Abstract
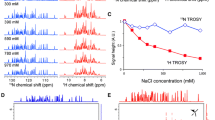
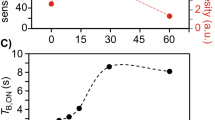
Detection of 15N in multidimensional NMR experiments of proteins has sparsely been utilized because of the low gyromagnetic ratio (γ) of nitrogen and the presumed low sensitivity of such experiments. Here we show that selecting the TROSY components of proton-attached 15N nuclei (TROSY 15NH) yields high quality spectra in high field magnets (>600 MHz) by taking advantage of the slow 15N transverse relaxation and compensating for the inherently low 15N sensitivity. The 15N TROSY transverse relaxation rates increase modestly with molecular weight but the TROSY gain in peak heights depends strongly on the magnetic field strength. Theoretical simulations predict that the narrowest line width for the TROSY 15NH component can be obtained at 900 MHz, but sensitivity reaches its maximum around 1.2 GHz. Based on these considerations, a 15N-detected 2D 1H–15N TROSY-HSQC (15N-detected TROSY-HSQC) experiment was developed and high-quality 2D spectra were recorded at 800 MHz in 2 h for 1 mM maltose-binding protein at 278 K (τc ~ 40 ns). Unlike for 1H detected TROSY, deuteration is not mandatory to benefit 15N detected TROSY due to reduced dipolar broadening, which facilitates studies of proteins that cannot be deuterated, especially in cases where production requires eukaryotic expression systems. The option of recording 15N TROSY of proteins expressed in H2O media also alleviates the problem of incomplete amide proton back exchange, which often hampers the detection of amide groups in the core of large molecular weight proteins that are expressed in D2O culture media and cannot be refolded for amide back exchange. These results illustrate the potential of 15NH-detected TROSY experiments as a means to exploit the high resolution offered by high field magnets near and above 1 GHz.





Similar content being viewed by others
Change history
11 December 2017
The authors regret a mistake appeared in the supplement of this paper.
References
Allegrozzi M, Bertini I, Janik MBL, Lee Y-M, Liu G, Luchinat C (2000) Lanthanide-induced pseudocontact shifts for solution structure refinements of macromolecules in shells up to 40 Å from the metal ion. J Am Chem Soc 122:4154–4161
Allen KN, Imperiali B (2010) Lanthanide-tagged proteins—an illuminating partnership. Curr Opin Chem Biol 14:247–254
Arnesano F, Banci L, Piccioli M (2005) NMR structures of paramagnetic metalloproteins. Q Rev Biophys 38:167–219
Balayssac S, Bertini I, Luchinat C, Parigi G, Piccioli M (2006) 13C direct detected NMR increases the detectability of residual dipolar couplings. J Am Chem Soc 128:15042–15043
Banci L, Bertini I, Cavallaro G, Giachetti A, Luchinat C, Parigi G (2004) Paramagnetism-based restraints for Xplor-NIH. J Biomol NMR 28:249–261
Bermel W, Bertini I, Felli IC, Kummerle R, Pierattelli R (2003) 13C Direct detection experiments on the paramagnetic oxidized monomeric copper, zinc superoxide dismutase. J Am Chem Soc 125:16423–16429
Bermel W, Bertini I, Felli IC, Lee YM, Luchinat C, Pierattelli R (2006a) Protonless NMR experiments for sequence-specific assignment of backbone nuclei in unfolded proteins. J Am Chem Soc 128:3918–3919
Bermel W, Bertini I, Felli IC, Piccioli M, Pierattelli R (2006b) 13C-detected protonless NMR spectroscopy of proteins in solution. Prog Nucl Magn Res Spec 48:25–45
Bohlen JM, Bodenhausen G (1993) Experimental aspects of chirp NMR spectroscopy. J Magn Res A 102:293–301
Felli I, Brutscher B (2009) Recent advances in solution NMR: fast methods and heteronuclear direct detection. Chem Phys Chem 10:1356–1368
