Abstract
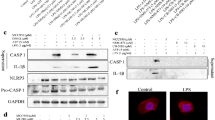
SIL1 acts as a co-chaperone for the major ER-resident chaperone BiP and thus plays a role in many BiP-dependent cellular functions such as protein-folding control and unfolded protein response. Whereas the increase of BiP upon cellular stress conditions is a well-known phenomenon, elevation of SIL1 under stress conditions was thus far solely studied in yeast, and different studies indicated an adverse effect of SIL1 increase. This is seemingly in contrast with the beneficial effect of SIL1 increase in surviving neurons in neurodegenerative disorders such as amyotrophic lateral sclerosis and Alzheimer’s disease. Here, we addressed these controversial findings. Applying cell biological, morphological and biochemical methods, we demonstrated that SIL1 increases in various mammalian cells and neuronal tissues upon cellular stress. Investigation of heterozygous SIL1 mutant cells and tissues supported this finding. Moreover, SIL1 protein was found to be stabilized during ER stress. Increased SIL1 initiates ER stress in a concentration-dependent manner which agrees with the described adverse SIL1 effect. However, our results also suggest that protective levels are achieved by the secretion of excessive SIL1 and GRP170 and that moderately increased SIL1 also ameliorates cellular fitness under stress conditions. Our immunoprecipitation results indicate that SIL1 might act in a BiP-independent manner. Proteomic studies showed that SIL1 elevation alters the expression of proteins including crucial players in neurodegeneration, especially in Alzheimer’s disease. This finding agrees with our observation of increased SIL1 immunoreactivity in surviving neurons of Alzheimer’s disease autopsy cases and supports the assumption that SIL1 plays a protective role in neurodegenerative disorders.









Similar content being viewed by others
References
Hetz C (2013) The biological meaning of the UPR. Nat Rev Mol Cell Biol 14(7):404. doi:10.1038/nrm3606
Dudek J, Benedix J, Cappel S, Greiner M, Jalal C, Muller L, Zimmermann R (2009) Functions and pathologies of BiP and its interaction partners. Cell Mol Life Sci 66(9):1556–1569. doi:10.1007/s00018-009-8745-y
Easton DP, Kaneko Y, Subjeck JR (2000) The hsp110 and Grp1 70 stress proteins: newly recognized relatives of the Hsp70s. Cell Stress Chaperones 5(4):276–290
Tyson JR, Stirling CJ (2000) LHS1 and SIL1 provide a lumenal function that is essential for protein translocation into the endoplasmic reticulum. EMBO J 19(23):6440–6452. doi:10.1093/emboj/19.23.6440
Chung KT, Shen Y, Hendershot LM (2002) BAP, a mammalian BiP-associated protein, is a nucleotide exchange factor that regulates the ATPase activity of BiP. J Biol Chem 277(49):47557–47563. doi:10.1074/jbc.M208377200
Quinones QJ, de Ridder GG, Pizzo SV (2008) GRP78: a chaperone with diverse roles beyond the endoplasmic reticulum. Histol Histopathol 23(11):1409–1416
Wang M, Ye R, Barron E, Baumeister P, Mao C, Luo S, Fu Y, Luo B et al (2010) Essential role of the unfolded protein response regulator GRP78/BiP in protection from neuronal apoptosis. Cell Death Differ 17(3):488–498. doi:10.1038/cdd.2009.144
Anttonen AK, Mahjneh I, Hamalainen RH, Lagier-Tourenne C, Kopra O, Waris L, Anttonen M, Joensuu T et al (2005) The gene disrupted in Marinesco-Sjogren syndrome encodes SIL1, an HSPA5 cochaperone. Nat Genet 37(12):1309–1311. doi:10.1038/ng1677
