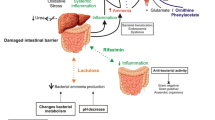
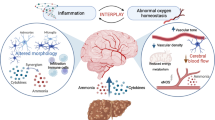
Abstract
The incidence of chronic liver disease is on the rise. One of the primary causes of hospital admissions for patients with cirrhosis is hepatic encephalopathy (HE), a debilitating neurological complication. HE is defined as a reversible syndrome, yet there is growing evidence stating that, under certain conditions, HE is associated with permanent neuronal injury and irreversibility. The pathophysiology of HE primarily implicates a strong role for hyperammonemia, but it is believed other pathogenic factors are involved. The fibrotic scarring of the liver during the progression of chronic liver disease (cirrhosis) consequently leads to increased hepatic resistance and circulatory anomalies characterized by portal hypertension, hyperdynamic circulatory state and systemic hypotension. The possible repercussions of these circulatory anomalies on brain perfusion, including impaired cerebral blood flow (CBF) autoregulation, could be implicated in the development of HE and/or permanent brain injury. Furthermore, hypotensive insults incurring during gastrointestinal bleed, infection, or liver transplantation may also trigger or exacerbate brain dysfunction and cell damage. This review will focus on the role of hypotension in the onset of HE as well as in the occurrence of neuronal cell loss in cirrhosis.



Similar content being viewed by others
Abbreviations
- BBB:
-
Blood-brain barrier
- CBF:
-
Cerebral blood flow
- CLD:
-
Chronic liver disease
- EC:
-
Endothelial cells
- GI:
-
Gastrointestinal
- HE:
-
Hepatic encephalopathy
- LT:
-
Liver transplant
- MAP:
-
Mean arterial pressure
- NO:
-
Nitric oxide
- RI:
-
Reperfusion injury
References
Tapper EB, Parikh ND (2018) Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: observational study. BMJ 362:k2817. https://doi.org/10.1136/BMJ.K2817
National Center for Health Statistics Chronic Liver Disease and Cirrhosis. https://www.cdc.gov/nchs/fastats/liver-disease.htm
Zhou WC, Zhang QB, Qiao L (2014) Pathogenesis of liver cirrhosis. World J Gastroenterol 20:7312–7324. https://doi.org/10.3748/wjg.v20.i23.7312
Ochoa-Sanchez R, Rose CF (2018) Pathogenesis of hepatic Encephalopathy in Chronic Liver Disease. J Clin Exp Hepatol 8:262–271. https://doi.org/10.1016/j.jceh.2018.08.001
Shifflet A, Wu GY (2009) Non-alcoholic steatohepatitis: an overview. J Formos Med Assoc 108:4–12
Bataller R, Brenner DA (2005) Liver fibrosis. J Clin Invest 115:209–218. https://doi.org/10.1172/JCI24282
Yang L, Yang C, Thomes PG et al (2019) Lipophagy and alcohol-induced fatty liver. Front Pharmacol 10:495. https://doi.org/10.3389/fphar.2019.00495
Parola M, Pinzani M (2019) Liver fibrosis: pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med 65:37–55
Gustot T, Stadlbauer V, Laleman W et al (2021) Transition to decompensation and acute-on-chronic liver failure: role of predisposing factors and precipitating events. J Hepatol 75:S36–S48. https://doi.org/10.1016/j.jhep.2020.12.005
Poordad FF (2015) Presentation and complications associated with cirrhosis of the liver. Curr Med Res Opin 31:925–937
