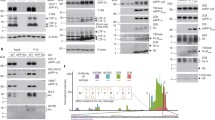
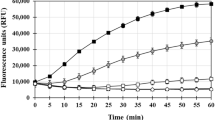
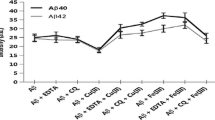
Abstract
The extracellular deposition of amyloid β (Aβ) is known to be the fundamental cause of Alzheimer’s disease (AD). Aβ1-42, generated by β-secretases from the amyloid precursor protein (APP), is the main component of neuritic plaque, and the aggregation of this protein is shown to be dependent to an extent on metal ions such as copper and zinc. However, the mechanism by which Cu2+ affects the physicochemical properties of Aβ1-42 or the central nervous system is still under debate. A recent series of studies have demonstrated that both the soluble-type matrix metalloproteinases (MMP-2 and MMP-9) and the membrane-type matrix metalloproteinase (MT1-MMP) are capable of degrading Aβ peptides. MMP-7, one of the soluble-type matrix metalloproteinases, is expressed in hippocampal tissue; however, less information is available concerning the pathophysiological roles of this enzyme in the process and/or progress of Alzheimer’s disease. In this study, we examined the degradation activity of MMP-7 against various Aβ1-42’s fragment peptides and the effect of Cu2+. Although Aβ22-40 was degraded by MMP-7 regardless of Cu2+, Cu2+ inhibited the degradation of Aβ1-19, Aβ11-20, and Aβ11-29 by MMP-7. These results indicate that MMP-7 is capable of degrading Aβ1-42, and that Aβ1-42 acquired resistance against MMP-7 cleavage through Cu2+-binding and structure changes. Our results demonstrate that MMP-7 may play an important role in the defensive mechanism against the aggregation of Aβ1-42, which gives rise to the pathology of AD.





Similar content being viewed by others
References
Allinson TM, Parkin ET, Turner AJ, Hooper NM (2003) ADAMs family members as amyloid precursor protein alpha-secretases. J Neurosci Res 74:342–352. doi:10.1002/jnr.10737
Atwood CS, Moir RD, Huang X, Scarpa RC, Bacarra NM, Romano DM, Hartshorm MA, Tanzi RE, Bush AI (1998) Dramatic aggregation of Alzheimer abeta by Cu(II) is induced by conditions representing physiological acidosis. J Biol Chem 273:12817–12826
Backstrom JR, Lim GP, Cullen MJ, Tökés ZA (1996) Matrix metalloproteinase-9 (MMP-9) is synthesized in neurons of the human hippocampus and is capable of degrading the amyloid-beta peptide (1-40). J Neurosci 16:7910–7919
Barnham KJ, Bush AI (2014) Biological metals and metal-targeting compounds in major neurodegenerative diseases. Chem Soc Rev 43(19):6727–6749. doi:10.1039/c4cs00138a
Bohnen N, Jolles J, Degenaar CP (1994) Levels of trace elements in blood in healthy aging subjects. Z Gerontol 27(5):324–327
Bolognin S, Messori L, Drago D, Gabbiani C, Cendron L, Zatta P (2011) Aluminum, copper, iron and zinc differentially alter amyloid-Aβ(1-42) aggregation and toxicity. Int J Biochem Cell Biol 43(6):877–885. doi:10.1016/j.biocel.2011.02.009
Chen Z, Thangiah G, Marla G, Jeganathan R (2015) Amyloid β-abrogated TrkA ubiquitination in PC12 cells analogous to Alzheimer’s disease. J Neurochem 133(6):919–925. doi:10.1111/jnc.13076
Chiti F, Dobson CM (2006) Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem 75:333–366. doi:10.1146/annurev.biochem.75.101304.123901