Senderek J, Krieger M, Stendel C, Bergmann C, Moser M, Breitbach-Faller N, Rudnik-Schoneborn S, Blaschek A et al (2005) Mutations in SIL1 cause Marinesco-Sjogren syndrome, a cerebellar ataxia with cataract and myopathy. Nat Genet 37(12):1312–1314. doi:10.1038/ng1678
Zhao L, Longo-Guess C, Harris BS, Lee JW, Ackerman SL (2005) Protein accumulation and neurodegeneration in the woozy mutant mouse is caused by disruption of SIL1, a cochaperone of BiP. Nat Genet 37(9):974–979. doi:10.1038/ng1620
Zhao L, Rosales C, Seburn K, Ron D, Ackerman SL (2010) Alteration of the unfolded protein response modifies neurodegeneration in a mouse model of Marinesco-Sjogren syndrome. Hum Mol Genet 19(1):25–35. doi:10.1093/hmg/ddp464
Roos A, Buchkremer S, Kollipara L, Labisch T, Gatz C, Zitzelsberger M, Brauers E, Nolte K et al (2014) Myopathy in Marinesco-Sjogren syndrome links endoplasmic reticulum chaperone dysfunction to nuclear envelope pathology. Acta Neuropathol 127(5):761–777. doi:10.1007/s00401-013-1224-4
Buchkremer S, González Coraspe JA, Weis J, Roos A (2016) Sil1-mutant mice elucidate chaperone function in neurological disorders. J Neuromuscul Dis 3:169–181. doi:10.3233/JND-160152
Krieger M, Roos A, Stendel C, Claeys KG, Sonmez FM, Baudis M, Bauer P, Bornemann A et al (2013) SIL1 mutations and clinical spectrum in patients with Marinesco-Sjogren syndrome. Brain J Neurol 136(Pt 12):3634–3644. doi:10.1093/brain/awt283
Roos A, Schwanitz G, Diepolder I, Senderek J, Eggermann K (2012) Search for cryptic subtelomeric aberrations in patients with non-classical Marinesco-Sjogren phenotype. J Pediatr Neurol 10(3):167–172. doi:10.3233/JPN-2012-0557
Roos A, Kollipara L, Buchkremer S, Labisch T, Brauers E, Gatz C, Lentz C, Gerardo-Nava J et al (2015) Cellular signature of SIL1 depletion: disease pathogenesis due to alterations in protein composition beyond the ER machinery. Mol Neurobiol. doi:10.1007/s12035-015-9456-z
Benedix J, Lajoie P, Jaiswal H, Burgard C, Greiner M, Zimmermann R, Rospert S, Snapp EL et al (2010) BiP modulates the affinity of its co-chaperone ERj1 for ribosomes. J Biol Chem 285(47):36427–36433. doi:10.1074/jbc.M110.143263
Schauble N, Lang S, Jung M, Cappel S, Schorr S, Ulucan O, Linxweiler J, Dudek J et al (2012) BiP-mediated closing of the Sec61 channel limits Ca2+ leakage from the ER. EMBO J 31(15):3282–3296. doi:10.1038/emboj.2012.189
Yan M, Li J, Sha B (2011) Structural analysis of the Sil1-Bip complex reveals the mechanism for Sil1 to function as a nucleotide-exchange factor. Biochem J 438(3):447–455. doi:10.1042/BJ20110500
Awe K, Lambert C, Prange R (2008) Mammalian BiP controls posttranslational ER translocation of the hepatitis B virus large envelope protein. FEBS Lett 582(21–22):3179–3184. doi:10.1016/j.febslet.2008.07.062
Howes J, Shimizu Y, Feige MJ, Hendershot LM (2012) C-terminal mutations destabilize SIL1/BAP and can cause Marinesco-Sjogren syndrome. J Biol Chem 287(11):8552–8560. doi:10.1074/jbc.M111.333286
Filezac de L’Etang A, Maharjan N, Cordeiro Brana M, Ruegsegger C, Rehmann R, Goswami A, Roos A, Troost D et al (2015) Marinesco-Sjogren syndrome protein SIL1 regulates motor neuron subtype-selective ER stress in ALS. Nat Neurosci 18(2):227–238. doi:10.1038/nn.3903
Liu ZC, Chu J, Lin L, Song J, Ning LN, Luo HB, Yang SS, Shi Y et al (2016) SIL1 rescued Bip elevation-related tau hyperphosphorylation in ER stress. Mol Neurobiol 53(2):983–994. doi:10.1007/s12035-014-9039-4
Penas C, Font-Nieves M, Fores J, Petegnief V, Planas A, Navarro X, Casas C (2011) Autophagy, and BiP level decrease are early key events in retrograde degeneration of motoneurons. Cell Death Differ 18(10):1617–1627. doi:10.1038/cdd.2011.24