Tsoris A, Marlar C (2020) Use Of The Child Pugh Score In Liver Disease. In: StatPearls. [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan. 2022 Mar 18. https://www.ncbi.nlm.nih.gov/books/NBK542308/
Shah NJ, Mousa OY, Syed K, et al. Acute On Chronic Liver Failure. [Updated 2022 Oct 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499902/
Arroyo V, Moreau R, Jalan R (2020) Acute-on-Chronic Liver Failure. 382:2137-2145 https://doi.org/10.1056/NEJMra1914900
Moreau R, Jalan R, Gines P et al (2013) Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 144:1426–1437e9. https://doi.org/10.1053/j.gastro.2013.02.042
Weissenborn K (2019) Hepatic encephalopathy: definition, clinical Grading and Diagnostic Principles. Drugs 79:5–9
Labenz C, Toenges G, Schattenberg JM et al (2019) Health-related quality of life in patients with compensated and decompensated liver cirrhosis. Eur J Intern Med 70:54–59. https://doi.org/10.1016/j.ejim.2019.09.004
Dhiman RK, Sawhney MS, Chawla YK et al (2000) Efficacy of lactulose in cirrhotic patients with subclinical hepatic encephalopathy. Dig Dis Sci 45:1549-52. https://doi.org/10.1023/a:1005556826152
Allampati S, Duarte-Rojo A, Thacker LR et al (2016) Diagnosis of minimal hepatic Encephalopathy using Stroop EncephalApp: a multicenter US-Based, norm-based study. Am J Gastroenterol 111:78–86. https://doi.org/10.1038/ajg.2015.377
Afzal S, Ahmad M (2011) Precipitating factors of Hepatic Coma. Annals of KEMU 7:213.
Romero-Gómez M, Jover M, Galán JJ, Ruiz A (2009) Gut ammonia production and its modulation. Metab Brain Dis 24:147–157. https://doi.org/10.1007/s11011-008-9124-3
Litwack G (2018) Chap. 13- metabolism of amino acids. Human biochemistry. Academic Press, pp 359–394
Ahluwalia V, Betrapally NS, Hylemon PB et al (2016) Impaired gut-liver-brain Axis in patients with cirrhosis. Sci Rep 6:26800. https://doi.org/10.1038/srep26800
Bosoi CR, Rose CF (2009) Identifying the direct effects of ammonia on the brain. Metab Brain Dis 25:95–102. https://doi.org/10.1142/7114
Rose C, Felipo V (2005) Limited capacity for ammonia removal by brain in chronic liver failure: potential role of nitric oxide. Metab Brain Dis 20:275–283. https://doi.org/10.1007/s11011-005-7906-4
Sepehrinezhad A, Zarifkar A, Namvar G et al (2020) Astrocyte swelling in hepatic encephalopathy: molecular perspective of cytotoxic edema. Metab Brain Dis. https://doi.org/10.1007/s11011-020-00549-8
Leite MC, Brolese G, de Almeida LMV et al (2006) Ammonia-induced alteration in S100B secretion in astrocytes is not reverted by creatine addition. Brain Res Bull 70:179–185. https://doi.org/10.1016/j.brainresbull.2006.05.003
Brusilow SW, Koehler RC, Traystman RJ, Cooper AJL (2010) Astrocyte glutamine synthetase: Importance in Hyperammonemic Syndromes and potential target for Therapy. Neurotherapeutics 7:452–470. https://doi.org/10.1016/j.nurt.2010.05.015
Cichoz-Lach H, Michalak A (2013) Current pathogenetic aspects of hepatic encephalopathy and noncirrhotic hyperammonemic encephalopathy. World J Gastroenterol 19:26–34. https://doi.org/10.3748/wjg.v19.i1.26
Butterworth RF (2008) Pathophysiology of hepatic encephalopathy: the concept of synergism. Hepatol Res 38:S116-21 https://doi.org/10.1111/j.1872-034X.2008.00436.x.
