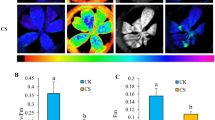
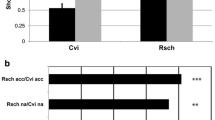
Abstract
Background
As an alpine plant, Rhododendron chrysanthum (R. chrysanthum) has evolved cold resistance mechanisms and become a valuable plant resource with the responsive mechanism of cold stress.
Methods and results
We adopt the phosphoproteomic and proteomic analysis combining with physiological measurement to illustrate the responsive mechanism of R. chrysanthum seedling under cold (4 °C) stress. After chilling for 12 h, 350 significantly changed proteins and 274 significantly changed phosphoproteins were detected. Clusters of Orthologous Groups (COG) analysis showed that significantly changed phosphoproteins and proteins indicated cold changed energy production and conversion and signal transduction.
Conclusions
The results indicated photosynthesis was inhibited under cold stress, but cold induced calcium-mediated signaling, reactive oxygen species (ROS) homeostasis and other transcription regulation factors could protect plants from the destruction caused by cold stress. These data provide the insight to the cold stress response and defense mechanisms of R. chrysanthum leaves at the phosphoproteome level.






Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ALDH:
-
NAD(P) + − dependent aldehyde dehydrogenases
- CaM-BP:
-
CaM-binding protein
- CaM:
-
Calcium
- CAT:
-
Catalase
- COG:
-
Clusters of Orthologous Groups of proteins
- MAP3K:
-
Epsilon protein kinase 1
- NDPK2:
-
Nucleoside diphosphate kinase 2 protein kinase
- OEC:
-
Photosystem II oxygen evolving complex
- POD:
-
Peroxidase
- SOD:
-
Superoxide dismutase
- TMT Labeling:
-
Tandem mass tags labeling
- STP:
-
Serine/threonine-protein kinase
References
Song JB, Gao S, Wang Y et al (2016) miR394 and its target gene LCR are involved in cold stress response in Arabidopsis. Plant Gene 5:56–64. https://doi.org/10.1016/j.plgene.2015.12.001
Orvar BL, Sangwan V, Omann F et al (2000) Early steps in cold sensing by plant cells: the role of actin cytoskeleton and membrane fluidity. Plant J 23:785–794. https://doi.org/10.1046/j.1365-313x.2000.00845.x
Ding Y, Shi Y, Yang S (2019) Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol 222:1690–1704. https://doi.org/10.1111/nph.15696
Huang W, Zhang S-B, Cao K-F (2010) The different effects of chilling stress under moderate light intensity on photosystem II compared with photosystem I and subsequent recovery in tropical tree species. Photosynth Res 103:175–182. https://doi.org/10.1007/s11120-010-9539-7
Janmohammadi M, Zolla L, Rinalducci S (2015) Low temperature tolerance in plants: Changes at the protein level. Phytochemistry 117:76–89. https://doi.org/10.1016/j.phytochem.2015.06.003
Wilkins KA, Matthus E, Swarbreck SM et al (2016) Calcium-mediated abiotic stress signaling in roots. Front Plant Sci 7:1296. https://doi.org/10.3389/fpls.2016.01296
Yuan P, Yang T, Poovaiah BW (2018) Calcium signaling-mediated plant response to cold stress. IJMS 19:3896. https://doi.org/10.3390/ijms19123896
Gill MB, Zeng F, Shabala L et al (2019) Identification of QTL related to ROS formation under hypoxia and their association with waterlogging and salt tolerance in Barley. Int J Mol Sci. https://doi.org/10.3390/ijms20030699
Mittler R, Vanderauwera S, Gollery M et al (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498. https://doi.org/10.1016/j.tplants.2004.08.009
Kamal MM, Ishikawa S, Takahashi F et al (2020) Large-scale phosphoproteomic study of arabidopsis membrane proteins reveals early signaling events in response to cold. Int J Mol Sci. https://doi.org/10.3390/ijms21228631
Rampitsch C, Bykova NV (2012) The beginnings of crop phosphoproteomics: exploring early warning systems of stress. Front Plant Sci 3:144. https://doi.org/10.3389/fpls.2012.00144
Hsu C-C, Zhu Y, Arrington JV et al (2018) Universal plant phosphoproteomics workflow and its application to tomato signaling in response to cold stress. Mol Cell Proteomics 17:2068–2080. https://doi.org/10.1074/mcp.TIR118.000702
Pi Z, Zhao M-L, Peng X-J et al (2017) Phosphoproteomic analysis of paper mulberry reveals phosphorylation functions in chilling tolerance. J Proteome Res 16:1944–1961. https://doi.org/10.1021/acs.jproteome.6b01016
Lu Z-S, Chen Q-S, Zheng Q-X et al (2019) Proteomic and phosphoproteomic analysis in tobacco mosaic virus-infected tobacco (Nicotiana tabacum). Biomolecules. https://doi.org/10.3390/biom9020039
Zhou X, Chen S, Wu H et al (2017) Biochemical and proteomics analyses of antioxidant enzymes reveal the potential stress tolerance in Rhododendron chrysanthum Pall. Biol Direct 12:10. https://doi.org/10.1186/s13062-017-0181-6
Singh M (2000) Turnover of D1 Protein Encoded by psbA Gene in Higher Plants and Cyanobacteria Sustains Photosynthetic Efficiency to Maintain Plant Productivity Under Photoinhibitory Irradiance. Photosynthetica 38:161–169. https://doi.org/10.1023/A:1007297227403