Kimata Y, Ishiwata-Kimata Y, Yamada S, Kohno K (2006) Yeast unfolded protein response pathway regulates expression of genes for anti-oxidative stress and for cell surface proteins. Genes Cells 11(1):59–69. doi:10.1111/j.1365-2443.2005.00921.x
Doyon JB, Liu DR (2007) Identification of eukaryotic promoter regulatory elements using nonhomologous random recombination. Nucleic Acids Res 35(17):5851–5860. doi:10.1093/nar/gkm634
Payne T, Finnis C, Evans LR, Mead DJ, Avery SV, Archer DB, Sleep D (2008) Modulation of chaperone gene expression in mutagenized Saccharomyces cerevisiae strains developed for recombinant human albumin production results in increased production of multiple heterologous proteins. Appl Environ Microbiol 74(24):7759–7766. doi:10.1128/AEM.01178-08
Chang JY (1997) A two-stage mechanism for the reductive unfolding of disulfide-containing proteins. J Biol Chem 272(1):69–75
Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P (2000) Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 101(3):249–258
Inesi G, Wade R, Rogers T (1998) The sarcoplasmic reticulum Ca2+ pump: inhibition by thapsigargin and enhancement by adenovirus-mediated gene transfer. Ann N Y Acad Sci 853:195–206
George SJ, Johnson JL, Angelini GD, Jeremy JY (1997) Short-term exposure to thapsigargin inhibits neointima formation in human saphenous vein. Arterioscler Thromb Vasc Biol 17(11):2500–2506
Shaw G, Morse S, Ararat M, Graham FL (2002) Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. FASEB J 16(8):869–871. doi:10.1096/fj.01-0995fje
Moha ou Maati H, Peyronnet R, Devader C, Veyssiere J, Labbal F, Gandin C, Mazella J, Heurteaux C et al (2011) A human TREK-1/HEK cell line: a highly efficient screening tool for drug development in neurological diseases. PLoS One 6(10):e25602. doi:10.1371/journal.pone.0025602
Kleine H, Poreba E, Lesniewicz K, Hassa PO, Hottiger MO, Litchfield DW, Shilton BH, Luscher B (2008) Substrate-assisted catalysis by PARP10 limits its activity to mono-ADP-ribosylation. Mol Cell 32(1):57–69. doi:10.1016/j.molcel.2008.08.009
Brauers E, Dreier A, Roos A, Wormland B, Weis J, Kruttgen A (2010) Differential effects of myopathy-associated caveolin-3 mutants on growth factor signaling. Am J Pathol 177(1):261–270. doi:10.2353/ajpath.2010.090741
Manza LL, Stamer SL, Ham AJ, Codreanu SG, Liebler DC (2005) Sample preparation and digestion for proteomic analyses using spin filters. Proteomics 5(7):1742–1745. doi:10.1002/pmic.200401063
Wisniewski JR, Zougman A, Nagaraj N, Mann M (2009) Universal sample preparation method for proteome analysis. Nat Methods 6(5):359–362. doi:10.1038/nmeth.1322
Kollipara L, Zahedi RP (2013) Protein carbamylation: in vivo modification or in vitro artefact? Proteomics 13(6):941–944. doi:10.1002/pmic.201200452
Burkhart JM, Schumbrutzki C, Wortelkamp S, Sickmann A, Zahedi RP (2012) Systematic and quantitative comparison of digest efficiency and specificity reveals the impact of trypsin quality on MS-based proteomics. J Proteome 75(4):1454–1462. doi:10.1016/j.jprot.2011.11.016
Vaudel M, Burkhart JM, Zahedi RP, Oveland E, Berven FS, Sickmann A, Martens L, Barsnes H (2015) PeptideShaker enables reanalysis of MS-derived proteomics data sets. Nat Biotechnol 33(1):22–24. doi:10.1038/nbt.3109
Uttara B, Singh AV, Zamboni P, Mahajan RT (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7(1):65–74. doi:10.2174/157015909787602823
Richardson K, Allen SP, Mortiboys H, Grierson AJ, Wharton SB, Ince PG, Shaw PJ, Heath PR (2013) The effect of SOD1 mutation on cellular bioenergetic profile and viability in response to oxidative stress and influence of mutation-type. PLoS One 8(6):e68256. doi:10.1371/journal.pone.0068256