Cornett CR, Markesbery WR, Ehmann WD (1998) Imbalances of trace elements related to oxidative damage in Alzheimer’s disease brain. Neurotoxicology 19(3):339–345
Corso DL, Pastine F, Protti MA, Romanelli AM, Moruzzo D, Ruocco L, Pentimone F (2000) Blood zinc, copper and magnesium in aging. A study in healthy home-living elderly. Panminerva Med 42(4):273–277
Crouch PJ, Tew DJ, Du T, Nguyen DN, Caragounis A, Filiz G, Blake RE, Trounce IA, Soon CP, Laughton K, Perez KA, Li QX, Cherny RA, Masters CL, Barnham KJ, White AR (2009) Restored degradation of the Alzheimer’s amyloid-beta peptide by targeting amyloid formation. J Neurochem 108(5):1198–1207. doi:10.1111/j.1471-4159.2009.05870.x
Deibel MA, Ehmann WD, Markesbery WR (1996) Copper, iron, and zinc imbalances in severely degenerated brain regions in Alzheimer’s disease: possible relation to oxidative stress. J Neurol Sci 143(1–2):137–142
Dewachter I, Van Leuwen F (2002) Secretases as targets for the treatment of Alzheimer’s disease: the prospects. Lancet Neurol 1(7):409–416
Esch FS, Keim PS, Beattie EC, Blacher RW, Culwell AR, Oltersdorf T, McClure D, Ward PJ (1990) Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science 248(4959):1122–1124
Faller P, Hureau C, La Penna G (2014) Metal ions and intrinsically disordered proteins and peptides: from Cu/Zn amyloid-β to general principles. Acc Chem Res 47(8):2252–2259. doi:10.1021/ar400293h
Francis R, McGrath G, Zhang J, Ruddy DA, Sym M, Apfeld J, Nicoll M, Maxwell M, Hai B, Ellis MC, Parks AL, Xu W, Li J, Gurney M, Myers RL, Himes CS, Hiebsch R, Ruble C, Nye JS, Curtis D (2002) aph-1 and pen-2 are required for Notch pathway signaling, gamma-secretase cleavage of betaAPP, and presenilin protein accumulation. Dev Cell 3(1):85–97
Haass C, Selkoe DJ (1993) Cellular processing of beta-amyloid precursor protein and the genesis of amyloid beta-peptide. Cell 75(6):1039–1042
Hamley IW (2012) The amyloid beta peptide: a chemist’s perspective. Role in Alzheimer’s and fibrillization. Chem Rev 112(10):5147–5192. doi:10.1021/cr3000994
Hozumi I, Hasegawa T, Honda A, Ozawa K, Hayashi Y, Hashimoto K, Yamada M, Koumura A, Sakurai T, Kimura A, Tanaka Y, Satoh M, Inuzuka T (2011) Patterns of levels of biological metals in CSF differ among neurodegenerative diseases. J Neurol Sci 303(1–2):95–99. doi:10.1016/j.jns.2011.01.003
Hussain I, Powell D, Howlett DR, Tew DG, Meek TD, Chapman C, Gloger IS, Murphy KE, Southan CD, Ryan DM, Smith TS, Simmons DL, Waish FS, Dingwall C, Christie G (1999) Identification of a novel aspartic protease (Asp 2) as beta-secretase. Mol Cell Neurosci 14(6):419–427. doi:10.1006/mcne.1999.0811
Itoh M, Masuda K, Ito Y, Akizawa T, Yoshioka M, Imai K, Okada Y, Sato H, Seiki M (1996) Purification and refolding of recombinant human proMMP-7 (pro-matrilysin) expressed in Escherichia coli and its characterization. J Biochem 119(4):667–673
Jarrett JT, Berger EP, Lansbury PT Jr (1993) The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer’s disease. Biochemistry 32(18):4693–4697
Kojima A, Konishi M, Akizawa T (2014) Prion fragment peptides are digested with membrane type matrix metalloproteinases and acquire enzyme resistance through Cu2+-binding. Biomolecules 4(2):510–526. doi:10.3390/biom4020510
