Abstract
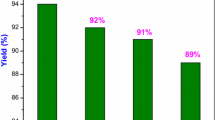
A new type of the four-component tandem Knoevenagel—Michael reaction was discovered. The transformation of arylaldehydes, N,N′-dimethylbarbituric acid, 4-hydroxy-6-methyl-2H-pyran-2-one, and morpholine in alcohols, other organic solvents, and water without a catalyst or any other additives and without heating leads to the selective formation of a new substituted unsymmetric ionic scaffold with three different heterocyclic rings in 81–98% yields. The structure of the obtained compounds was confirmed by the data of X-ray diffraction analysis.
Similar content being viewed by others
References
Multicomponent Reactions in Organic Synthesis, Eds J. Zhu, Q. Wang, M. Wang, Wiley-VCH, Weinheim, 2015.
R. C. Cioc, E. Ruijter, R. V. Orru, Green Chem., 2014, 16, 2958; DOI: https://doi.org/10.1039/c4gc00013g.
M. N. Elinson, Y. E. Ryzhkova, F. V. Ryzhkov, Russ. Chem. Rev., 2021, 90, 94; DOI: https://doi.org/10.1070/RCR4972.
T. L. Ho, Tandem Organic Reactions, John Wiley & Sons, New York, USA, 1992.
L. F. Tietze, U. Beifuss, Angew. Chem., Int. Ed. Engl., 1993, 32, 131; DOI: https://doi.org/10.1002/anie.199301313.
S. N. Jadhav, S. P. Patil, D. P. Sahoo, D. Rath, K. Parida, C. V. Rode, Catal. Lett., 2020, 150, 2331; DOI: https://doi.org/10.1007/s10562-019-03089-8.
M. N. Elinson, A. N. Vereshchagin, Y. E. Ryzhkova, S. K. Krymov, N. A. Leonova, A. S. Goloveshkin, M. P. Egorov, Mendeleev Commun., 2020, 30, 223; DOI: https://doi.org/10.1016/j.mencom.2020.03.031.
M. N. Elinson, A. N. Vereshchagin, Y. E. Anisina, N. A. Leonova, M. P. Egorov, Mendeleev Commun., 2020, 30, 15; DOI: https://doi.org/10.1016/j.mencom.2020.03.031.
M. Liu, M.; C-F. Liu, J. Zhang, Y-J. Xu, L. Dong, Org. Chem. Front., 2019, 6, 664; DOI: https://doi.org/10.1039/C8QO01378K.
M. N. Elinson, M. G. Medvedev, A. I. Ilovaisky, V. M. Merkulova, T. A. Zaimovskaya, G. I. Nikishin, Mendeleev Commun., 2013, 23, 94; DOI: https://doi.org/10.1016/j.mencom.2013.03.014.
A. N. Vereshchagin, M. N. Elinson, T. A. Zaymovskaya, G. I. Nikishin, Tetrahedron, 2008, 64, 9766; DOI: https://doi.org/10.1016/j.tet.2008.07.060.
M. Baumann, I. R. Baxendale, Beilstein J. Org. Chem., 2013, 9, 2265; DOI: https://doi.org/10.3762/bjoc.9.265.
R. W. DeSimone, K. S. Currie, S. A. Mitchell, J. W. Darrow, D. A. Pippin, Comb. Chem High Throughput Screen, 2004, 7, 473; DOI: https://doi.org/10.2174/1386207043328544.
K. C. Nicolaou, J. A. Pfefferkorn, A. J. Roecker, G.-Q. Cao, S. Barluenga, H. J. Mitchell, J. Am. Chem. Soc., 2000, 122, 9939; DOI: https://doi.org/10.1021/ja002033k.
L. L. Brunton, J. S. Lazo, K. L. Parker, I. Buxton, D. Blumenthal, Goodman and Gilman’s The pharmacological basis of therapeutics; 11th edn, The McGraw-Hill Companies Inc., New-York, USA, 2006.
M. W. Johns, Drugs, 1975, 9, 448; DOI: https://doi.org/10.2165/00003495-197509060-00004.
C. Uhlmann, W. Froscher, CNS Neurosci. Ther., 2009, 15, 24; DOI: https://doi.org/10.1111/j.1755-5949.2008.00073.x.
F. N. M. Naguib, D. L. Levesque, W. Eng-Chi, R. P. Panzica, M. H. El Kouni, Biochem. Pharmacol., 1993, 46, 1273; DOI: https://doi.org/10.1016/0006-2952(93)90477-E.
