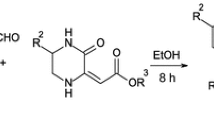
Multicomponent reactions employ three or more reactants to obtain heterocycles containing fragments of all starting materials in a onepot process. All new bonds are formed at once, hence multicomponent reactions are characterized by high bond-forming index. A new multicomponent, one-pot reaction yielding previously unknown 5-(4-hydroxy-2-oxo-2H-chromen-3-yl)-5H-chromeno[2,3-b]pyridines in 52–94% yield has been found. This multicomponent approach allows to construct four new bonds to synthesize some 5H-chromeno[2,3-b]-pyridines under mild conditions. Molecular docking and dynamics studies of the synthesized structures were carried out to identify their interaction with the binding pocket of the neuropeptide Y1 receptor.










Similar content being viewed by others
References
Rössner, S. Int. J. Obes. 2002, 26, S2.
Ogden, C. L.; Carroll, M. D.; Kit, B. K.; Flegal, K. M. JAMA 2014, 311, 806.
Holtcamp, W. Environ. Health Perspect. 2012, 120, a62.
Garfinkel, L. Ann. Intern. Med. 1985, 103, 1034.
Hammoud, A. O.; Gibson, M.; Peterson, C. M.; Meikle, A. W.; Carrell, D. T. Fertil. Steril. 2008, 90, 897.
Sandmark, H.; Hogstedt, C.; Lewold, S.; Vingård, E. Ann. Rheum. Dis. 1999, 58, 151.
Zhang, L.; Bijker, M. S.; Herzog, H. Pharmacol. Ther. 2011, 131, 91.
Morales-Medina, J. C.; Dumont, Y.; Quirion, R. Brain Res. 2010, 1314, 194.
Yulyaningsih, E.; Zhang, L.; Herzog, H.; Sainsbury, A. Br. J. Pharmacol. 2011, 163, 1170.
Clarke, P. A.; Santos, S.; Martin, W. H. C. Green Chem. 2007, 9, 438.
Newhouse, T.; Baran, P. S.; Hoffmann, R. W. Chem. Soc. Rev. 2009, 38, 3010.
Hayashi, Y. Chem. Sci. 2016, 7, 866.
Khasanov, A. F.; Kopchuk, D. S.; Kim, G. A.; Slepukhin, P. A.; Kovalev, I. S.; Santra, S.; Zyryanov, G. V.; Majee, A.; Chupakhin, O. N.; Charushin, V. N. ChemistrySelect 2018, 3, 340.
Charushin, V. N.; Chupakhin, O. N. Russ. Chem. Bull., Int. Ed. 2019, 68, 453. [Izv. Akad. Nauk, Ser. Khim. 2019, 453.]
Dömling, A.; Wang, K.; Wang, W. Chem Rev. 2012, 112, 3083.
Tu, X.-J.; Fan, W.; Hao, W.-J.; Jiang, B.; Tu, S.-J. ACS Comb. Sci. 2014, 16, 647.
Abdelmoniem, A. M.; Ghozlan, S. A. S.; Abdelmoniem, D. M.; Elwahy, A. H. M.; Abdelhamid, I. A. J. Heterocycl. Chem. 2017, 54, 2844.
Bardasov, I. N.; Alekseeva, A. U.; Mihailov, D. L.; Ershov, O. V.; Grishanov, D. A. Tetrahedron Lett. 2015, 56, 1830.
Zhang, W.; Wang, J.; Mao, J.; Hu, L.; Wu, X.; Guo, C. Tetrahedron Lett. 2016, 57, 1985.
Olyaei, A.; Shahsavari, M. S.; Sadeghpour, M. Res. Chem. Intermed. 2018, 44, 943.
Elinson, M. N.; Ryzhkov, F. V.; Nasybullin, R. F.; Vereshchagin, A. N.; Egorov, M. P. Helv. Chim. Acta 2016, 99, 724.
Elinson, M. N.; Vereshchagin, A. N.; Ryzhkov, F. V.; Anisina, Y. E. ARKIVOC 2018, (iv), 276.
Elinson, M. N.; Ryzhkov, F. V.; Korolev, V. A.; Egorov, M. P. Heterocycl. Commun. 2016, 22, 11.
Vereshchagin, A. N.; Elinson, M. N.; Anisina, Y. E.; Ryzhkov, F. V.; Goloveshkin, A. S.; Bushmarinov, I. S.; Zlotin, S. G.; Egorov, M. P. Mendeleev Commun. 2015, 25, 424.
Elinson, M. N.; Vereshchagin, A. N.; Anisina, Y. E.; Fakhrutdinov, A. N.; Goloveshkin, A. S.; Egorov, M. P. Eur. J. Org. Chem. 2019, 4171.