Salonen M, Aro E-M, Rintamäki E (1998) Reversible phosphorylation and turnover of the D1 protein under various redox states of Photosystem II induced by low temperature photoinhibition. Photosynth Res 58:143–151. https://doi.org/10.1023/A:1006155223221
Koivuniemi A, Aro EM, Andersson B (1995) Degradation of the D1- and D2-proteins of photosystem II in higher plants is regulated by reversible phosphorylation. Biochemistry 34:16022–16029. https://doi.org/10.1021/bi00049a016
Nishiyama Y, Allakhverdiev SI, Yamamoto H et al (2004) Singlet oxygen inhibits the repair of photosystem II by suppressing the translation elongation of the D1 protein in Synechocystis sp. PCC 6803. Biochemistry 43:11321–11330. https://doi.org/10.1021/bi036178q
Merry R, Jerrard J, Frebault J et al (2017) A comparison of pine and spruce in recovery from winter stress; changes in recovery kinetics, and the abundance and phosphorylation status of photosynthetic proteins during winter. Tree Physiol 37:1239–1250. https://doi.org/10.1093/treephys/tpx065
Grebe S, Trotta A, Bajwa AA et al (2020) Specific thylakoid protein phosphorylations are prerequisites for overwintering of Norway spruce (Picea abies) photosynthesis. Proc Natl Acad Sci U S A 117:17499–17509. https://doi.org/10.1073/pnas.2004165117
Allahverdiyeva Y, Suorsa M, Rossi F et al (2013) Arabidopsis plants lacking PsbQ and PsbR subunits of the oxygen-evolving complex show altered PSII super-complex organization and short-term adaptive mechanisms. Plant J 75:671–684. https://doi.org/10.1111/tpj.12230
Tola AJ, Jaballi A, Germain H et al (2020) Recent development on plant aldehyde dehydrogenase enzymes and their functions in plant development and stress signaling. Genes (Basel). https://doi.org/10.3390/genes12010051
Piattoni CV, Ferrero DML, Dellaferrera I et al (2017) Cytosolic glyceraldehyde-3-phosphate dehydrogenase is phosphorylated during seed development. Front Plant Sci 8:522. https://doi.org/10.3389/fpls.2017.00522
Hsu CH, Hsu YT (2019) Biochemical responses of rice roots to cold stress. Bot Stud 60:14. https://doi.org/10.1186/s40529-019-0262-1
Qi W, Wang F, Ma L et al (2020) Physiological and biochemical mechanisms and cytology of cold tolerance in brassica napus. Front Plant Sci 11:1241. https://doi.org/10.3389/fpls.2020.01241
Mhamdi A, Queval G, Chaouch S et al (2010) Catalase function in plants: a focus on Arabidopsis mutants as stress-mimic models. J Exp Bot 61:4197–4220. https://doi.org/10.1093/jxb/erq282
Chang T-S, Jeong W, Choi SY et al (2002) Regulation of peroxiredoxin I activity by Cdc2-mediated phosphorylation. J Biol Chem 277:25370–25376. https://doi.org/10.1074/jbc.M110432200
Han F, Chen H, Li X-J et al (2009) A comparative proteomic analysis of rice seedlings under various high-temperature stresses. Biochim Biophys Acta 1794:1625–1634. https://doi.org/10.1016/j.bbapap.2009.07.013
Moon H, Lee B, Choi G et al (2003) NDP kinase 2 interacts with two oxidative stress-activated MAPKs to regulate cellular redox state and enhances multiple stress tolerance in transgenic plants. Proc Natl Acad Sci U S A 100:358–363. https://doi.org/10.1073/pnas.252641899
Andrews C, Xu Y, Kirberger M et al (2020) Structural aspects and prediction of calmodulin-binding proteins. Int J Mol Sci. https://doi.org/10.3390/ijms22010308
Reddy ASN, Ali GS, Celesnik H et al (2011) Coping with stresses: roles of calcium- and calcium/calmodulin-regulated gene expression. Plant Cell 23:2010–2032. https://doi.org/10.1105/tpc.111.084988
Garg G, Yadav S et al (2015) Key roles of calreticulin and calnexin proteins in plant perception under stress conditions: A review. Advances in Life Sciences 5:18–26
Takemiya A, Sugiyama N, Fujimoto H et al (2013) Phosphorylation of BLUS1 kinase by phototropins is a primary step in stomatal opening. Nat Commun 4:2094. https://doi.org/10.1038/ncomms3094
Liu H, Wang F-F, Peng X-J et al (2019) Global phosphoproteomic analysis reveals the defense and response mechanisms of jatropha curcas seedling under chilling stress. Int J Mol Sci. https://doi.org/10.3390/ijms20010208
Funding
This work was mainly supported by the National Natural Science Foundation of China (31070224) and the Science and Technology Department of Jilin Province (20130206059NY).
Author information
Authors and Affiliations
Contributions
XZ and HX designed the research; YL and HF prepared the plant materials for sequencing. YL carried out bioinformatics analysis of data; YL, HF and JC performed the experiments and statistical analyses; YL and JD collected data and researched literature, YL interpreted the data and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, Y., Fan, H., Dong, J. et al. Phosphoproteomics of cold stress-responsive mechanisms in Rhododendron chrysanthum. Mol Biol Rep 49, 303–312 (2022). https://doi.org/10.1007/s11033-021-06874-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06874-0