Abstract
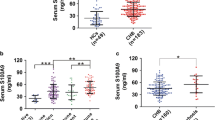
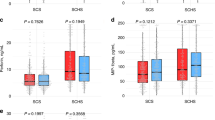
Hepatitis B virus (HBV) infection is a major cause of chronic liver diseases including hepatocellular carcinoma (HCC). CD14 and its soluble form sCD14 play important roles in immunity and are involved in the translocation of bacteria and their products which is related to the pathogenesis in chronic HBV infection. This study investigated serum sCD14 levels in HBV chronically infected patients with various clinical diseases. Serum sCD14 levels in HBV patients were significantly elevated compared with those of healthy controls. HCC patients had significantly highest levels of serum sCD14 across all the HBV-related diseases. Serum sCD14 levels significantly discriminated HCC from other HBV-related non-HCC diseases. The area under the receiver operating characteristic curve (AUC) of sCD14 levels for HCC was significantly higher in comparison with other HBV-related non-HCC diseases. The AUC of sCD14 for HCC (0.868, 95 % CI 0.791–0.946, P < 0.001) was higher than that of alpha-fetoprotein (0.660, 95 % CI 0.508–0.811, P = 0.039). Serum level of sCD14 was associated with the overall survival (OS) of HCC patients, with sCD14 levels >20 ng/mL being significantly related to poorer OS (P = 0.017). Multivariate regression showed that serum sCD14 level was an independent factor associated with the OS rates of HBV-related HCC patients (HR 2.544, 95 % CI 1.169–5.538, P = 0.019). HCC resection resulted in a significant decrease of sCD14 levels (P < 0.001). These findings suggest the potential role of sCD14 in the pathogenesis of chronic HBV infection, especially the development of HCC, and the potential usefulness of sCD14 as a biomarker for discriminating clinical diseases and predicting survival of HCC patients in chronic HBV infection.







Similar content being viewed by others
Abbreviations
- AFP:
-
Alpha-fetoprotein
- ALT:
-
Alanine aminotransferase
- ASC:
-
Asymptomatic HBV carrier status
- AST:
-
Aspartate aminotransferase
- AUC:
-
Area under the ROC curve
- CH:
-
Chronic hepatitis
- CT:
-
Computerized tomography
- CI:
-
Confidence interval
- CV:
-
Coefficient of variation
- ELISA:
-
Enzyme-linked immunosorbent assay
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- HCV:
-
Hepatitis C virus
- HIV:
-
Human immunodeficiency virus
- HR:
-
Hazard ratio
- IL:
-
Interleukin
- LC:
-
Liver cirrhosis
- LPS:
-
Lipopolysaccharide
- MDD:
-
Minimum detectable dose
- MRI:
-
Magnetic resonance imaging
- mCD14:
-
Membrane CD14
- OS:
-
Overall survival
- ROC curve:
-
Receiver operating characteristic curve
- sCD14:
-
Soluble CD14
- TLR:
-
Toll-like receptor
- TNF-α:
-
Tumor necrosis factor-α
References
European Association For The Study Of The Liver. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 2012;57(1):167–85.
Bertoletti A, Gehring AJ. The immune response during hepatitis B virus infection. J Gen Virol. 2006;87(Pt 6):1439–49.
Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81(8):4215–25.
Molloy MJ, Bouladoux N, Belkaid Y. Intestinal microbiota: shaping local and systemic immune responses. Semin Immunol. 2012;24(1):58–66.
Ivanov II, Honda K. Intestinal commensal microbes as immune modulators. Cell Host Microbe. 2012;12(4):496–508.
Kamada N, Seo SU, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13(5):321–35.
Littman DR, Pamer EG. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe. 2011;10(4):311–23.
Cesaro C, Tiso A, Del Prete A, Cariello R, Tuccillo C, Cotticelli G, et al. Gut microbiota and probiotics in chronic liver diseases. Dig Liver Dis. 2011;43(6):431–8.
Sandler NG, Koh C, Roque A, Eccleston JL, Siegel RB, Demino M, et al. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology. 2011;141(4):1220–30. 1230.e1-3.
