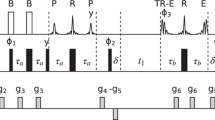
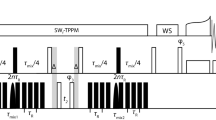
Abstract
Replacement of non-exchangeable protons by deuterons has become a standard tool in structural studies of proteins on the order of 30–40 kDa to overcome problems arising from rapid 1H and 13C transverse relaxation. However, 1H nuclei are required at exchangeable sites to maintain the benefits of proton detection. Protein expression in D2O-based media containing deuterated carbon sources yields protein deuterated in all positions. Subsequent D/H-exchange is commonly used to reintroduce protons in labile positions. Since this strategy may fail for large proteins with strongly inhibited exchange we propose to express the protein in fully deuterated algal lysate medium in 100% H2O. As a side-effect partial Cα protonation occurs in a residue-type dependent manner. Samples obtained by this protocol are suitable for complementary 1HN- and 1Hα-based triple resonance experiments allowing complete backbone resonance assignments in cases where back-exchange of amide protons is very slow after expression in D2O and refolding of chemically denatured protein is not feasible. This approach is explored using a 35-kDa protein as a test case. The degree of Cα protonation of individual amino acids is determined quantitatively and transverse relaxation properties of 1HN and 15N nuclei of the partially deuterated protein are investigated and compared to the fully protonated and perdeuterated species. Based on the deviations of assigned chemical shifts from random coil values its solution secondary structure can be established.
Similar content being viewed by others
References
Arrowsmith, C.H. and Wu, Y.-S. (1998) Prog. Nucl. Magn. Reson. Spectrosc., 32, 277-286.
Bax, A., Griffey, R.H. and Hawkins, B.L. (1983) J. Magn. Reson., 55, 301-315.
Bazzo, R., Cicero, D.O. and Barbato, G. (1995) J. Magn. Reson., B107, 189-191.
Bendall, M.R., Pegg, D.T. and Doddrell, D.M. (1983) J. Magn. Reson., 52, 81-117.
Bodenhausen, G. and Ruben, D.J. (1980) Chem. Phys. Lett., 69, 185-189.
Boisbouvier, J., Gans, P., Blackledge, M., Brutscher, B. and Marion, D. (1999) J. Am. Chem. Soc., 121, 7700-7701.
Boucher, W., Laue, E.D., Campbell-Burk, S.L. and Domaille, P.J. (1992) J. Biomol. NMR, 2, 631-637.
Boyd, J. and Soffe, N. (1989) J. Magn. Reson., 85, 406-413.
Carr, H.Y. and Purcell, E.M. (1954) Phys. Rev., 94, 630-638.
Clubb, R.T., Thanabal, V. and Wagner, G. (1992) J. Magn. Reson., 97, 213-217.
Constantine, K.L., Mueller, L., Goldfarb, V., Wittekind, M., Metzler, W.J., Yanchunas Jr, J., Robertson, J.G., Malley, M.F., Friedrichs, M.S. and Farmer II, B.T. (1997) J. Mol. Biol., 267, 1223-1246.
Czisch, M. and Boelens, R. (1998) J. Magn. Reson., 134, 158-160.
Delaglio, F., Grzesiek, S., Vuister, G.W., Zhu, G., Pfeifer, J. and Bax, A. (1995) J. Biomol. NMR, 6, 277-293.
Deverell, C., Morgan, R.E. and Strange, J.H. (1970) Mol. Phys., 18, 553-559.
Dingley, A.J. and Grzesiek, S. (1998) J. Am. Chem. Soc., 120, 8293-8297.
Eletsky, A., Kienhöfer, A. and Pervushin, K. (2001) J. Biomol. NMR, 20, 177-180.
Emsley, L. and Bodenhausen, G. (1990) Chem. Phys. Lett., 165, 469-476.
Farmer II, B.T. and Venters, R.A. (1995) J. Am. Chem. Soc., 117, 4187-4188.
Farmer II, B.T. and Venters, R.A. (1998) In Biological MagneticResonance, Vol. 16, Krishna, N.R. and Berliner, L.J. (Eds.), Kluwer Academic/Plenum Publishers, New York, NY, pp. 75-120.
Fiala, R., Czernek, J. and Sklenář, V. (2000) J. Biomol. NMR, 16, 291-302.
Gardner, K.H. and Kay, L.E. (1998) Annu. Rev. Biophys. Biomol. Struct., 27, 357-406.
Gardner, K.H., Rosen, M.K. and Kay, L.E. (1997) Biochemistry, 36, 1389-1401.
Geen, H. and Freeman, R. (1991) J. Magn. Reson., 93, 93-141.
Griffey, R.H. and Redfield, A.G. (1987) Quart. Rev. Biophys., 19, 51-82.
Grzesiek, S. and Bax, A (1992a) J. Magn. Reson., 96, 432-440.
Grzesiek, S. and Bax, A. (1992b) J. Am. Chem. Soc., 114, 6291-6293.
Grzesiek, S. and Bax, A. (1993a) J. Biomol. NMR, 3, 185-204.
