Abstract
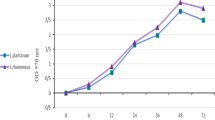
The present study aimed to assess the activity of cell-free supernatant (CFS) containing bacteriocins on the formation and maintenance of biofilms developed by Listeria monocytogenes, and the associated effect of bacteriocins and ethylene-diamine-tetra-acetic acid (EDTA) on the formed biofilm. CFS from 9 lactic acid bacteria (LAB) strains was tested for inhibitory activity against 85 L. monocytogenes isolates and 21 LAB strains. Then, 12 L. monocytogenes strains were selected based on genetic profiles and sensitivity to CFS and were subjected to an in vitro assay to assess biofilm formation in microtiter plates, considering different culture media and incubation conditions. Based on these results, 6 L. monocytogenes strains were subjected to the same in vitro procedure to assess biofilm formation, being co-inoculated with CFS. In addition, these strains were subjected to the same in vitro procedure, modified by adding the CFS after biofilm formation. Relevant decrease in biofilm formation was observed in the first experiment, but CFS added after biofilm formation did not eliminate them. CFS from Lactobacillus curvatus ET31 were selected due to its anti-biofilm activity, being associated to EDTA at different concentrations and tested for biofilm control of three strains of L. monocytogenes, using the same in vitro procedure described previously. Concentrated bacteriocin presented poor performance in eliminating formed biofilms, and EDTA concentration presented no evident interference on biofilm elimination. Twelve selected L. monocytogenes strains were positive for investigated virulence makers and negative for luxS gene, recognized as being involved in biofilm formation. Selected L. monocytogenes strains were able to produce biofilms under different conditions. CFSs have the potential to prevent biofilm formation, but they were not able to destroy already formed biofilms. Nevertheless, low concentrations of CFS combined with EDTA caused a relevant reduction in already formed biofilms, but this association was not able to eliminate them. The activity of selected CFS was demonstrated against L. monocytogenes-formed biofilms, being more effective when associated to EDTA at different concentrations.


Similar content being viewed by others
References
Chen, Y., Zhang, W., & Knabel, S. J. (2007). Multi-virulence-locus sequence typing identifies single nucleotide polymorphisms which differentiate epidemic clones and outbreak strains of Listeria monocytogenes. Journal of Clinical Microbiology, 45, 835–846.
Swaminathan, B., & Gerner-Smidt, P. (2007). The epidemiology of human listeriosis. Microbes and Infection, 9, 1236–1243.
Behravesh, C. B., Jones, T. F., Vugia, D. J., Long, C., Marcus, R., Smith, K., Thomas, S., Zansky, S., Fullerton, K. E., & Henao, O. L. (2011). Deaths associated with bacterial pathogens transmitted commonly through food: foodborne diseases active surveillance network (FoodNet), 1996–2005. Journal of Infectious Diseases, 204, 263–267.
Carpentier, B., & Cerf, O. (2011). Review: persistence of Listeria monocytogenes in food industry equipment and premises. International Journal of Food Microbiology, 145, 1–8.
Ibarreche, M. P., Castellano, P., & Vignolo, G. (2014). Evaluation of anti-Listeria meat borne Lactobacillus for biofilm formation on selected abiotic surfaces. Meat Science, 96, 295–303.
Bonsaglia, E. C. R., Silva, N. C. C., Júnior, A. F., Júnior, J. P. A., Tsunemi, M. H., & Rall, V. L. M. (2014). Production of biofilm by Listeria monocytogenes in different materials and temperatures. Food Control, 35, 386–391.
Daines, D. A., Bothwell, M., Furrer, J., Unrath, W., Nelson, K., Jarisch, J., Melrose, N., Greiner, L., Apicella, M., & Smith, A. L. (2005). Haemophilus influenzae luxS mutants form a biofilm and have increased virulence. Microb Pathogenesis, 39, 87–96.
Kong, K.-F., Vuong, C., & Otto, M. (2006). Staphylococcus quorum sensing in biofilm formation and infection. International Journal Medical Microbiology, 296, 133–139.
Stroeher, U. H., Paton, A. W., Ogunniyi, A. D., & Paton, J. C. (2003). Mutation of luxS of Streptococcus pneumoniae affects virulence in a mouse model. Infection and Immunity, 71, 3206–3212.
Xu, L., Li, H., Vuong, C., Vadyvaloo, V., Wang, J., Yao, Y., Otto, M., & Gao, Q. (2006). Role of the luxS quorum-sensing system in biofilm formation and virulence of Staphylococcus epidermidis. Infection and Immunity, 74, 488–496.
Belval, S. C., Gal, L., Margiewes, S., Garmyn, D., Piveteau, P., & Guzzo, J. (2006). Assessment of the roles of luxS, S-ribosyl homocysteine, and autoinducer 2 in cell attachment during biofilm formation by Listeria monocytogenes EGD-e. Applied and Environmental Microbiology, 72, 2644–2650.
