Abstract
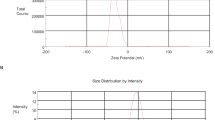
Baicalein-loaded cinnamon essential oil nanoemulsion (BaiCN) was formulated to enhance the solubility and oral bioavailability of the drug, and its potential combined anticancer efficacy with cinnamon oil was evaluated against the MDA-MB-231 breast cancer cell line. The formulation was optimized using full factorial design, and the optimized formulation was characterized for its size distribution, morphology, FTIR analysis, release, and storage stability at 4 °C. The cytotoxicity of developed formulation was assessed via MTT assay on MDA-MB-231 cell line. The average droplet size was found to be 139.05 ± 0.05 nm with a polydispersity index (PDI) value of 0.154 ± 0.029. The zeta potential of BaiCN was −14.35 ± 0.21 mV. Upon storage up to 6 months at 4 °C, there was no change in physical appearance or phase separation. However, a little increase in droplet size and PDI was observed. BaiCN exhibited good in vitro anticancer results against MDA-MB-231. There was about 19-fold and 23-fold enhancements in anticancer activity of BaiCN compared to that of free baicalein after 12 h and 24 h of treatment, respectively. Moreover, the cinnamon oil nanoemulsion without baicalein also showed good cytotoxicity against MDA-MB-231 cells and provided a collaborative effect to baicalein. In conclusion, cinnamon oil nanoemulsion is a promising drug carrier for encapsulating baicalein and enhancing their anticancer effect.
Graphical abstract








Similar content being viewed by others
References
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., & Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 68(6), 394–424. https://doi.org/10.3322/caac.21492
Singh, S., Sharma, B., Kanwar, S. S., & Kumar, A. (2016). Lead phytochemicals for anticancer drug development. Frontiers in Plant Science, 7, 1667. https://doi.org/10.3389/fpls.2016.01667
Li-Weber, M. (2009). New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents Wogonin. Baicalein and Baicalin. Cancer Treatment Reviews, 35(1), 57–68. https://doi.org/10.1016/j.ctrv.2008.09.005
Scutellaria baicalensis Georgi GRIN-Global. (n.d.). Retrieved February 7, 2021, from https://npgsweb.ars-grin.gov/gringlobal/taxon/taxonomydetail?id=33424
Kang, K. A., Zhang, R., Piao, M. J., et al. (2012). Baicalein inhibits oxidative stress-induced cellular damage via antioxidant effects. Toxicology and Industrial Health, 28(5), 412–421. https://doi.org/10.1177/0748233711413799
Johari, J., Kianmehr, A., Mustafa, M. R., Abubakar, S., & Zandi, K. (2012). Antiviral activity of baicalein and quercetin against the Japanese encephalitis virus. International Journal of Molecular Sciences, 13(12), 16020–16045. https://doi.org/10.3390/ijms131216785
Lee, W., Ku, S. K., & Bae, J. S. (2014). Anti-inflammatory effects of baicalin, baicalein, and wogonin in vitro and in vivo. Inflammation, 38(1), 110–125. https://doi.org/10.1007/s10753-014-0013-0
Ying, G., Snyder, S. A., Smith, J. N., & Chen, Y. C. (2016). Anticancer properties of baicalein: A review. Medicinal Chemistry Research, 25(8), 1515–1523. https://doi.org/10.1007/s00044-016-1607-x
Ling, Y., Chen, Y., Chen, P., et al. (2011). Baicalein potently suppresses angiogenesis induced by vascular endothelial growth factor through the p53/Rb signaling pathway leading to G1/S cell cycle arrest. Experimental Biology and Medcine, 236(7), 851–858. https://doi.org/10.1258/ebm.2011.010395
Lee, J. H., Li, Y. C., Ip, S. W., et al. (2008). The role of Ca2+ in baicalein-induced apoptosis in human breast MDA-MB-231 cancer cells through mitochondria- and caspase-3-dependent pathway. Anticancer Res, 28(3 A), 1701–1711.