Ji L, Chauhan A, Chauhan V (2010) Upregulation of cytoplasmic gelsolin, an amyloid-beta-binding protein, under oxidative stress conditions: involvement of protein kinase C. J Alzheimers Dis 19(3):829–838. doi:10.3233/JAD-2010-1281
Kon M, Cuervo AM (2010) Chaperone-mediated autophagy in health and disease. FEBS Lett 584(7):1399–1404. doi:10.1016/j.febslet.2009.12.025
Raciti M, Lotti LV, Valia S, Pulcinelli FM, Di Renzo L (2012) JNK2 is activated during ER stress and promotes cell survival. Cell Death Dis 3:e429. doi:10.1038/cddis.2012.167
Weitzmann A, Volkmer J, Zimmermann R (2006) The nucleotide exchange factor activity of Grp170 may explain the non-lethal phenotype of loss of Sil1 function in man and mouse. FEBS Lett 580(22):5237–5240. doi:10.1016/j.febslet.2006.08.055
Hassdenteufel S, Schauble N, Cassella P, Leznicki P, Muller A, High S, Jung M, Zimmermann R (2011) Ca2+-calmodulin inhibits tail-anchored protein insertion into the mammalian endoplasmic reticulum membrane. FEBS Lett 585(21):3485–3490. doi:10.1016/j.febslet.2011.10.008
Gulow K, Bienert D, Haas IG (2002) BiP is feed-back regulated by control of protein translation efficiency. J Cell Sci 115(Pt 11):2443–2452
Hoyer-Hansen M, Jaattela M (2007) Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ 14(9):1576–1582. doi:10.1038/sj.cdd.4402200
Li J, Ni M, Lee B, Barron E, Hinton DR, Lee AS (2008) The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ 15(9):1460–1471. doi:10.1038/cdd.2008.81
Clark HF, Gurney AL, Abaya E, Baker K, Baldwin D, Brush J, Chen J, Chow B et al (2003) The secreted protein discovery initiative (SPDI), a large-scale effort to identify novel human secreted and transmembrane proteins: a bioinformatics assessment. Genome Res 13(10):2265–2270. doi:10.1101/gr.1293003
Kobayashi T, Takita Y, Suzuki A, Katsu Y, Iguchi T, Ohta Y (2008) Vacuolar degeneration of skeletal muscle in transgenic mice overexpressing ORP150. J Vet Med Sci 70(1):115–118
Scheper W, Hoozemans JJ (2015) The unfolded protein response in neurodegenerative diseases: a neuropathological perspective. Acta Neuropathol 130(3):315–331. doi:10.1007/s00401-015-1462-8
Ullrich S, Munch A, Neumann S, Kremmer E, Tatzelt J, Lichtenthaler SF (2010) The novel membrane protein TMEM59 modulates complex glycosylation, cell surface expression, and secretion of the amyloid precursor protein. J Biol Chem 285(27):20664–20674. doi:10.1074/jbc.M109.055608
Sui X, Ren X, Huang P, Li S, Ma Q, Ying M, Ni J, Liu J et al (2014) Proteomic analysis of serum proteins in triple transgenic Alzheimer’s disease mice: implications for identifying biomarkers for use to screen potential candidate therapeutic drugs for early Alzheimer’s disease. J Alzheimers Dis 40(3):575–586. doi:10.3233/JAD-131823
Uchiumi T, Ohgaki K, Yagi M, Aoki Y, Sakai A, Matsumoto S, Kang D (2010) ERAL1 is associated with mitochondrial ribosome and elimination of ERAL1 leads to mitochondrial dysfunction and growth retardation. Nucleic Acids Res 38(16):5554–5568. doi:10.1093/nar/gkq305
Redmann M, Darley-Usmar V, Zhang J (2016) The role of autophagy, mitophagy and lysosomal functions in modulating bioenergetics and survival in the context of redox and proteotoxic damage: implications for neurodegenerative diseases. Aging Dis 7(2):150–162. doi:10.14336/AD.2015.0820
Garringer HJ, Murrell J, Sammeta N, Gnezda A, Ghetti B, Vidal R (2013) Increased tau phosphorylation and tau truncation, and decreased synaptophysin levels in mutant BRI2/tau transgenic mice. PLoS One 8(2):e56426. doi:10.1371/journal.pone.0056426