Fujiwara T, Nagayama K (1988) Composite inversion pulses with frequency switching and their application to broadband decoupling. J Magn Res (1969) 77:53–63
Gal M, Edmonds KA, Milbradt AG, Takeuchi K, Wagner G (2011) Speeding up direct (15)N detection: hCaN 2D NMR experiment. J Biomol NMR 51:497–504
Gardner KH, Zhang X, Gehring K, Kay LE (1998) Solution NMR studies of a 42 KDa Escherichia Coli maltose binding protein/β-cyclodextrin complex: chemical shift assignments and analysis. J Am Chem Soc 120:11738–11748
Hsu S-TD, Bertoncini CW, Dobson CM (2009) Use of protonless NMR spectroscopy to alleviate the loss of information resulting from exchange-broadening. J Am Chem Soc 131:7222–7223
Inagaki F, Miyazawa T (1980) NMR analyses of molecular conformations and conformational equilibria with the lanthanide probe method. Prog Nuc Magn Res Spec 14:67–111
Kay L, Keifer P, Saarinen T (1992) Pure absorption gradient enhanced heteronuclear single quantum correlation spectroscopy with improved sensitivity. J Am Chem Soc 114:10663–10665
Keizers PH, Desreux JF, Overhand M, Ubbink M (2007) Increased paramagnetic effect of a lanthanide protein probe by two-point attachment. J Am Chem Soc 129:9292–9293
Kelly AE, Ou HD, Withers R, Dötsch V (2002) Low-conductivity buffers for high-sensitivity NMR measurements. J Am Chem Soc 124:12013–12019
Kofuku Y, Ueda T, Okude J, Shiraishi Y, Kondo K, Mizumura T, Suzuki S, Shimada I (2014) Functional dynamics of deuterated β2 -adrenergic receptor in lipid bilayers revealed by NMR spectroscopy. Angew Chem Int Ed 53:13376–13379
Kupce E, Nishida T, Freeman R (2003) Hadamard NMR spectroscopy. Magn Reson Chem 42:95–122
Lee D, Vögeli B, Pervushin K (2005) Detection of C′, Cα correlations in proteins using a new time- and sensitivity-optimal experiment. J Biomol NMR 31:273–278
Lee D, Hilty C, Wider G, Wüthrich K (2006) Effective rotational correlation times of proteins from NMR relaxation interference. J Magn Reson 178:72–76
Levy G, Richter R (1979) Nitrogen 15 nuclear magnetic resonance spectroscopy. Wiley, Hoboken
Lohr F, Katsemi V, Hartleib J, Gunther U, Ruterjans H (2003) A strategy to obtain backbone resonance assignments of deuterated proteins in the presence of incomplete amide 2H/1H back-exchange. J Biomol NMR 25:291–311
Miyazawa-Onami M, Takeuchi K, Takano T, Sugiki T, Shimada I, Takahashi H (2013) Perdeuteration and methyl-selective 1H, 13C-labeling by using a Kluyveromyces lactis expression system. J Biomol NMR 57:297–304
Morgan WD, Kragt A, Feeney J (2000) Expression of deuterium-isotope-labelled protein in the yeast pichia pastoris for NMR studies. J Biomol NMR 17:337–347
Peng J, Thanabal V, Wagner G (1991) Improved accuracy of heteronuclear transverse relaxation time measurements in macromolecules. Elimination of antiphase contributions. J Magn Res 95:421–427
Pervushin K (2000) Impact of transverse relaxation optimized spectroscopy (TROSY) on NMR as a technique in structural biology. Q Rev Biophys 33:161–197
Pervushin K, Riek R, Wider G, Wuthrich K (1997) Attenuated T2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc Natl Acad Sci 94:12366–12371
Pervushin KV, Wider G, Wüthrich K (1998) Single transition-to-single transition polarization transfer (ST2-PT) in [15N,1H]-TROSY. J Biomol NMR 12:345–348
Piotto M, Saudek V, Sklenar V (1992) Gradient-tailored excitation for single-quantum NMR spectroscopy of aqueous solutions. J Biomol NMR 2:661–665
Rovnyak D, Hoch JC, Stern AS, Wagner G (2004) Resolution and sensitivity of high field nuclear magnetic resonance spectroscopy. J Biomol NMR 30:1–10
