production: the effects of temperature and substrate concentration. Int J Hydrog Energy 39:6407–6418. https://doi.org/10.1016/j.ijhydene.2014.02.058
Hwang J-H, Choi J-A, Abou-Shanab RAI, Bhatnagar A, Min B, Song H, Kumar E, Choi J, Lee ES, Kim YJ, Um S, Lee DS, Jeon BH (2009) Effect of pH and sulfate concentration on hydrogen production using anaerobic mixed microflora. Int J Hydrog Energy 34:9702–9710. https://doi.org/10.1016/j.ijhydene.2009.10.022
Lopes SIC, Capela MI, Lens PNL (2010) Sulfate reduction during the acidification of sucrose at pH 5 under thermophilic (55 °C) conditions. II: effect of sulfide and COD/SO42− ratio. Bioresour Technol 101:4278–4284. https://doi.org/10.1016/j.biortech.2010.01.010
Chen C-C, Chen H-P, Wu J-H, Lin C-Y (2008) Fermentative hydrogen production at high sulfate concentration. Int J Hydrog Energy 33:1573–1578. https://doi.org/10.1016/j.ijhydene.2007.09.042
Searmsirimongkol P, Rangsunvigit P, Leethochawalit M, Chavadej S (2011) Hydrogen production from alcohol distillery wastewater containing high potassium and sulfate using an anaerobic sequencing batch reactor. Int J Hydrog Energy 36:12810–12821. https://doi.org/10.1016/j.ijhydene.2011.07.080
Gao M, Guo B, Zhang L, Zhang Y, Yu N, Liu Y (2020) Biomethane recovery from source-diverted household Blackwater: impacts from feed sulfate. Process Saf Environ Prot 136:28–38. https://doi.org/10.1016/j.psep.2020.01.010
Alain K, Holler T, Musat F, Elvert M, Treude T, Kruger M (2006) Microbiological investigation of methane- and hydrocarbon-discharging mud volcanoes in the Carpathian Mountains, Romania. Environ Microbiol 8:574–590. https://doi.org/10.1111/j.1462-2920.2005.00922.x
Buitrón G, Prato-Garcia D, Zhang A (2014) Biohydrogen production from tequila vinasse using a fixed bed reactor. Water Sci Technol 70:1919–1925. https://doi.org/10.2166/wst.2014.433
Cabrol L, Marone A, Tapia-Venegas E, Steyer J-P, Ruiz-Filippi G, Trably E (2017) Microbial ecology of fermentative hydrogen producing bioprocesses: useful insights for driving the ecosystem function. FEMS Microbiol Rev 41:158–181. https://doi.org/10.1093/femsre/fuw043
de Menezes CA, Silva EL (2019) Hydrogen production from sugarcane juice in expanded granular sludge bed reactors under mesophilic conditions: the role of homoacetogenesis and lactic acid production. Ind Crop Prod 138:111586. https://doi.org/10.1016/j.indcrop.2019.111586
García-Depraect O, León-Becerril E (2018) Fermentative biohydrogen production from tequila vinasse via the lactate-acetate pathway: operational performance, kinetic analysis and microbial ecology. Fuel 234:151–160. https://doi.org/10.1016/j.fuel.2018.06.126
García-Becerra M, Macías-Muro M, Arellano-García L, Aguilar-Juárez O (2019) Bio-hydrogen production from tequila vinasses: effect of detoxification with activated charcoal on dark fermentation performance. Int J Hydrog Energy 44:31860–31872. https://doi.org/10.1016/j.ijhydene.2019.10.059
Chowdhary P, Raj A, Bharagava RN (2018) Environmental pollution and health hazards from distillery wastewater and treatment approaches to combat the environment threats: a review. Chemosphere 194:229–246. https://doi.org/10.1016/j.chemosphere.2017.11.163
Corona VM, Razo-Flores E (2018) Continuous hydrogen and methane production from Agave tequilana bagasse hydrolysate by sequential process to maximize energy recovery efficiency. Bioresour Technol 249:334–341. https://doi.org/10.1016/j.biortech.2017.10.032
Kim M, Ahn Y-H, Speece RE (2002) Comparative process stability and efficiency of anaerobic digestion; mesophilic vs. thermophilic. Water Res 36:4369–4385. https://doi.org/10.1016/S0043-1354(02)00147-1
Bárcenas-Ruiz CD, Carrillo-Reyes J, Arellano-García L, Celis LB, Alatriste-Mondragón F, Razo-Flores E (2016) Pretreatment and upward liquid velocity effects over granulation in hydrogen producing EGSB reactors. Biochem Eng J 107:75–84. https://doi.org/10.1016/j.bej.2015.12.010