Faldu KG, Shah JS, Patel SS (2015) Anti-viral agents in neurodegenerative disorders: new paradigm for targeting Alzheimer’s disease. Recent Pat Antiinfect Drug Discov 10(2):76–83
Ploen D, Hafirassou ML, Himmelsbach K, Schille SA, Biniossek ML, Baumert TF, Schuster C, Hildt E (2013) TIP47 is associated with the hepatitis C virus and its interaction with Rab9 is required for release of viral particles. Eur J Cell Biol 92(12):374–382. doi:10.1016/j.ejcb.2013.12.003
Rodrigues EM, Scudder SL, Goo MS, Patrick GN (2016) Abeta-induced synaptic alterations require the E3 ubiquitin ligase Nedd4-1. J Neurosci 36(5):1590–1595. doi:10.1523/JNEUROSCI.2964-15.2016
Shearwin-Whyatt LM, Brown DL, Wylie FG, Stow JL, Kumar S (2004) N4WBP5A (Ndfip2), a Nedd4-interacting protein, localizes to multivesicular bodies and the Golgi, and has a potential role in protein trafficking. J Cell Sci 117(Pt 16):3679–3689. doi:10.1242/jcs.01212
Ostroumova OS, Schagina LV, Mosevitsky MI, Zakharov VV (2011) Ion channel activity of brain abundant protein BASP1 in planar lipid bilayers. FEBS J 278(3):461–469. doi:10.1111/j.1742-4658.2010.07967.x
Thiede-Stan NK, Tews B, Albrecht D, Ristic Z, Ewers H, Schwab ME (2015) Tetraspanin-3 is an organizer of the multi-subunit Nogo-A signaling complex. J Cell Sci 128(19):3583–3596. doi:10.1242/jcs.167981
Xu YQ, Sun ZQ, Wang YT, Xiao F, Chen MW (2015) Function of Nogo-A/Nogo-A receptor in Alzheimer’s disease. CNS Neurosci Ther 21(6):479–485. doi:10.1111/cns.12387
Wang Q, Xu Y, Chen JC, Qin YY, Liu M, Liu Y, Xie MJ, Yu ZY et al (2012) Stromal cell-derived factor 1alpha decreases beta-amyloid deposition in Alzheimer’s disease mouse model. Brain Res 1459:15–26. doi:10.1016/j.brainres.2012.04.011
Malik B, Fernandes C, Killick R, Wroe R, Usardi A, Williamson R, Kellie S, Anderton BH et al (2012) Oligomeric amyloid-beta peptide affects the expression of genes involved in steroid and lipid metabolism in primary neurons. Neurochem Int 61(3):321–333. doi:10.1016/j.neuint.2012.05.006
Valdez CM, Phelix CF, Smith MA, Perry G, Santamaria F (2011) Modeling cholesterol metabolism by gene expression profiling in the hippocampus. Mol BioSyst 7(6):1891–1901. doi:10.1039/c0mb00282h
Yamin R, Bagchi S, Hildebrant R, Scaloni A, Widom RL, Abraham CR (2007) Acyl peptide hydrolase, a serine proteinase isolated from conditioned medium of neuroblastoma cells, degrades the amyloid-beta peptide. J Neurochem 100(2):458–467
Akhter R, Sanphui P, Das H, Saha P, Biswas SC (2015) The regulation of p53 up-regulated modulator of apoptosis by JNK/c-Jun pathway in beta-amyloid-induced neuron death. J Neurochem 134(6):1091–1103. doi:10.1111/jnc.13128
Doostzadeh-Cizeron J, Evans R, Yin S, Goodrich DW (1999) Apoptosis induced by the nuclear death domain protein p84N5 is inhibited by association with Rb protein. Mol Biol Cell 10(10):3251–3261
Varisli L, Gonen-Korkmaz C, Debelec-Butuner B, Erbaykent-Tepedelen B, Muhammed HS, Bogurcu N, Saatcioglu F, Korkmaz KS (2011) Ubiquitously expressed hematological and neurological expressed 1 downregulates Akt-mediated GSK3beta signaling, and its knockdown results in deregulated G2/M transition in prostate cells. DNA Cell Biol 30(6):419–429. doi:10.1089/dna.2010.1128
McArthur S, Cristante E, Paterno M, Christian H, Roncaroli F, Gillies GE, Solito E (2010) Annexin A1: a central player in the anti-inflammatory and neuroprotective role of microglia. J Immunol 185(10):6317–6328. doi:10.4049/jimmunol.1001095