Giaouris, E., Heir, E., Hébraud, M., Chorianopoulos, N., Langsrud, S., Møretrø, T., Habimana, O., Desvaux, M., Renier, S., & Nychas, G.-J. (2014). Attachment and biofilm formation by foodborne bacteria in meat processing environments: causes, implications, role of bacterial interactions and control by alternative novel methods. Meat Science, 97, 298–309.
Srey, S., Jahid, I. K., & Ha, S.-D. (2013). Biofilm formation in food industries: a food safety concern. Food Control, 31, 572–585.
de las Heras, A., Cain, R. J., Bielecka, M. K., & Vázquez-Boland, J. A. (2011). Regulation of Listeria virulence: PrfA master and commander. Current Opinion in Biotechnology, 14, 118–127.
Gálvez, A., Abriouel, H., Benomar, N., & Lucas, R. (2010). Microbial antagonists to food-borne pathogens and biocontrol. Current Opinion in Biotechnology, 21, 142–148.
Hartmann, H. A., Wilke, T., & Erdmann, R. (2011). Efficacy of bacteriocin-containing cell-free culture supernatants from lactic acid bacteria to control Listeria monocytogenes in food. International Journal of Food Microbiology, 146, 192–199.
Gómez, N. C., Abriouel, H., Grande, M. J., Pulido, R. P., & Gálvez, A. (2012). Effect of enterocin AS-48 in combination with biocides on planktonic and sessile Listeria monocytogenes. Food Microbiology, 30, 51–58.
García-Almendárez, B. E., Cann, I. K. O., Martin, S. E., Guerrero-Legarreta, I., & Regalado, C. (2008). Effect of Lactococcus lactis UQ2 and its bacteriocin on Listeria monocytogenes biofilms. Food Control, 19, 670–680.
Winkelströter, L. K., Tulini, F. L., & de Martinis, E. C. P. (2015). Identification of the bacteriocin produced by cheese isolate Lactobacillus paraplantarum FT259 and its potential influence on Listeria monocytogenes biofilm formation. LWT - Food Science Technology, 64, 586–592.
Tomé, E., Gibbs, P. A., & Teixeira, P. C. (2008). Growth control of Listeria innocua 2030c on vacuum-packaged cold-smoked salmon by lactic acid bacteria. International Journal of Food Microbiology, 121, 285–294.
Tomé, E., Pereira, V. L., Lopes, C. I., Gibbs, P. A., & Teixeira, P. C. (2008). In vitro tests of suitability of bacteriocin-producing lactic acid bacteria, as potential biopreservation cultures in vacuum-packaged cold-smoked salmon. Food Control, 19, 535–543.
Tomé, E., Todorov, S. D., Gibbs, P. A., & Teixeira, P. C. (2009). Partial characterization of nine bacteriocins produced by lactic acid bacteria isolated from cold-smoked salmon with activity against Listeria monocytogenes. Food Biotechnology, 23, 50–73.
Camargo, A. C., Dias, M. R., Cossi, M. V. C., Lanna, F. G. P. A., Cavicchioli, V. Q., Vallim, D. C., Pinto, P. S. A., Hofer, E., & Nero, L. A. (2015). Serotypes and pulsotypes diversity of Listeria monocytogenes in a beef-processing environment. Foodborne Pathogens and Disease, 12, 323–326.
Todorov, S. D. (2009). Bacteriocins from Lactobacillus plantarum production, genetic organization and mode of action: produção, organização genética e modo de ação. Brazilian Journal of Microbiology, 40, 209–221.
Djordjevic, D., Wiedmann, M., & McLandsborough, L. (2002). Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Applied and Environmental Microbiology, 68, 2950–2958.
Stepanović, S., Vuković, D., Hola, V., di Bonaventura, G., Djukić, S., Ćirković, I., & Ruzicka, F. (2007). Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS, 115, 891–899.
Galvão, N. N., Chiarini, E., Destro, M. T., Ferreira, M. A., & Nero, L. A. (2012). PFGE characterisation and adhesion ability of Listeria monocytogenes isolates obtained from bovine carcasses and beef processing facilities. Meat Science, 92, 635–643.
Liu, D., Lawrence, M. L., Austin, F. W., & Ainsworth, A. J. (2007). A multiplex PCR for species-and virulence-specific determination of Listeria monocytogenes. Journal Microbiology Methods, 71, 133–140.
Rawool, D., Malik, S., Barbuddhe, S., Shakuntala, I., & Aurora, R. (2007). A multiplex PCR for detection of virulence associated genes in Listeria monocytogenes. International Journal Food Safety, 9, 56–62.
Camargo, A. C., Lafisca, A., Cossi, M. V. C., Lanna, F. G. P. A., Dias, M. R., Pinto, P. S. A., & Nero, L. A. (2014). Low occurrence of Listeria monocytogenes on bovine hides and carcasses in Minas Gerais State, Brazil: molecular characterization and antimicrobial resistance. Journal of Food Protection, 77, 1148–1152.