Wang, L., Ling, Y., Chen, Y., et al. (2010). Flavonoid baicalein suppresses adhesion, migration and invasion of MDA-MB-231 human breast cancer cells. Cancer Letters, 297(1), 42–48. https://doi.org/10.1016/j.canlet.2010.04.022
Chung, H., Choi, H. S., Seo, E. K., Kang, D. H., & Oh, E. S. (2015). Baicalin and baicalein inhibit transforming growth factor-β1-mediated epithelial-mesenchymal transition in human breast epithelial cells. Biochemical and Biophysical Research Communications, 458(3), 707–713. https://doi.org/10.1016/j.bbrc.2015.02.032
Wang, Y., Han, E., Xing, Q., et al. (2015). Baicalein upregulates DDIT4 expression which mediates mTOR inhibition and growth inhibition in cancer cells. Cancer Letters, 358(2), 170–179. https://doi.org/10.1016/j.canlet.2014.12.033
Ma, X. C., Yan, W., Dai, Z., et al. (2016). Baicalein suppresses metastasis of breast cancer cells by inhibiting EMT via downregulation of SATB1 and Wnt/β-catenin pathway. Drug Design Development and Therapy, 10, 1419–1441. https://doi.org/10.2147/DDDT.S102541
Zhang, L., Lin, G., Chang, Q., & Zuo, Z. (2005). Role of intestinal first-pass metabolism of baicalein in its absorption process. Pharmaceutical Research, 22(7), 1050–1058. https://doi.org/10.1007/s11095-005-5303-7
Zhang, L., Lin, G., Kovács, B., et al. (2007). Mechanistic study on the intestinal absorption and disposition of baicalein. European Journal of Pharmaceutical Sciences, 31(3–4), 221–231. https://doi.org/10.1016/j.ejps.2007.04.001
Zhang, J., Lv, H., Jiang, K., & Gao, Y. (2011). Enhanced bioavailability after oral and pulmonary administration of baicalein nanocrystal. International Journal of Pharmaceutics, 420(1), 180–188. https://doi.org/10.1016/j.ijpharm.2011.08.023
He, X., Pei, L., Tong, H. H. Y., & Zheng, Y. (2011). Comparison of spray freeze drying and the solvent evaporation method for preparing solid dispersions of baicalein with pluronic F68 to improve dissolution and oral bioavailability. An Official Journal of the American Association of Pharmaceutical Scientists, 12(1), 104–113. https://doi.org/10.1208/s12249-010-9560-3
Wang, S. X., Wen, X., Bell, C., & Appiah, S. (2018). Liposome-delivered baicalein induction of myeloid leukemia K562 cell death via reactive oxygen species generation. Molecular Medicine Reports, 17(3), 4524–4530. https://doi.org/10.3892/mmr.2018.8396
Liao, H., Gao, Y., Lian, C., et al. (2019). Oral absorption and lymphatic transport of baicalein following drug–phospholipid complex incorporation in self-microemulsifying drug delivery systems. International journal of Nanomedicine, 14, 7291–7306. https://doi.org/10.2147/IJN.S214883
Yin, J., Xiang, C., Wang, P., Yin, Y., & Hou, Y. (2017). Biocompatible nanoemulsions based on hemp oil and less surfactants for oral delivery of baicalein with enhanced bioavailability. International Journal of Nanomedicine, 12, 2923–2931.
Shen, H., Liu, Y., Zhang, H., et al. (2019). Enhancing the oral bioavailability of baicalein via Solutol® HS15 and Poloxamer 188 mixed micelles system. Journal of Pharmacy and Pharmacology, 71(5), 765–773. https://doi.org/10.1111/jphp.13058
Ganta, S., Talekar, M., Singh, A., Coleman, T. P., & Amiji, M. M. (2014). Nanoemulsions in translational research - Opportunities and challenges in targeted cancer therapy. An Official Journal of the American Association of Pharmaceutical Scientists, 15(3), 694–708. https://doi.org/10.1208/s12249-014-0088-9
Li, Y. Q., Kong, D. X., & Wu, H. (2013). Analysis and evaluation of essential oil components of cinnamon barks using GC-MS and FTIR spectroscopy. Industrial Crops and Products, 41(1), 269–278. https://doi.org/10.1016/j.indcrop.2012.04.056
Han, X., & Parker, T. L. (2017). Antiinflammatory activity of cinnamon (Cinnamomum zeylanicum) bark essential oil in a human skin disease model. Phytotherapy Research, 31(7), 1034–1038. https://doi.org/10.1002/ptr.5822
Kallel I, Hadrich B, Gargouri B et al. (2019) Optimization of cinnamon (Cinnamomum zeylanicum Blume) essential oil extraction: Evaluation of antioxidant and antiproliferative effects. Evidence-Based Complementary and Alternative Medicine 2019 (6498347). https://doi.org/10.1155/2019/6498347
Sim, J. X. F., Khazandi, M., Pi, H., Venter, H., Trott, D. J., & Deo, P. (2019). Antimicrobial effects of cinnamon essential oil and cinnamaldehyde combined with EDTA against canine otitis externa pathogens. Journal of Applied Microbiology, 127(1), 99–108. https://doi.org/10.1111/jam.14298
Thompson, M., Schmelz, E. M., & Bickford, L. (2019). Anti-cancer properties of cinnamon oil and its active component, trans-cinnamaldehyde. Journal of Nutrition & Food Sciences, 9(1), 750. https://doi.org/10.4172/2155-9600.1000750