Bajaj JS, Schubert CM, Heuman DM et al (2010) Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology 138:2332–2340. https://doi.org/10.1053/j.gastro.2010.02.015
Agarwal AN, Mais DD (2019) Sensitivity and specificity of Alzheimer type II astrocytes in hepatic encephalopathy. Arch Pathol Lab Med 143:1256–1258. https://doi.org/10.5858/arpa.2018-0455-OA
Zeneroli ML, Cioni G, Vezzelli C et al (1987) Prevalence of brain atrophy in liver cirrhosis patients with chronic persistent encephalopathy. Evaluation by computed tomography. J Hepatol 4:283–292. https://doi.org/10.1016/S0168-8278(87)80536-6
Albhaisi SAM, Bajaj JS (2020) Cognitive function in liver transplantation. Curr Transpl Rep 7:31–37. https://doi.org/10.1007/s40472-020-00274-2
Weiss N, Thabut D (2019) Neurological complications occurring after liver transplantation: role of risk factors, hepatic Encephalopathy, and Acute (on Chronic) Brain Injury. Liver Transpl 25:469–487. https://doi.org/10.1002/lt.25420
Dhar R, Young GB, Marotta P (2008) Perioperative neurological complications after liver transplantation are best predicted by pre-transplant hepatic encephalopathy. Neurocrit Care 8:253–258. https://doi.org/10.1007/s12028-007-9020-4
García-Lezana T, Oria M, Romero-Giménez J et al (2017) Cerebellar neurodegeneration in a new rat model of episodic hepatic encephalopathy. J Cereb Blood Flow Metab 37:927–937. https://doi.org/10.1177/0271678X16649196
Ochoa-Sanchez R, Tamnanloo F, Rose CF (2021) Hepatic encephalopathy: from metabolic to neurodegenerative. Neurochem Res 46:2612-2625 https://doi.org/10.1007/s11064-021-03372-4
Granger DN, Kvietys PR (2004) Circulation, overview. Encyclopedia of Gastroenterology. Elsevier, pp 351–355
Buob S, Johnston AN, Webster CRL (2011) Portal hypertension: pathophysiology, diagnosis, and treatment. J Vet Intern Med 25:169–186
Kumar A, Praveen S, Sarin S (2008) Hepatic venous pressure gradient measurement:time to learn! Indian J Gastroenterol 27:74–80
Groszmann RJ, Wongcharatrawee S (2004) The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology 39:280–282
McConnell M, Iwakiri Y (2018) Biology of portal hypertension. Hepatol Int 12:11–23
Jairath V, Desborough MJR (2015) Modern-day management of upper gastrointestinal haemorrhage. Transfus Med 25:351–357. https://doi.org/10.1111/tme.12266
Vora RS, Subramanian RM (2019) Hypotension in cirrhosis. Clin Liver Dis (Hoboken) 13:149–153
Iwakiri Y, Groszmann RJ (2006) The Hyperdynamic Circulation of Chronic Liver Diseases: From the Patient to the Molecule. Hepatology 43:S121-31 https://doi.org/10.1002/hep.20993
Tarquini R, Masini E, LaVilla G et al (2009) Increased plasma Carbon Monoxide in patients with viral cirrhosis and hyperdynamic circulation. Am J Gastroenterol 104:891–897. https://doi.org/10.1038/ajg.2009.2
García-Pagán JC, Gracia-Sancho J, Bosch J (2012) Functional aspects on the pathophysiology of portal hypertension in cirrhosis. J Hepatol 57:458–461. https://doi.org/10.1016/j.jhep.2012.03.007
Vilar Gomez E, Torres Gonzalez A, Calzadilla Bertot L et al (2014) Arterial blood pressure is closely related to ascites development in compensated HCV-related cirrhosis. PLoS ONE 9:e95736. https://doi.org/10.1371/journal.pone.0095736
Fernandez-Seara J, Prieto J, Quiroga J et al (1989) Systemic and regional hemodynamics in patients with liver cirrhosis and ascites with and without functional renal failure. Gastroenterology 97:1304–1312. https://doi.org/10.1016/0016-5085(89)91704-6