F. Grams, H. Brandstetter, S. D’Alo, D. Geppert, H. W. Krell, H. Leinert, V. Livi, E. Menta, A. Oliva, G. Zimmermann, Biol. Chem., 2001, 382, 1277; DOI: https://doi.org/10.1515/BC.2001.159.
M. Liu, D. Cao, R. Russell, R. E. Handschumacher, G. Pizzorno, Cancer. Res., 1998, 58, 5418; https://cancerres.aacrjournals.org/content/canres/58/23/5418.full.pdf.
H. C. Swannie, S.B. Kaye, Curr. Oncol. Rep., 2002, 4, 37; DOI: https://doi.org/10.1007/s11912-002-0046-7.
P. Gruber, F. Rechfeld, J. Kirchmair, N. Hauser, M. Boehler, D. Garczarczyk, T. Langer, J. Hofmann, J. Biochem., 2011, 149, 331; DOI: https://doi.org/10.1093/jb/mvq147.
P. H. Yin, X. Liu, Y. Y. Qiu, J. F. Cai, J. M. Qin, H. R. Zhu, Q. Li, Asian. Pac. J. Cancer. Prev., 2012, 13, 5329; DOI: https://doi.org/10.7314/APJCP.2012.13.11.5339.
K. Tsuchiya, S. Kobayashi, T. Nishikiori, T. Nakagawa, K. Tatsuta, J. Antibiot., 1997, 50, 259; DOI: https://doi.org/10.7164/antibiotics.50.259.
S. R. Turner, J. W. Strohbach, R. A. Tommasi, P.A. Aristoff, P. D. Johnson, H. I. Shulnick, L. A. Dolak, E. P. Seest, P. K. Tomich, M. J. Bohanon, M. M. Horng, J. C. Lynn, K. T. Chong, R. R. Hinshaw, K. D. Waterpaugh, M. N. Janakiraman, S. Thaisrivongs, J. Med. Chem., 1998, 41, 3467; DOI: https://doi.org/10.1021/jm9802158.
J. V. N. V. Prasad, A. Pavlovsky, K. S. Para, E. L. Ellsworth, P. J. Tummino, C. Nouhan, D. Ferguson, Bioorg. Med. Chem. Lett., 1996, 6, 1133; DOI: https://doi.org/10.1016/0960-894X(96)00180-1.
Q. Y. Lan, Q. L. Liu, J. Cai, A. W. Liu, Int. J. Clin. Exp. Pathol., 2015, 8, 155.
M. Kondoh, T. Usui, S. Kobayashi, K. Tsuchiya, K. Nishikawa, T. Nishikiori, T. Mayumi, H. Osada, Cancer. Lett., 1998, 126, 29; DOI: https://doi.org/10.1016/s0304-3835(97)00528-4.
J. M. Dickinson, Nat. Prod. Rep., 1993, 10, 71; DOI: https://doi.org/10.1039/np9931000071.
H. Tomoda, N. Tabata, D. J. Yang, I. Namatame, H. Tanaka, S. Omura, T. Kaneko, J. Antibiot., 1996, 49, 292; DOI: https://doi.org/10.7164/antibiotics.49.292.
A. P. Kourounakis, D. Xanthopoulos, A. Tzara, Med. Res. Rev., 2020, 40, 709; DOI: https://doi.org/10.1002/med.21634.
J. F. Cotten, B. Keshavaprasad, M. J. Laster, E. I. Eger, C. S. Yost, Anesth. Analg., 2006, 102, 779; DOI: https://doi.org/10.1213/01.ane.0000194289.34345.63.
W. W. Stoops, J. C. Strickland, J. L. Alcorn, L. R. Hays, A. O. Rayapati, J. A. Lile, C. R. Rush, Psychopharmacology, 2019, 236, 2569; DOI: https://doi.org/10.1007/s00213-019-05227-x.
U. Bonnet, CNS Drug Rev., 2003, 9, 97; DOI: https://doi.org/10.1111/j.1527-3458.2003.tb00245.x.
C. Naidu, J. Kulkarni, Aust. N. Z. J. Psychiatry, 2019, 53, 1227; DOI: https://doi.org/10.1177/0004867419865612.
S. L. Walsh, M. Heilig, P. A. Nuzzo, P. Henderson, M. R. Lofwall, Addict. Biol., 2013, 18, 332; DOI: https://doi.org/10.1111/j.1369-1600.2011.00419.x.
M. S. N. Patel, M. H. Ahmed, M. Saqib, S. N. Shaikh, J. Drug. Deliv. Ther., 2019, 9, 542; DOI: https://doi.org/10.2270/jddt.v9i2.2432.