Grzesiek, S. and Bax, A. (1993b) J. Am. Chem. Soc., 115, 12593-12594.
Grzesiek, S. and Bax, A. (1995) J. Biomol. NMR, 6, 335-339.
Grzesiek, S., Anglister, J., Ren, H. and Bax, A. (1993) J. Am. Chem. Soc., 115, 4369-4370.
Grzesiek, S., Kuboniwa, H., Hinck, A.P. and Bax, A. (1995) J. Am. Chem. Soc., 117, 5312-5315.
Günther, U.L., Ludwig, C. and Rüterjans, H. (2000) J. Magn. Reson., 145, 201-208.
Hartleib, J. and Rüterjans, H. (2001a) Protein Expr. Purif., 21, 210-219.
Hartleib, J. and Rüterjans, H. (2001b) Biochim. Biophys. Acta, 1546, 312-324.
Hartleib, J., Geschwindner, S., Scharff, E.I. and Rüterjans, H. (2001) Biochem. J., 353, 579-589.
Heikkinen, S. and Kilpeläinen, I. (2001) J. Magn. Reson., 151, 314-319.
Ikura, M., Kay, L.E. and Bax, A. (1990) Biochemistry, 29, 4659-4667.
Kay, L.E., Ikura, M. and Bax, A. (1991) J. Magn. Reson., 91, 84-92.
Kay, L.E., Keifer, P. and Saarinen, T. (1992) J. Am. Chem. Soc., 114, 10663-10665.
Kong, X.M., Sze, K.H. and Zhu, G. (1999) J. Biomol. NMR, 14, 133-140.
Konrat, R., Yang, D. and Kay, L.E. (1999) J. Biomol. NMR, 15, 309-313.
Kontaxis, G., Clore, G.M. and Bax, A. (2000) J. Magn. Reson., 143, 184-196.
Kupče, E.and Freeman, R. (1995) J. Magn. Reson., A115, 273-276.
Kushlan, D.M. and LeMaster, D.M. (1993) J. Biomol. NMR, 3, 701-708.
Larsson, G., Wijmenga, S. S. and Schleucher, J. (1999) J. Biomol. NMR, 14, 169-174.
LeMaster, D.M. (1994) Prog. Nucl. Magn. Reson. Spectrosc., 26, 371-419.
LeMaster, D.M. and Richards, F.M. (1988) Biochemistry, 27, 142-150.
Logan, T.M., Olejniczak, E.T., Xu, R.X. and Fesik, S.W. (1992) FEBS Lett., 314, 413-418.
Löhr, F. and Rüterjans, H. (1995a) J. Biomol. NMR, 6, 189-197.
Löhr, F. and Rüterjans, H. (1995b) J. Magn. Reson., B109, 80-87.
Löhr, F., Pfeiffer, S., Lin, Y.-J., Hartleib, J., Klimmek, O. and Rüterjans, H. (2000) J. Biomol. NMR, 18, 337-346.
Loria, J.P., Rance, M. and Palmer III, A.G. (1999) J. Magn. Reson., 141, 180-184.
Marion, D., Ikura, M. and Bax, A. (1989a) J. Magn. Reson., 84, 425-430.
Marion, D., Ikura, M., Tschudin, R. and Bax, A. (1989b) J. Magn. Reson., 85, 393-399.
Markus, M.A., Dayie, K.T., Matsudaira, P. and Wagner, G. (1994) J. Magn. Reson., B105, 192-195.
Matsuo, H., Kupče, E., Li, H. and Wagner, G. (1996) J. Magn. Reson., B111, 194-198.
McCallum, S.A., Hitchens, T.K. and Rule, G.S. (1999) J. Mol. Biol., 285, 2119-2132.
McCoy, M.A. and Mueller, L. (1992) J. Magn. Reson., 99, 18-36.
Meiboom, S. and Gill, D. (1958) Rev. Sci. Instrum., 29, 688-691.
Mohebbi, A and Shaka, A.J. (1991) Chem. Phys. Lett., 178, 374-378.
Montelione, G.T. and Wagner, G. (1990) J. Magn. Reson., 87, 183-188.
Morris, G.A. and Freeman, R. (1979) J. Am. Chem. Soc., 101, 760-762.
Mulder, F.A.A., Ayed, A., Yang, D., Arrowsmith, C.H. and Kay, L.E. (2000) J. Biomol. NMR, 18, 173-176.
Nietlispach, D., Clowes, R.T., Broadhurst, R.W., Ito, Y., Keeler, J., Kelly, M., Ashurst, J., Oschkinat, H., Domaille, P.J. and Laue, E.D. (1996) J. Am. Chem. Soc., 118, 407-415.
Palmer III, A.G., Cavanagh, J., Wright, P.E. and Rance, M. (1991) J. Magn. Reson., 93, 151-170.
Patt, S. (1992) J. Magn. Reson., 96, 94-102.
Pervushin, K., Riek, R., Wider, G. and Wüthrich, K. (1997) Proc. Natl. Acad. Sci. USA, 94, 12366-12371.
Pervushin, K., Wider, G. and Wüthrich, K. (1998) J. Biomol. NMR, 12, 345-348.