Sastry M, Xu L, Georgiev IS, Bewley CA, Nabel GJ, Kwong PD (2011) Mammalian production of an isotopically enriched outer domain of the HIV-1 gp120 glycoprotein for NMR spectroscopy. J Biomol NMR 50:197–207
Schanda P, Brutscher B (2005) Very fast two-dimensional NMR spectroscopy for real-time investigation of dynamic events in proteins on the time scale of seconds. J Am Chem Soc 127:8014–8015
Schanda P, Brutscher B (2006) Hadamard frequency-encoded SOFAST-HMQC for ultrafast two-dimensional protein NMR. J Magn Reson 178:334–339
Serber Z, Richter C, Dotsch V (2001) Carbon-detected NMR experiments to investigate structure and dynamics of biological macromolecules. ChemBioChem 2:247–251
Shaka AJ, Keeler J, Frenkiel T, Freeman R (1983) An improved sequence for broadband decoupling: WALTZ-16. J Magn Reson 52:335–338
Takeuchi K, Sun ZY, Wagner G (2008) Alternate 13C–12C labeling for complete mainchain resonance assignments using Ca direct-detection with applicability toward fast relaxing protein systems. J Am Chem Soc 130:17210–17211
Takeuchi K, Frueh DP, Hyberts SG, Sun ZJ, Wagner G (2010a) High-resolution 3D CANCA NMR experiments for complete mainchain assignments using Ca direct-detection. J Am Chem Soc 132:2945–2951
Takeuchi K, Heffron G, Sun ZY, Frueh DP, Wagner G (2010b) Nitrogen-detected CAN and CON experiments as alternative experiments for main chain NMR resonance assignments. J Biomol NMR 47:271–282
Takeuchi K, Gal M, Shimada I, Wagner G (2012) Low γ-nuclei detection experiments for bimolecular NMR. In: Clore M, Potts J (eds) Recent Developments in Biomolecular NMR, Royal Society of Chemistry
Tjandra N, Bax A (1997) Direct measurement of distances and angles in biomolecules by NMR in a dilute liquid crystalline medium. Science 278:1111–1114
Tolman JR, Flanagan JM, Kennedy MA, Prestegard JH (1995) Nuclear magnetic dipole interactions in field-oriented proteins: information for structure determination in solution. Proc Natl Acad Sci 92:9279–9283
Tugarinov V, Kanelis V, Kay LE (2006) Isotope labeling strategies for the study of high-molecular-weight proteins by solution NMR spectroscopy. Nat Protocols 1:749–754
Tycko R, Blanco FJ, Ishii Y (2000) Alignment of biopolymers in strained gels: a new way to create detectable dipole–dipole couplings in high-resolution biomolecular NMR. J Am Chem Soc 122:9340–9341
Vasos PR, Hall JB, Kummerle R, Fushman D (2006) Measurement of 15N relaxation in deuterated amide groups in proteins using direct nitrogen detection. J Biomol NMR 36:27–36
Acknowledgments
This work was supported by NIH Grants GM047467 and AI37581 to GW and by METI (Grant Name: development of core technologies for innovative drug development based upon IT) to IS. This work was also partly supported by JST, PRESTO to KT. Maintenance of NMR instruments was in part supported by NIH grant EB002026. We would like thanks Dominique Frueh, Wolfgang Bermel and Arthur Palmer for useful discussions. We would like to thank Ms. Misaki Imai for making the protein samples used in the paper.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Koh Takeuchi and Haribabu Arthanari have contributed equally to this work.
An erratum to this article is available at https://doi.org/10.1007/s10858-017-0131-8.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Takeuchi, K., Arthanari, H., Shimada, I. et al. Nitrogen detected TROSY at high field yields high resolution and sensitivity for protein NMR. J Biomol NMR 63, 323–331 (2015). https://doi.org/10.1007/s10858-015-9991-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-015-9991-y