Y. E. Ryzhkova, A. N. Fakhrutdinov, M. N. Elinson, Molbank, 2021, M1219; DOI: https://doi.org/10.3390/M1219.
N. N. Pesyan, H. Rashidnejad, M. A. Esmaeili, E. Safari, T. Tunç, M. Alilou, R. Safavi-Sohi, E. Şahin, J. Chin. Chem. Soc., 2020, 67, 1679; DOI: https://doi.org/10.1002/jccs.202000057.
S. Katsamakas, A. G. Papadopoulos, M. G. Kouskoura, C. K. Markopoulou, D. Hadjipavlou-Litina, Future Med. Chem., 2019, 11, 2063; DOI: https://doi.org/10.4155/fmc-2018-0541.
A. Barakat, M. Ali, A. M. Al Majid, S. Yousuf, M. I. Choudhary, US Patent 9527820, 2016.
A. Barakat, A. M. Al-Majid, H. J. Al-Najjar, Y. N. Mabkhot, S. Javaid, S. Yousuf, M. I. Choudhary, Eur. J. Med. Chem., 2014, 84, 146; DOI: https://doi.org/10.1016/j.ejmech.2014.07.026.
M. N. Elinson, A. N. Vereshchagin, Y. E. Anisina, N. A. Leonova, M. P. Egorov, Mendeleev Commun., 2020, 30, 15; DOI: https://doi.org/10.1016/j.mencom.2020.01.005.
M. N. Elinson, A. N. Vereshchagin, Y. E. Ryzhkova, S. K. Krymov, N. A. Leonova, A. S. Goloveshkin, M. P. Egorov, Mendeleev Commun., 2020, 30, 223; DOI: https://doi.org/10.1016/j.mencom.2020.03.031.
M. N. Elinson, O. O. Sokolova, R. F. Nasybullin, Heterocycl. Commun., 2015, 21, 97; DOI: https://doi.org/10.1515/hc-2015-0046.
M. N. Elinson, R. F. Nasybullin, F. V. Ryzhkov, M. P. Egorov, C. R. Chim., 2014, 17, 437; DOI: https://doi.org/10.1016/j.crci.2013.08.002.
A. N. Vereshchagin, M. N. Elinson, T. A. Zaimovskaya, G. I. Nikishin, Tetrahedron, 2008, 64, 9766; DOI: https://doi.org/10.1016/j.tet.2008.07.060.
M. N. Elinson, A. N. Vereshchagin, Y. E. Ryzhkova, K. A. Karpenko, I. E. Ushakov, Mendeleev Commun., 2021, 31, 698; DOI: https://doi.org/10.1016/j.mencom.2021.09.035.
A. N. Vereshchagin, M. N. Elinson, F. V. Ryzhkov, R. F. Nasybullin, S. I. Bobrovsky, A. S. Goloveshkin, M. P. Egorov, C. R. Chim., 2015, 18, 1344; DOI: https://doi.org/10.1016/j.crci.2015.02.005.
M. N. Elinson, E. O. Dorofeeva, G. I. Nikishin, Russ. Chem. Rev., 2015, 84, 485; DOI: https://doi.org/10.1070/RCR4465.
M. N. Elinson, R. F. Nasybullin, G. I. Nikishin, C. R. Chim., 2013, 16, 789; DOI: https://doi.org/10.1016/j.crci.2013.03.003.
M. N. Elinson, A. N. Vereshchagin, Y. E. Ryzhkova, K. A. Karpenko, F. V. Ryzhkov, M. P. Egorov, Chem. Heterocycl. Comp., 2021, 57, 274; DOI: https://doi.org/10.1007/s10593-021-02904-8.
F. V. Ryzhkov, Y. E. Ryzhkova, M. N. Elinson, A. N. Vereshchagin, V. A. Korolev, M. P. Egorov, Chem. Heterocycl. Comp., 2020, 56, 1560; DOI: https://doi.org/10.1007/s10593-020-02850-x.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no competing interests.
Additional information
No human or animal subjects were used in this research.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 3, pp. 464–473, March, 2022.
Rights and permissions
About this article
Cite this article
Elinson, M.N., Vereshchagin, A.N., Ryzhkova, Y.E. et al. Four-component transformation of benzaldehydes, dimethylbarbituric acid, 4-hydroxy-6-methyl-2H-pyran-2-one, and morpholine into the unsymmetrical ionic scaffold with three different heterocyclic rings. Russ Chem Bull 71, 464–473 (2022). https://doi.org/10.1007/s11172-022-3434-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-022-3434-1