Thierry A, Deutsch S-M, Falentin H, Dalmasso M, Cousin FJ, Jan G (2011) New insights into physiology and metabolism of Propionibacterium freudenreichii. Int J Food Microbiol 149:19–27. https://doi.org/10.1016/j.ijfoodmicro.2011.04.026
Yu H-Q, Mu Y, Fang HHP (2004) Thermodynamic analysis of product formation in mesophilic acidogenesis of lactose. Biotechnol Bioeng 87:813–822. https://doi.org/10.1002/bit.20190
Khemkhao M, Nuntakumjorn B, Techkarnjanaruk S, Phalakornkule C (2012) Comparative mesophilic and thermophilic anaerobic digestion of palm oil mill effluent using upflow anaerobic sludge blanket. Water Environ Res 84:577–587. https://doi.org/10.2175/106143012X13378023685637
Soares LA, Braga JK, Motteran F, Sakamoto IK, Monteiro PAS, Seleghim Jr. P, Varesche MBA (2019) Bioconversion of sugarcane bagasse into value-added products by bioaugmentation of endogenous cellulolytic and fermentative communities. Waste Biomass Valor 10:1899–1912. https://doi.org/10.1007/s12649-018-0201-5
Valdez-Vazquez I, Ríos-Leal E, Esparza-García F, Cecchi F, Poggi-Varaldo HM (2005) Semi-continuous solid substrate anaerobic reactors for H2 production from organic waste: Mesophilic versus thermophilic regime. Int J Hydrog Energy 30:1383–1391. https://doi.org/10.1016/j.ijhydene.2004.09.016
Koesnandar NN, Yamamoto A, Nagai S (1991) Enzymatic reduction of cystine into cysteine by cell-free extract of Clostridium thermoaceticum. J Ferment Bioeng 72:11–14. https://doi.org/10.1016/0922-338X(91)90138-7
Acknowledgments
The authors thank the Research Support Foundation of the State of São Paulo, the Coordination for the Improvement of Higher Education Personnel, and the National Council for Scientific and Technological Development for the financial support, the Laboratory of Environmental Control II, and the Laboratory of Biological Processes.
Funding
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)-Finance Code 001, Conselho Nacional de Desenvolvimento Científico e Tecnológico–Brasil (CNPq) (process 422223/2018-2 and 304723/2019-3), and Fundação de Amparo à Pesquisa do Estado de São Paulo–Brasil (FAPESP) (Grant No. 2015/06246-7).
Author information
Authors and Affiliations
Contributions
Gabriel Catucci Rego (investigation; conceptualization; formal analysis; writing-original draft; writing–review & editing); Tiago Borges Ferreira (investigation; conceptualization; formal analysis; writing-original draft; writing–review & editing); Lucas Rodrigues Ramos (investigation; conceptualization; formal analysis; writing-original draft; writing–review & editing); Camila Aparecida de Menezes (conceptualization; formal analysis; writing-original draft; writing–review & editing); Laís Américo Soares (conceptualization; formal analysis; writing-original draft; writing–review & editing); Isabel Kimiko Sakamoto (Conceptualization; formal analysis; writing-original draft; writing–review & editing); Maria Bernadete Amâncio Varesche (conceptualization; writing-review & editing; funding acquisition); Edson Luiz Silva (conceptualization; formal analysis; writing-review & editing; funding acquisition; project administration).
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have are no conflicts of interest.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding authors on request.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 128 kb)
Rights and permissions
About this article
Cite this article
Rego, G.C., Ferreira, T.B., Ramos, L.R. et al. Bioconversion of pretreated sugarcane vinasse into hydrogen: new perspectives to solve one of the greatest issues of the sugarcane biorefinery. Biomass Conv. Bioref. 12, 5527–5541 (2022). https://doi.org/10.1007/s13399-020-00984-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00984-8