Lin Y, Yu LX, Yan HX, Yang W, Tang L, Zhang HL, et al. Gut-derived lipopolysaccharide promotes T-cell-mediated hepatitis in mice through toll-like receptor 4. Cancer Prev Res (Phila). 2012;5(9):1090–102.
Quigley EM, Stanton C, Murphy EF. The gut microbiota and the liver. Pathophysiological and clinical implications. J Hepatol. 2013;58(5):1020–7.
Lu H, Wu Z, Xu W, Yang J, Chen Y, Li L. Intestinal microbiota was assessed in cirrhotic patients with hepatitis B virus infection. Intestinal microbiota of HBV cirrhotic patients. Microb Ecol. 2011;61(3):693–703.
Chou HH, Chien WH, Wu LL, Cheng CH, Chung CH, Horng JH, et al. Age-related immune clearance of hepatitis B virus infection requires the establishment of gut microbiota. Proc Natl Acad Sci U S A. 2015;112(7):2175–80.
Tobias PS, Ulevitch RJ. Lipopolysaccharide binding protein and CD14 in LPS dependent macrophage activation. Immunobiology. 1993;187(3–5):227–32.
Labeta MO, Landmann R, Obrecht JP, Obrist R. Human B cells express membrane-bound and soluble forms of the CD14 myeloid antigen. Mol Immunol. 1991;28(1–2):115–22.
Verhasselt V, Buelens C, Willems F, De Groote D, Haeffner-Cavaillon N, Goldman M. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: evidence for a soluble CD14-dependent pathway. J Immunol. 1997;158(6):2919–25.
Su GL. Lipopolysaccharides in liver injury: molecular mechanisms of Kupffer cell activation. Am J Physiol Gastrointest Liver Physiol. 2002;283(2):G256–65.
Beutler B. Tlr4: central component of the sole mammalian LPS sensor. Curr Opin Immunol. 2000;12(1):20–6.
Triantafilou M, Triantafilou K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 2002;23(6):301–4.
Landmann R, Ludwig C, Obrist R, Obrecht JP. Effect of cytokines and lipopolysaccharide on CD14 antigen expression in human monocytes and macrophages. J Cell Biochem. 1991;47(4):317–29.
Devitt A, Moffatt OD, Raykundalia C, Capra JD, Simmons DL, Gregory CD. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature. 1998;392(6675):505–9.
Grunwald U, Fan X, Jack RS, Workalemahu G, Kallies A, Stelter F, et al. Monocytes can phagocytose Gram-negative bacteria by a CD14-dependent mechanism. J Immunol. 1996;157(9):4119–25.
Haziot A, Chen S, Ferrero E, Low MG, Silber R, Goyert SM. The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J Immunol. 1988;141(2):547–52.
Ziegler-Heitbrock HW, Ulevitch RJ. CD14: cell surface receptor and differentiation marker. Immunol Today. 1993;14(3):121–5.
Bazil V, Strominger JL. Shedding as a mechanism of down-modulation of CD14 on stimulated human monocytes. J Immunol. 1991;147(5):1567–74.
Bufler P, Stiegler G, Schuchmann M, Hess S, Krüger C, Stelter F, et al. Soluble lipopolysaccharide receptor (CD14) is released via two different mechanisms from human monocytes and CD14 transfectants. Eur J Immunol. 1995;25(2):604–10.
Pugin J, Schürer-Maly CC, Leturcq D, Moriarty A, Ulevitch RJ, Tobias PS. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci U S A. 1993;90(7):2744–8.
Kirschning CJ, Wesche H, Merrill Ayres T, Rothe M. Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med. 1998;188(11):2091–7.
Bas S, Gauthier BR, Spenato U, Stingelin S, Gabay C. CD14 is an acute-phase protein. J Immunol. 2004;172(7):4470–9.
Su GL, Dorko K, Strom SC, Nüssler AK, Wang SC. CD14 expression and production by human hepatocytes. J Hepatol. 1999;31(3):435–42.