Kino Y, Washizu C, Kurosawa M et al (2016) FUS/TLS acts as an aggregation-dependent modifier of polyglutamine disease model mice. Sci Rep 6:35236. doi:10.1038/srep35236
Senderek J, Muller JS, Dusl M, Strom TM, Guergueltcheva V, Diepolder I, Laval SH, Maxwell S et al (2011) Hexosamine biosynthetic pathway mutations cause neuromuscular transmission defect. Am J Hum Genet 88(2):162–172. doi:10.1016/j.ajhg.2011.01.008
Lo AS, Liew CT, Ngai SM, Tsui SK, Fung KP, Lee CY, Waye MM (2005) Developmental regulation and cellular distribution of human cytosolic malate dehydrogenase (MDH1). J Cell Biochem 94(4):763–773. doi:10.1002/jcb.20343
Zhi G, Ryder JW, Huang J, Ding P, Chen Y, Zhao Y, Kamm KE, Stull JT (2005) Myosin light chain kinase and myosin phosphorylation effect frequency-dependent potentiation of skeletal muscle contraction. Proc Natl Acad Sci U S A 102(48):17519–17524. doi:10.1073/pnas.0506846102
Tian G, Thomas S, Cowan NJ (2010) Effect of TBCD and its regulatory interactor Arl2 on tubulin and microtubule integrity. Cytoskeleton (Hoboken) 67(11):706–714. doi:10.1002/cm.20480
Sengle G, Carlberg V, Tufa SF, Charbonneau NL, Smaldone S, Carlson EJ, Ramirez F, Keene DR et al (2015) Abnormal activation of BMP signaling causes myopathy in Fbn2 null mice. PLoS Genet 11(6):e1005340. doi:10.1371/journal.pgen.1005340
Ansseau E, Eidahl JO, Lancelot C, Tassin A, Matteotti C, Yip C, Liu J, Leroy B et al (2016) Homologous transcription factors DUX4 and DUX4c associate with cytoplasmic proteins during muscle differentiation. PLoS One 11(1):e0146893. doi:10.1371/journal.pone.0146893
Covington JD, Galgani JE, Moro C, LaGrange JM, Zhang Z, Rustan AC, Ravussin E, Bajpeyi S (2014) Skeletal muscle perilipin 3 and coatomer proteins are increased following exercise and are associated with fat oxidation. PLoS One 9(3):e91675. doi:10.1371/journal.pone.0091675
Moreau MM, Piguel N, Papouin T, Koehl M, Durand CM, Rubio ME, Loll F, Richard EM et al (2010) The planar polarity protein Scribble1 is essential for neuronal plasticity and brain function. J Neurosci 30(29):9738–9752. doi:10.1523/JNEUROSCI.6007-09.2010
Acknowledgements
Financial support by the Ministerium für Innovation, Wissenschaft und Forschung des Landes Nordrhein-Westfalen, the Senatsverwaltung für Wirtschaft, Technologie und Forschung des Landes Berlin and the Bundesministerium für Bildung und Forschung is gratefully acknowledged. This work was also supported by a grant from the START program of RWTH Aachen University (to A. R.; grant No. 41/12) and by the German Research Foundation (DFG to R. Z.; grant no. ZA 639/1-1) as well as by the Deutsche Gesellschaft für Muskelkranke (DGM) and the IZKF Aachen (to J. W.; grant no. N5-3). We thank Mrs. Claudia Krude, Mrs. Hannelore Mader, Mrs. Astrid Knichewski, Mr. Julian Kaufmann and Mrs. Sarah Waide for expert technical assistance. Immortalized lymphoblastoid cells were kindly provided by Professor Jan Senderek (Friedrich-Baur-Institute Munich, Germany).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Online Resource 1
(DOCX 699 kb)
Online Resource 2
(DOCX 1278 kb)
Online Resource 3
(DOCX 120 kb)
Online Resource 4
(DOCX 464 kb)
Online Resource 5
(DOCX 60 kb)
Online Resource 6
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Labisch, T., Buchkremer, S., Phan, V. et al. Tracking Effects of SIL1 Increase: Taking a Closer Look Beyond the Consequences of Elevated Expression Level. Mol Neurobiol 55, 2524–2546 (2018). https://doi.org/10.1007/s12035-017-0494-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0494-6