Balciunas, E. M., Martinez, F. A. C., Todorov, S. D., Franco, B. D. G. M., Converti, A., & Oliveira, R. P. S. (2013). Novel biotechnological applications of bacteriocins: a review. Food Control, 32, 134–142.
Sobrino-López, A., & Martín-Belloso, O. (2008). Use of nisin and other bacteriocins for preservation of dairy products. International Dairy Journal, 18, 329–343.
Todorov, S. D., Furtado, D. N., Saad, S. M. I., Tome, E., & Franco, B. D. G. M. (2011). Potential beneficial properties of bacteriocin producing lactic acid bacteria isolated from smoked salmon. Journal of Applied Microbiology, 110, 971–986.
Moltz, A. G. (2005). Formation of biofilms by Listeria monocytogenes under various growth conditions. Journal of Food Protection, 68, 92–97.
Borucki, M. K., Peppin, J. D., White, D., Loge, F., & Call, D. R. (2003). Variation in biofilm formation among strains of Listeria monocytogenes. Applied and Environmental Microbiology, 69, 7336–7342.
Di Bonaventura, G., Piccolomini, R., Paludi, D., D’Orio, V., Vergara, A., Conter, M., & Ianieri, A. (2008). Influence of temperature on biofilm formation by Listeria monocytogenes on various food-contact surfaces: relationship with motility and cell surface hydrophobicity. Journal of Applied Microbiology, 104, 1552–1561.
Lundén, J. M., Miettinen, M. K., Autio, T. J., & Korkeala, H. J. (2000). Persistent Listeria monocytogenes strains show enhanced adherence to food contact surface after short contact times. Journal of Food Protection, 63, 1204–1207.
Nilsson, R. E., Ross, T., & Bowman, J. P. (2011). Variability in biofilm production by Listeria monocytogenes correlated to strain origin and growth conditions. International Journal of Food Microbiology, 150, 14–24.
Norwood, D. E., & Gilmour, A. (1999). Adherence of Listeria monocytogenes strains to stainless steel coupons. Journal of Applied Microbiology, 86, 576–582.
Pan, Y., Breidt, F., & Gorski, L. (2010). Synergistic effects of sodium chloride, glucose, and temperature on biofilm formation by Listeria monocytogenes serotype 1/2a and 4b strains. Applied and Environmental Microbiology, 76, 1433–1441.
Orsi, R. H., den Bakker, H. C., & Wiedmann, M. (2011). Listeria monocytogenes lineages: genomics, evolution, ecology, and phenotypic characteristics. International Journal Medical Microbiology, 301, 79–96.
Ferreira, V., Wiedmann, M., Teixeira, P., & Stasiewicz, M. (2014). Listeria monocytogenes persistence in food-associated environments: epidemiology, strain characteristics, and implications for public health. Journal of Food Protection, 77, 150–170.
Winkelstroeter, L. K., Gomes, B. C., Thomaz, M. R. S., Souza, V. M., & de Martinis, E. C. P. (2011). Lactobacillus sakei 1 and its bacteriocin influence adhesion of Listeria monocytogenes on stainless steel surface. Food Control, 22, 1404–1407.
Cotter, P. D., Hill, C., & Ross, R. P. (2005). Bacteriocins: developing innate immunity for food. Nature Reviews Microbiology, 3, 777–788.
Chang, Y., Gu, W., & McLandsborough, L. (2012). Low concentration of ethylenediaminetetraacetic acid (EDTA) affects biofilm formation of Listeria monocytogenes by inhibiting its initial adherence. Food Microbiology, 29, 10–17.
Alonso, A. N., Perry, K. J., Regeimbal, J. M., Regan, P. M., & Higgins, D. E. (2014). Identification of Listeria monocytogenes determinants required for biofilm formation. PLoS ONE, 9, e113696.
Lemon, K. P., Freitag, N. E., & Kolter, R. (2010). The virulence regulator PrfA promotes biofilm formation by Listeria monocytogenes. Journal of Bacteriology, 192, 3969–3976.
Wassinger, A., Zhang, L., Tracy, E., Munson, R. S., Jr., Kathariou, S., & Wang, H. H. (2013). Role of a GntR-family response regulator LbrA in Listeria monocytogenes biofilm formation. PLoS ONE, 8, e70448.
Zhu, X., Liu, W., Lametsch, R., Aarestrup, F., Shi, C., She, Q., Shi, X., & Knøchel, S. (2011). Phenotypic, proteomic, and genomic characterization of a putative ABC-transporter permease involved in Listeria monocytogenes biofilm formation. Foodborne Pathogens and Disease, 8, 495–501.
Acknowledgments
The authors want to express their gratitude to Prof. Elisabetta Tome from Universidad Central de Venezuela, Caracas, Venezuela for providing bacteriocinogenic strains used in this study. Authors were supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Camargo, A.C., de Paula, O.A.L., Todorov, S.D. et al. In Vitro Evaluation of Bacteriocins Activity Against Listeria monocytogenes Biofilm Formation. Appl Biochem Biotechnol 178, 1239–1251 (2016). https://doi.org/10.1007/s12010-015-1941-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1941-3