Hetherington CJ, Kingsley PD, Crocicchio F, Zhang P, Rabin MS, Palis J, et al. Characterization of human endotoxin lipopolysaccharide receptor CD14 expression in transgenic mice. J Immunol. 1999;162(1):503–9.
Meuleman P, Steyaert S, Libbrecht L, Couvent S, Van Houtte F, Clinckspoor F, et al. Human hepatocytes secrete soluble CD14, a process not directly influenced by HBV and HCV infection. Clin Chim Acta. 2006;366(1–2):156–62.
Vanlandschoot P, Van Houtte F, Roobrouck A, Farhoudi A, Stelter F, Peterson DL, et al. LPS-binding protein and CD14-dependent attachment of hepatitis B surface antigen to monocytes is determined by the phospholipid moiety of the particles. J Gen Virol. 2002;83(Pt 9):2279–89.
Steyaert S, Vanlandschoot P, Van Vlierberghe H, Diepolder H, Leroux-Roels G. Soluble CD14 levels are increased and inversely correlated with the levels of hepatitis B surface antigen in chronic hepatitis B patients. J Med Virol. 2003;71(2):188–94.
Wu CC, Hsu CW, Chen CD, Yu CJ, Chang KP, Tai DI, et al. Candidate serological biomarkers for cancer identified from the secretomes of 23 cancer cell lines and the human protein atlas. Mol Cell Proteomics. 2010;9(6):1100–17.
Mikuls TR, LeVan TD, Sayles H, Yu F, Caplan L, Cannon GW, et al. Soluble CD14 and CD14 polymorphisms in rheumatoid arthritis. J Rheumatol. 2011;38(12):2509–16.
Landmann R, Zimmerli W, Sansano S, Link S, Hahn A, Glauser MP, et al. Increased circulating soluble CD14 is associated with high mortality in gram-negative septic shock. J Infect Dis. 1995;171(3):639–44.
Ogawa Y, Imajo K, Yoneda M, Kessoku T, Tomeno W, Shinohara Y, et al. Soluble CD14 levels reflect liver inflammation in patients with nonalcoholic steatohepatitis. PLoS One. 2013;8(6):e65211.
Roderburg C, Luedde T. The role of the gut microbiome in the development and progression of liver cirrhosis and hepatocellular carcinoma. Gut Microbes. 2014;5(4):441–5.
Jing YY, Han ZP, Sun K, Zhang SS, Hou J, Liu Y, et al. Toll-like receptor 4 signaling promotes epithelial-mesenchymal transition in human hepatocellular carcinoma induced by lipopolysaccharide. BMC Med. 2012;10:98.
Wang L, Zhu R, Huang Z, Li H, Zhu H. Lipopolysaccharide-induced toll-like receptor 4 signaling in cancer cells promotes cell survival and proliferation in hepatocellular carcinoma. Dig Dis Sci. 2013;58(8):2223–36.
Liu WT, Jing YY, Yu GF, Han ZP, Yu DD, Fan QM, et al. Toll like receptor 4 facilitates invasion and migration as a cancer stem cell marker in hepatocellular carcinoma. Cancer Lett. 2015;358(2):136–43.
Llovet JM, Peña CE, Lathia CD, Shan M, Meinhardt G, Bruix J, et al. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2012;18(8):2290–300.
Medhat E, Salama H, Fouad H, Abd E, Haleem H, Said M, et al. Serum soluble CD14 in Egyptian patients with chronic hepatitis C: its relationship to disease progression and response to treatment. J Interferon Cytokine Res. 2015;35(7):563–8.
Acknowledgments
This work was supported in part by funding from the National Natural Science Foundation of China (grant no. 81371798). The authors are indebted to Dr. Guoyu Zhang and Dr. Zhu Li for their help in this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Li, N., Zhu, Q., Yang, C. et al. Elevated serum soluble CD14 levels in chronic HBV infection are significantly associated with HBV-related hepatocellular carcinoma. Tumor Biol. 37, 6607–6617 (2016). https://doi.org/10.1007/s13277-015-4423-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4423-x