Salerno F, Gerbes A, Ginès P et al (2008) Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Postgrad Med J 84:662–670
Ruiz-Del-Arbol L, Serradilla R (2015) Cirrhotic cardiomyopathy. World J Gastroenterol 21:11502–11521. https://doi.org/10.3748/wjg.v21.i41.11502
Pere P, Höckerstedt K, Isoniemi H, Lindgren L (2000) Cerebral blood flow and oxygenation in liver transplantation for acute or chronic hepatic disease without venovenous bypass. Liver Transpl 6:471–479. https://doi.org/10.1053/jlts.2000.8186
de Oliveira THC, Marques PE, Proost P, Teixeira MMM (2018) Neutrophils: a cornerstone of liver ischemia and reperfusion injury. Lab Invest 98:51–62. https://doi.org/10.1038/labinvest.2017.90
Bañares R, Bañares B, Moitinho E et al (1999) Carvedilol, a New Nonselective Beta-Blocker with intrinsic Anti-Alpha 1-Adrenergic activity. Has a Greater Portal Hypotensive Effect Than Propranolol in Patients With Cirrhosis. Hepatology 1999 30:79-83 https://doi.org/10.1002/hep.510300124
Tripathi D, Hayes PC (2014) Beta-blockers in portal hypertension: new developments and controversies. Liver Int 34:655–667. https://doi.org/10.1111/liv.12360
Leithead JA, Hayes PC, Ferguson JW (2014) Review article: advances in the management of patients with cirrhosis and portal hypertension-related renal dysfunction. Aliment Pharmacol Ther 39:699–711
Garcia-Tsao G (2001) Current management of the complications of cirrhosis and portal hypertension: Variceal hemorrhage, ascites, and spontaneous bacterial peritonitis. Gastroenterology 120:726–748. https://doi.org/10.1053/gast.2001.22580
Duschek S, Schandry R (2007) Reduced brain perfusion and cognitive performance to constitutional hypotension. Clin Auton Res 17:69–76
Morris M, Scherr P, Hebert L et al (2002) Association between blood pressure and cognitive function in a Biracial Community Population of older persons. Neuroepidemiology 21:123–130
Lécuyer M-AA, Kebir H, Prat A (2016) Glial influences on BBB functions and molecular players in immune cell trafficking. 1862:472–482
Finnerty FA, Witkins L, Fazekas JF (1954) Cerebral hemodynamics during cerebral ischemia induced by acute hypotension. J Clin Invest 33:1227–1232. https://doi.org/10.1172/JCI102997
Armstead WM (2016) Cerebral blood Flow Autoregulation and Dysautoregulation. Anesthesiol Clin 34:465–477
Berne RM, Winn HR, Rubio R (1981) The Local Regulation of Cerebral Blood Flow. Prog Cardiovasc Dis 24:243-60. https://doi.org/0.1016/0033-0620(81)90030-x
Peterson EC, Wang Z, Britz G (2011) Regulation of cerebral blood flow. Int J Vasc Med 2011:823525. https://doi.org/10.1155/2011/823525
Duschek S, Schandry R (2007) Reduced brain perfusion and cognitive performance due to constitutional hypotension. Clin Auton Res 17:69–76. https://doi.org/10.1007/s10286-006-0379-7
Frederiksen SD, Haanes KA, Warfvinge K, Edvinsson L (2019) Perivascular neurotransmitters: regulation of cerebral blood flow and role in primary headaches. J Cereb Blood Flow Metab 39:610–632
Ogoh S (2017) Relationship between cognitive function and regulation of cerebral blood flow. J Physiological Sci 67:345–351. https://doi.org/10.1007/s12576-017-0525-0
Koehler RC, Roman RJ, Harder DR (2009) Astrocytes and the regulation of cerebral blood flow. Trends Neurosci 32:160–169
Trewby PN, Williams R (1977) Pathophysiology of hypotension in patients with fulminant hepatic failure. Gut 18:1021–1026. https://doi.org/10.1136/gut.18.12.1021