Powers, R., Gronenborn, A.M., Clore, G.M. and Bax, A. (1991) J. Magn. Reson., 94, 209-213.
Riek, R., Fiaux, J., Bertelsen, E.B., Horwich, A.L. and Wüthrich, K. (2002) J. Am. Chem. Soc., 124, 12144-12153.
Rosen, M.K., Gardner, K.H., Willis, R.C., Parris, W.E., Pawson, T. and Kay, L.E. (1996) J. Mol. Biol., 263, 627-636.
Salzmann, M., Pervushin, K., Wider, G., Senn, H. and Wüthrich, K. (1998) Proc. Natl. Acad. Sci. USA, 95, 13585-13590.
Salzmann, M., Pervushin, K., Wider, G., Senn, H. and Wüthrich, K. (2000) J. Am. Chem. Soc., 122, 7543-7548.
Salzmann, M., Wider, G., Pervushin, K., Senn, H. and Wüthrich, K. (1999a) J. Am. Chem. Soc., 121, 844-848.
Salzmann, M., Wider, G., Pervushin, K. and Wüthrich, K. (1999b) J. Biomol. NMR, 15, 181-184.
Salzmann, M., Pervushin, K., Wider, G., Senn, H. and Wüthrich, K. (1999c) J. Biomol. NMR, 14, 85-88.
Sattler, M. and Fesik, S.W. (1996) Structure, 4, 1245-1249.
Scharff, E.I., Koepke, J., Fritzsch, G., Lücke, C. and Rüterjans, H. (2001) Structure, 9, 493-502.
Seip, S., Balbach, J. and Kessler, H. (1992) J. Magn. Reson., 100, 406-410.
Serber, Z., Ledwidge, R., Miller, S.M. and Dötsch, V. (2001) J. Am. Chem. Soc., 123, 8895-8901.
Shaka, A.J., Barker, P.B. and Freeman, R. (1985) J. Magn. Reson., 64, 547-552.
Shaka, A.J., Keeler, J., Frenkiel, T. and Freeman, R. (1983) J. Magn. Reson., 52, 335-338.
Shaka, A.J., Lee, C.J. and Pines, A. (1988) J. Magn. Reson., 77, 274-293.
Shan, X., Gardner, K.H., Muhandiram, D.R., Rao, N.S., Arrowsmith, C.H. and Kay, L.E. (1996) J. Am. Chem. Soc., 118, 6570-6579.
Stonehouse, J., Shaw, G.L., Keeler, J. and Laue, E.D. (1994) J. Magn. Reson., A107, 178-184.
Swapna, G.V.T., Rios, C.B., Shang, Z. and Montelione, G.T. (1997) J. Biomol. NMR, 9, 105-111.
Tessari, M., Gentile, L.N., Taylor, S.J., Shalloway, D.I., Nicholson, L.K. and Vuister, G.W. (1997) Biochemistry, 36, 14561-14571.
Tugarinov, V., Muhandiram, R., Ayed, A. and Kay, L.E. (2002) J. Am. Chem. Soc., 124, 10025-10035.
Venters, R.A., Farmer II, B.T., Fierke, C.A. and Spicer, L.D. (1996) J. Mol. Biol., 264, 1101-1116.
Wang, Y.-X., Jacob, J., Cordier, F., Wingfield, P., Stahl, S.J., Lee-Huang, S., Torchia, D., Grzesiek, S. and Bax, A. (1999) J. Biomol. NMR, 14, 181-184.
Weigelt, J. (1998) J. Am. Chem. Soc., 120, 10778-10779.
Wienk, H.L.J., Martínez, M.M., Yalloway, G.N., Schmidt, J.M., Pérez, C., Rüterjans, H. and Löhr, F. (2003) J. Biomol. NMR, 25, 133-145.
Wishart, D.S., Bigam, C.G., Holm, A., Hodges, R.S. and Sykes, B.D. (1995) J. Biomol. NMR, 5, 67-81.
Wittekind, M. and Mueller, L. (1993) J. Magn. Reson., B101, 201-205.
Xia, Y., Kong, X., Smith, D.K., Liu, Y., Man, D. and Zhu, G. (2000) J. Magn. Reson., 143, 407-410.
Yamazaki, T., Lee, W., Arrowsmith, C.H., Muhandiram, D.R. and Kay, L.E. (1994) J. Am. Chem. Soc., 116, 11655-11666.
Yamazaki, T., Tochio, H., Furui, J., Aimoto, S. and Kyogoku, Y. (1997) J. Am. Chem. Soc., 119, 872-880.
Yang D. and Kay, L.E. (1999a) J. Am. Chem. Soc., 121, 2571-2575.
Yang, D. and Kay, L.E. (1999b) J. Biomol. NMR, 14, 273-276.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Löhr, F., Katsemi, V., Hartleib, J. et al. A strategy to obtain backbone resonance assignments of deuterated proteins in the presence of incomplete amide 2H/1H back-exchange. J Biomol NMR 25, 291–311 (2003). https://doi.org/10.1023/A:1023084605308
Issue Date:
DOI: https://doi.org/10.1023/A:1023084605308