Kumar J, Namsechi R, Sim LV (2015) Structure-based peptide design to modulate amyloid beta aggregation and reduce cytotoxicity. PLoS ONE 10(6):e0129087. doi:10.1371/journal.pone.0129087
LaFerla FM, Green KN, Oddo S (2007) Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci 8(7):499–509
Levitan D, Lee J, Song L, Manning R, Wong G, Parker E, Zhang L (2001) PS1N- and C-terminal fragments form a complex that functions in APP processing and Notch signaling. Proc Natl Acad Sci USA 98:12186–12190. doi:10.1073/pnas.211321898
Lin CJ, Huang HC, Jiang ZF (2010) Cu(II) interaction with amyloid-beta peptide: a review of neuroactive mechanisms in AD brains. Brain Res Bull 82(5–6):235–242. doi:10.1016/j.brainresbull.2010.06.003
Loeffler DA, LeWitt PA, Juneau PL, Sima AA, Nguyen HU, DeMaggio AJ, Brickman CM, Brewer GJ, Dick RD, Troyer MD, Kanaley L (1996) Increased regional brain concentrations of ceruloplasmin in neurodegenerative disorders. Brain Res 738(2):265–274
Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR (1998) Copper, iron and zinc in Alzheimer’s disease senile plaques. J Neurol Sci 158(1):47–52
Massie HR, Aiello VR, Iodice AA (1979) Changes with age in copper and superoxide dismutase levels in brains of C57BL/6J mice. Mech Ageing Dev 10(1–2):93–99
Morita A, Kimura M, Itokawa Y (1994) The effect of aging on the mineral status of female mice. Biol Trace Element Res 42(2):165–177
Munirah A, Takahisa T, Hisashi M, Tomokazu Y, Mitsuru F, Hiroshi S (2006) Cleavage of amyloid-beta precursor protein (APP) by membrane-type matrix metalloproteinases. J Biochem 139(3):517–526. doi:10.1093/jb/mvj054
Oku N, Matsukawa M, Yamakawa S, Asai T, Yahara S, Hashimoto F, Akizawa T (2003) Inhibitory effect of green tea polyphenols on membrane-type 1 matrix metalloproteinase, MT1-MMP. Biol Pharm Bull 26(9):1235–1238
Plantin LO, Lysing-Tunnell U, Kristensson K (1987) Trace elements in the human central nervous system studied with neutron activation analysis. Biol Trace Element Res 13(1):69–75. doi:10.1007/BF02796622
Rajendran R, Minqin R, Ynsa MD, Casadesus G, Smith MA, Perry G, Halliwell B, Watt F (2009) A novel approach to the identification and quantitative elemental analysis of amyloid deposits–insights into the pathology of Alzheimer’s disease. Biochem Biophys Res Commun 382(1):91–95. doi:10.1016/j.bbrc.2009.02.136
Religa D, Strozyk D, Cherny RA, Volitakis I, Haroutunian V, Winblad B, Naslund J, Bush AI (2006) Elevated cortical zinc in Alzheimer disease. Neurology 67(1):69–75. doi:10.1212/01.wnl.0000223644.08653.b5
Roher AE, Kasunic TC, Woods AS, Cotter RJ, Ball MJ, Fridman R (1994) Proteolysis of A beta peptide from Alzheimer disease brain by gelatinase A. Biochem Biophys Res Commun 205(3):1755–1761. doi:10.1006/bbrc.1994.2872
Roychaudhuri R, Yang M, Hoshi MM, Teplow DB (2009) Amyloid beta-protein assembly and Alzheimer disease. J Biol Chem 284(8):4749–4753. doi:10.1074/jbc.R800036200
Sarell CJ, Wilkinson SR, Viles JH (2010) Substoichiometric levels of Cu2+ions accelerate the kinetics of fiber formation and promote cell toxicity of amyloid-β from Alzheimer disease. J Biol Chem 285(53):41533–41540. doi:10.1074/jbc.M110.171355
Selkoe DJ (1996) Amyloid beta-protein and the genetics of Alzheimer’s disease. J Biol Chem 271(31):18295–18298