Brady KM, Hudson A, Hood R et al (2020) Personalizing the definition of hypotension to protect the brain. Anesthesiology 132:170–179. https://doi.org/10.1097/ALN.0000000000003005
Grace PA (1994) Ischemia-reperfusion injury. Br J Surg 81:631–641
Morrison H, McKee D, Ritter L (2011) Systemic neutrophil activation in a mouse model of ischemic stroke and reperfusion. Biol Res Nurs 13:154–163. https://doi.org/10.1177/1099800410384500
Ito D, Tanaka K, Suzuki S et al (2001) Enhanced expression of Iba1, Ionized calcium-binding adapter molecule 1, after transient focal cerebral ischemia in rat brain. Stroke 32:1208–1215
L’Ecuyer S, Gilbert K, Brochu B et al (2021) Targeting Uric Acid prevents Brain Injury and anxiety in a rat model of hemorrhagic shock. Shock 56:298–307. https://doi.org/10.1097/SHK.0000000000001708
Nemirovich-Danchenko NM, Khodanovich MY (2019) New neurons in the post-ischemic and injured brain: migrating or resident? Front Neurosci 13:588
Moretti R, Torre P, Antonello RM et al (2008) Risk factors for vascular dementia: hypotension as a key point. Vasc Health Risk Manag 4:395–402. https://doi.org/10.2147/VHRM.S2434
Mccarron RM, Yu Z-Y, Ono S, Spatz M (2002) Effect of hemorrhagic shock on apoptosis and energy-dependent Efflux System in the brain. Neurochem Res 27:1625–1632
Puig B, Brenna S, Magnus T (2018) Molecular communication of a dying neuron in stroke. Int J Mol Sci 19:2834. https://doi.org/10.3390/ijms19092834
Nour M, Scalzo F, Liebeskind DS (2013) Ischemia-Reperfusion Injury in Stroke. Interv Neurol 1:185–199. https://doi.org/10.1159/000353125
Lochhead JJ, McCaffrey G, Quigley CE et al (2010) Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J Cereb Blood Flow Metab 30:1625–1636. https://doi.org/10.1038/jcbfm.2010.29
Frøkjær VG, Strauss GI, Mehlsen J et al (2006) Autonomic dysfunction and impaired cerebral autoregulation in cirrhosis. Clin Auton Res 16:208–216. https://doi.org/10.1007/s10286-006-0337-4
Larsen FS, Olsen KS, Ejlersen E et al (1995) Cerebral blood flow autoregulation and transcranial doppler sonography in patients with cirrhosis. Hepatology 22:730–736. https://doi.org/10.1016/0270-9139(95)90290-2
Bjerring PN, Gluud LL, Larsen FS (2018) Cerebral blood Flow and metabolism in hepatic Encephalopathy—A Meta-analysis. J Clin Exp Hepatol 8:286–293. https://doi.org/10.1016/j.jceh.2018.06.002
Henriksen JH, Moller S (2006) Liver cirrhosis and arterial hypertension. World J Gastroenterol 12:678–685
Toyoda K, Fujii K, Ibayashi S et al (1997) Role of nitric oxide in regulation of brain stem circulation during hypotension. J Cereb Blood Flow Metab 17:1089–1096. https://doi.org/10.1097/00004647-199710000-00011
Hendrickse M, Triger D (1992) Peripheral and cardiovascular autonomic impairment in chronic liver disease: prevalence and relation to hepatic function. J Hepatol 16:177–183
Tzamouranis DG, Alexopoulou A, Dourakis SP, Stergiou GS (2010) Relationship of 24-hour ambulatory blood pressure and heart rate with markers of hepatic function in cirrhotic patients. BMC Gastroenterol 10:143. https://doi.org/10.1186/1471-230X-10-143
Merli M, Nicolini G, Angeloni S et al (2003) Incidence and natural history of small esophageal varices in cirrhotic patients. J Hepatol 38:266–272. https://doi.org/10.1016/S
Benigni A, Boccardo P, Galbusera M et al (1993) Reversible activation defect of the platelet glycoprotein IIb-IIIa Complex in Patients With Uremia Am J Kidney Dis 22:668-76. https://doi.org/10.1016/s0272-6386(12)80429-x.