Elinson, M. N.; Vereshchagin, A. N.; Anisina, Y. E.; Krymov, S. K.; Fakhrutdinov, A. N.; Egorov, M. P. Monatsh. Chem. 2019, 150, 1073.
Elinson, M. N.; Vereshchagin, A. N.; Anisina, Y. E.; Krymov, S. K.; Fakhrutdinov, A. N.; Goloveshkin, A. S.; Egorov, M. P. Mol. Diversity 2020, 24, 617.
Vereshchagin, A. N.; Elinson, M. N.; Anisina, Y. E.; Ryzhkov, F. V.; Novikov, R. A.; Egorov, M. P. ChemistrySelect 2017, 2, 4593.
Vereshchagin, A. N.; Elinson, M. N.; Anisina, Y. E.; Ryzhkov, F. V.; Goloveshkin, A. S.; Novikov, R. A.; Egorov, M. P. J. Mol. Struct. 2017, 1146, 766.
Elinson, M. N.; Nasybullin, R. F.; Ryzhkov, F. V; Egorov, M. P. C. R. Chim. 2014, 17, 437.
Elinson, M. N.; Nasybullin, R. F.; Ryzhkov, F. V.; Zaimovskaya, T. A.; Nikishin, G. I. Monatsh. Chem. 2015, 146, 631.
Elinson, M. N.; Vereshchagin, A. N.; Anisina, Y. E.; Krymov, S. K.; Fekhrutdinov, A. N.; Egorov, M. P. Mendeleev Commun. 2019, 29, 581.
Elinson, M. N.; Vereshchagin, A. N.; Anisina, Y. E.; Krymov, S. K.; Fakhrutdinov, A. N.; Egorov, M. P. ARKIVOC 2019, (ii), 38.
Elinson, M. N.; Vereshchagin, A. N.; Anisina, Y. E.; Goloveshkin, A. S.; Ushakov, I. E.; Egorov, M. P. Mendeleev Commun. 2018, 28, 372.
Patai, S.; Israeli, Y. J. Chem. Soc. 1960, 2025.
T.E.S.T., Version 4.2.; US EPA. 2016. https://www.epa.gov.edgekeystaging.net/chemical-research/toxicity-estimation-softwaretool- test; Accessed March 23, 2020.
Martin, T. M.; Lilavois, C. R.; Barron, M. G. SAR QSAR Environ. Res. 2017, 28, 525.
Calculation of Molecular Properties and Bioactivity, Molinspiration. http://www.molinspiration.com/; Accessed March 12, 2020.
Rudolf, K.; Eberlein, W.; Engel, W.; Wieland, H. A.; Willim, K. D.; Entzeroth, M.; Wienen, W.; Beck-Sickinger, A. G.; Doods, H. N. Eur. J. Pharmacol. 1994, 271, R11.
Stanley, B. G.; Kyrkouli, S. E.; Lampert, S.; Leibowitz, S. F. Peptides 1986, 7(6), 1189.
LeadFinder 1.0; Biomoltech, 2011. https://www.cressetgroup.com/software/lead-finder/ and http://www.biomoltech.com/;Accessed March 12, 2020.
Stroganov, O. V.; Novikov, F. N.; Stroylov, V. S.; Kulkov, V.; Chilov, G. G. J. Chem. Inf. Model. 2008, 48, 2371.
Yang, Z.; Han, S.; Keller, M.; Kaiser, A.; Bender, B. J.; Bosse, M.; Burkert, K.; Kögler, L. M.; Wifling, D.; Bernhardt, G.; Plank, N.; Littmann, T.; Schmidt, P.; Yi, C.; Li, B.; Ye, S.; Zhang, R.; Xu, B.; Larhammar, D.; Stevens, R. C.; Huster, D.; Meiler, J.; Zhao, Q.; Beck-Sickinger, A. G.; Buschauer, A.; Wu, B. Nature 2018, 556, 520.
The PyMOL Molecular Graphics System, Version 1.2r3pre; Schrödinger, LLC, 2019. https://pymol.org/; Accessed March 20, 2020.
Lemkul, J. A. Living J. Comput. Mol. Sci. 2018, 1, 5068.
Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; Mackerell, A. D. J. Comput. Chem. 2010, 31, 671.
Jorgensen, W. L.; Chandrasekhar, J.; Madura, J. D.; Impey, R. W.; Klein, M. L. J. Chem. Phys. 1983, 79, 926.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2020, 56(12), 1560–1568
Supplementary Information
ESM 1
(PDF 1803 kb)
Rights and permissions
About this article
Cite this article
Ryzhkov, F.V., Ryzhkova, Y.E., Elinson, M.N. et al. Quadruple Bond Forming Multicomponent Approach to 5-(3-chromenyl)-5H-chromeno[2,3-b]pyridines and Its Interaction with the Neuropeptide Y1 Receptor. Chem Heterocycl Comp 56, 1560–1568 (2020). https://doi.org/10.1007/s10593-020-02850-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-020-02850-x