Seubert P, Oltersdorf T, Lee MG, Barbour R, Blomquist C, Davis DL, Bryant K, Fritz LC, Galasko D, Thal LJ, Lieberburg I, Schenk DB (1993) Secretion of beta-amyloid precursor protein cleaved at the amino terminus of the beta-amyloid peptide. Nature 361(6409):260–263. doi:10.1038/361260a0
Sinha S, Anderson JP, Barbour R, Basi GS, Caccavello R, Davis D, Doan M, Dovey HF, Frigon N, Hong J, Jacobson-Croak K, Jewett N, Keim P, Knops J, Lieberburg I, Power M, Tan H, Tatsuno G, Tung J, Schenk D, Seubert P, Suomensaari SM, Wang S, Walker D, Zhao J, McConiogue L, Jhon V (1999) Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature 402(6761):537–540. doi:10.1038/990114
Sisodia SS (1992) Beta-amyloid precursor protein cleavage by a membrane-bound protease. Proc Natl Acad Sci USA 89(13):6075–6079
Smith DP, Ciccotosto GD, Tew DJ, Fodero-Tavoletti MT, Johanssen T, Masters CL, Barnham KJ, Cappai R (2007) Concentration dependent Cu2+ induced aggregation and dityrosine formation of the Alzheimer’s disease amyloid-beta peptide. Biochemisry 46(10):2881–2891. doi:10.1021/bi0620961
Steiner H, Winkler E, Edbauer D, Prokop S, Basset G, Yamasaki A, Kostka M, Haass C (2002) PEN-2 is an integral component of the gamma-secretase complex required for coordinated expression of presenilin and nicastrin. J Biol Chem 277(42):39062–39065. doi:10.1074/jbc.C200469200
Tõugu V, Tiiman A, Palumaa P (2011) Interactions of Zn(II) and Cu(II) ions with Alzheimer’s amyloid-beta peptide. Metal ion binding, contribution to fibrillization and toxicity. Metallomics 3(3):250–261. doi:10.1039/c0mt00073f
Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M (1999) Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286(5440):735–741
Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ (1999) Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature 398(6727):513–517. doi:10.1038/19077
Woodward WD, Filteau SM, Allen OB (1984) Decline in serum zinc level throughout adult life in the laboratory mouse. J Gerontol 39(5):521–524
Yan P, Hu X, Song H, Yin K, Bateman RJ, Cirrito JR, Xiao Q, Hsu FF, Turk JW, Xu J, Hsu CY, Holtzman DM, Lee JM (2006) Matrix metalloproteinase-9 degrades amyloid-beta fibrils in vitro and compact plaques in situ. J Biol Chem 281(34):24566–24574. doi:10.1074/jbc.M602440200
Yu G, Nishimura M, Arawaka S, Levitan D, Zhang L, Tandon A, Song YQ, Rogaeva E, Chen F, Kawarai T, Supala A, Levesque L, Yu H, Yang DS, Holmes E, Milman P, Liang Y, Zhang DM, Xu DH, Sato C, Rogaev E, Smith M, Janus C, Zhang Y, Aebersold R, Farrer LS, Sorbi S, Bruni A, Fraser P, St George-Hyslop P (2000) Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and betaAPP processing. Nature 407(6800):48–54. doi:10.1038/35024009
Acknowledgements
The authors are grateful to Dr. Hidemitsu Pan-Hou for his helpful discussions and advice, and we would like to thank Editage (www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Taniguchi, M., Matsuura, K., Nakamura, R. et al. MMP-7 cleaves amyloid β fragment peptides and copper ion inhibits the degradation. Biometals 30, 797–807 (2017). https://doi.org/10.1007/s10534-017-0048-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-017-0048-4