Alam I, Haider I, Humayun M et al(2005) Spectrum of precipitating factors of hepatic encephalopathy in liver cirrhosis.Pakistan J Med Res44
Mumtaz K, Ahmed U, Abid S et al (2010) Precipitating factors and the Outcome. of Hepatic Encephalopathy in Liver Cirrhosis. J Coll Physicians Surg Pak 20:514-8.
Olde Damink SWM, Dejong CHC, Deutz NEP, Soeters PB (1997) Effects of simulated Upper Gastrointestinal Hemorrhage on Ammonia and related amino acids in blood and. Brain of Chronic Portacaval-shunted Rats. Metab Brain Dis 12:121-35
Strnad P, Tacke F, Koch A, Trautwein C (2017) Liver-guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol 14:55–66
Bajaj JS, Heuman DM, Hylemon PB et al (2014) Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol 60:940–947. https://doi.org/10.1016/j.jhep.2013.12.019
Ghosh G, Jesudian AB (2019) Small intestinal bacterial overgrowth in patients with cirrhosis. J Clin Exp Hepatol 9:257–267. https://doi.org/10.1016/j.jceh.2018.08.006
Wong F, Bernardi M, Balk R et al (2005) Sepsis in cirrhosis: report on the 7th meeting of the International Ascites Club. Gut 54:718–725. https://doi.org/10.1136/gut.2004.038679
Mattos AA, Wiltgen D, Jotz RF et al (2020) Spontaneous bacterial peritonitis and extraperitoneal infections in patients with cirrhosis. Ann Hepatol 19:451–457. https://doi.org/10.1016/j.aohep.2020.04.010
Sipeki N, Antal-Szalmas P, Lakatos PL, Papp M (2014) Immune dysfunction in cirrhosis. World J Gastroenterol 20:2564–2577. https://doi.org/10.3748/wjg.v20.i10.2564
Guarner C, Soriano G (2005) Bacterial translocation and its consequences in patients with liver cirrhosis. Eur J Gastroenterol Hepatol 17:27–31
Navasa M, Rodés J (2004) Bacterial infections in cirrhosis. Liver Int 24:277–280. https://doi.org/10.1111/j.1478-3231.2004.0934.x
Sharshar T, Gray F, Poron F et al (2002) Multifocal necrotizing leukoencephalopathy in septic shock. Crit Care Med 30:2371–2375. https://doi.org/10.1097/00003246-200210000-00031
Rose CF, Amodio P, Bajaj JS et al (2020) Hepatic encephalopathy: novel insights into classification, pathophysiology and therapy. J Hepatol 73:1526–1547. https://doi.org/10.1016/j.jhep.2020.07.013
Thooft A, Favory R, Salgado DR et al (2011) Effects of changes in arterial pressure on organ perfusion during septic shock. Crit Care 15:5–12. https://doi.org/10.1186/cc10462
Sonneville R, Verdonk F, Rauturier C et al (2013) Understanding brain dysfunction in sepsis. Ann Intensive Care 3:1–11. https://doi.org/10.1186/2110-5820-3-15
Hofer S, Bopp C, Hoerner C et al (2008) Injury of the blood brain barrier and Up-Regulation of ICAM-1 in Polymicrobial Sepsis. J Surg Res 146:276–281. https://doi.org/10.1016/j.jss.2007.07.021
Schuppan D, Afdhal NH (2008) Liver cirrhosis. The Lancet 371:838–851. https://doi.org/10.1016/S0140-6736(08)60383-9
Lee H-C, Ryu H-G, Jung C-W (2017) Performance measurement of intraoperative systolic arterial pressure to predict in-hospital mortality in adult liver transplantation OPEN. 7:7030. https://doi.org/10.1038/s41598-017-07664-0
Joosten A, Lucidi V, Ickx B et al (2021) Intraoperative hypotension during liver transplant surgery is associated with postoperative acute kidney injury: a historical cohort study. BMC Anesthesiol 21:1–10. https://doi.org/10.1186/s12871-020-01228-y