Kubatka, P., Kello, M., Kajo, K., et al. (2020). Chemopreventive and therapeutic efficacy of Cinnamomum zeylanicum L. Bark in experimental breast carcinoma: Mechanistic in vivo and in vitro analyses. Molecules, 25(6), 1399. https://doi.org/10.3390/molecules25061399
Li K, Zhang H, Gao L et al. (2016) Preparation and characterization of baicalein-loaded nanoliposomes for antitumor therapy. Journal of Nanomaterials 2016 (2861915). https://doi.org/10.1155/2016/2861915
Pawar, V. K., Panchal, S. B., Singh, Y., Meher, , et al. (2014). Immunotherapeutic vitamin E nanoemulsion synergies the antiproliferative activity of paclitaxel in breast cancer cells via modulating Th1 and Th2 immune response. Journal of Controlled Release, 196, 295–306. https://doi.org/10.1016/j.jconrel.2014.10.010
Chuesiang, P., Siripatrawan, U., Sanguandeekul, R., McLandsborough, L., & Julian McClements, D. (2018). Optimization of cinnamon oil nanoemulsions using phase inversion temperature method: Impact of oil phase composition and surfactant concentration. Journal of Colloid and Interface Science, 514, 208–216. https://doi.org/10.1016/j.jcis.2017.11.084
Hidajat, M. J., Jo, W., Kim, H., & Noh, J. (2020). Effective droplet size reduction and excellent stability of limonene nanoemulsion formed by high-pressure homogenizer. Colloids and Interfaces, 4(1), 5. https://doi.org/10.3390/colloids4010005
Fofaria, N. M., Qhattal, H. S. S., Liu, X., & Srivastava, S. K. (2016). Nanoemulsion formulations for anti-cancer agent piplartine - Characterization, toxicological, pharmacokinetics and efficacy studies. International Journal of Pharmaceutics, 498(1–2), 12–22. https://doi.org/10.1016/j.ijpharm.2015.11.045
Chong, W. T., Tan, C. P., Cheah, Y. K., et al. (2018). Optimization of process parameters in preparation of tocotrienol-rich red palm oil-based nanoemulsion stabilized by Tween80-Span 80 using response surface methodology. PLoS ONE, 13(8), 1–22. https://doi.org/10.1371/journal.pone.0202771
Hadnadev, T. D., Dokic, P., Krstonosic, V., & Hadnadev, M. (2013). Influence of oil phase concentration on droplet size distribution and stability of oil-in-water emulsions. European Journal of Lipid Science and Technology, 115, 313–321. https://doi.org/10.1002/ejlt.201100321
Poorani, G., Uppuluri, S., & Uppuluri, K. B. (2016). Formulation, characterization, in vitro and in vivo evaluation of castor oil based self-nano emulsifying levosulpiride delivery systems. Journal of Miroencapsulation, 33(6), 535–543. https://doi.org/10.1080/02652048.2016.1223199
Ali HH, & Hussein AA (2017) Oral nanoemulsions of candesartan cilexetil: Formulation, characterization and in vitro drug release studies. AAPS Open 3(4). https://doi.org/10.1186/s41120-017-0016-7
Kumari, S., Kumaraswamy, R. V., Choudhary, R. C., et al. (2018). Thymol nanoemulsion exhibits potential antibacterial activity against bacterial pustule disease and growth promotory effect on soybean. Scientific Reports, 8, 6650. https://doi.org/10.1038/s41598-018-24871-5
Babu, V. N., & Kannan, S. (2012). International Journal of Biological Macromolecules. Enhanced delivery of baicalein using cinnamaldehyde cross-linked chitosan nanoparticle inducing apoptosis. Int J Biol Macromol, 51(5), 1103–1108. https://doi.org/10.1016/j.ijbiomac.2012.08.038
Liu, Q., Li, J., Pu, G., et al. (2016). Co-delivery of baicalein and doxorubicin by hyaluronic acid decorated nanostructured lipid carriers for breast cancer therapy. Drug Delivery, 23(4), 1364–1368. https://doi.org/10.3109/10717544.2015.1031295
Meng, L., Xia, X., Yang, Y., et al. (2016). Co-encapsulation of paclitaxel and baicalein in nanoemulsions to overcome multidrug resistance via oxidative stress augmentation and P-glycoprotein inhibition. International Journal of Pharmaceutics, 513(1–2), 8–16. https://doi.org/10.1016/j.ijpharm.2016.09.001
Kavithaa, K., Paulpandi, M., & Padma, R. (2016). Induction of intrinsic apoptotic pathway and cell cycle arrest via baicalein loaded iron oxide nanoparticles as a competent nano-mediated. RSC Advances, 6, 64531–64543. https://doi.org/10.1039/C6RA11658B
Acknowledgements
Author Shraddha Srivastava acknowledges CSIR, India, for CSIR SRF fellowship. The authors are thankful to the MRC department MNIT Jaipur for providing TEM and FTIR facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None
Research Involving Humans and Animals Statement
None
Informed Consent
None
Funding Statement
None
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Srivastava, S., Singh, S., Saraf, S.A. et al. Encapsulation of Baicalein in Cinnamon Essential Oil Nanoemulsion for Enhanced Anticancer Efficacy Against MDA-MB-231 Cells. BioNanoSci. 11, 1049–1060 (2021). https://doi.org/10.1007/s12668-021-00900-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-021-00900-y