Gregory A, Stapelfeldt WH, Khanna AK et al (2021) Intraoperative hypotension is Associated with adverse clinical outcomes after noncardiac surgery. Anesth Analg 132:1654–1665. https://doi.org/10.1213/ANE.0000000000005250
Feltracco P, Brezzi ML, Barbieri S et al (2013) Blood loss, predictors of bleeding, transfusion practice and strategies of blood cell salvaging during liver transplantation. World J Hepatol 5:1–15. https://doi.org/10.4254/wjh.v5.i1.1
Donohue CI, Mallett S, v, Perioperative RF (2015) Reducing transfusion requirements in liver transplantation. World J Transplant 5:165–182. https://doi.org/10.5500/wjt.v5.i4.165
Carrier FM, Sylvestre MP, Massicotte L et al (2020) Effects of intraoperative hemodynamic management on postoperative acute kidney injury in liver transplantation: an observational cohort study. PLoS ONE 15. https://doi.org/10.1371/journal.pone.0237503
Philips BJ, Armstrong IR, Pollock A, Alistair L (1998) Cerebral blood flow and metabolism in patients with chronic liver disease undergoing orthotopic liver transplantation. Hepatology 27:369–376. https://doi.org/10.1002/hep.510270209
Dar WA, Sullivan E, Bynon JS et al (2019) Ischaemia reperfusion injury in liver transplantation: Cellular and molecular mechanisms. Liver Int 39:788–801. https://doi.org/10.1111/liv.14091
Novak V, Novak P, Spies J, Low P (1998) Autoregulation of cerebral blood Flow in Orthostativ Hypotension. Stroke 21:104–111. https://doi.org/10.1016/B978-0-12-802381-5.00011-7
Felipo V, Urios A, Montesinos E et al (2012) Contribution of hyperammonemia and inflammatory factors to cognitive impairment in minimal hepatic encephalopathy. Metab Brain Dis 27:51–58. https://doi.org/10.1007/s11011-011-9269-3
Zheng G, Lu H, Yu W et al (2017) Severity-specific alterations in CBF, OEF and CMRO2 in cirrhotic patients with hepatic encephalopathy. Eur Radiol 27:4699–4709. https://doi.org/10.1007/s00330-017-4809-9
Dam M, Burra P, Tedeshi U et al (1998) Regional cerebral blood flow changes in patients with cirrhosis assessed with 99mTc-HM-PAO single-photon emission computed tomography: effect of liver transplantation. J Hepatol 29:79–84
Clément M, Bosoi CR, Oliveira MM et al (2021) Bile-duct ligation renders the brain susceptible to hypotension‐induced neuronal degeneration: implications of ammonia. J Neurochem 157:161-173 https://doi.org/10.1111/jnc.15290
Groiss SJ, Butz M, Baumgarten TJ et al (2019) GABA-ergic tone hypothesis in hepatic encephalopathy – revisited. Clin Neurophysiol 130:911–916. https://doi.org/10.1016/j.clinph.2019.03.011
Funding
SLE is a recipient of a Doctoral Training Scholarship from Fonds de Recherche du Québec-Santé. CFR is supported through CIHR grant..
Author information
Authors and Affiliations
Contributions
SLE lead the work for this manuscript. All the authors contributed to the literature research, format of the review and writing of the manuscript.
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
L’Écuyer, S., Charbonney, E., Carrier, F.M. et al. Implication of Hypotension in the Pathogenesis of Cognitive Impairment and Brain Injury in Chronic Liver Disease. Neurochem Res (2023). https://doi.org/10.1007/s11064-022-03854-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11064-022-03854-z