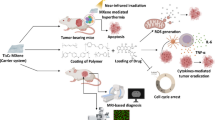
Abstract
Recently localized magnetic hyperthermia therapy is zell researched to treat cancer tumor as an independent therapy or an adjunct therapy to increase efficiency of the established radiation-, chemo-, and immuno-therapies. At the heart of the therapy is the generation of controlled heat by superparamagnetic nanoparticles (SPNPs) and their biocompatibility to human tissue and blood. Colloidal SPNPs produce heat under alternate magnetic field by electron magnetic spin relaxation mechanism. Magnetic spins relax through either Néel or Brown or both the mechanism in a nanocolloid. Néel relaxation is predominant in magnetic isotropic SPNPs, and Brown relaxation is predominant in magnetic anisotropic samples. Brown relaxation leads to rotation of whole magnetic nanoparticles under magnetic field to produce heat. Brown relaxation time (τB) strongly depends on hydrodynamic volume of nanoparticles (NPs) and dispersion medium viscosity. As the generation of magnetic hyperthermia power strongly depends on relaxation time, the recent literature is reviewed to understand the effect of dispersant type on hydrodynamic volume of SPNPs and relaxation time, and medium viscosity on relaxation time in nanocolloids. It is observed that dispersants on individual SPNPs increase both colloidal stability and spin relaxation time of NPs resulting in higher specific heat power generation compared to NPs without dispersants. Different amino acids, long-chain steric dispersants, and small-chain zwitterionic surfactants anchored on similar NPs show varied magnetic hyperthermia values due to varied hydrodynamic volume. Medium viscosity also played a significant role on hyperthermia values of NPs. The above characteristics are prominent in anisotropic magnetic materials, including core–shell bimagnetic nanoparticles. This review may help researchers in the field to design and synthesize new hyperthermia materials in future.




Adapted from Suriyanto et al. [36]



Adapted from reference [47]


adopted from the Department of Inorganic Biomaterials (Kawashita Lab) [206]











Similar content being viewed by others
References
Sun C, Lee JSH, Zhang M (2008) Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Deliv Rev 60(11):1252–1265. https://doi.org/10.1016/j.addr.2008.03.018
Hedayatnasab Z, Abnisa F, Daud WMAW (2017) Review on magnetic nanoparticles for magnetic nanofluid hyperthermia application. Mater Des 123:174–196. https://doi.org/10.1016/j.matdes.2017.03.036
Dulińska-Litewka J, Łazarczyk A, Hałubiec P, Szafrański O, Karnas K, Karewicz A (2019) Superparamagnetic iron oxide nanoparticles—current and prospective medical applications. Materials 12(4):617. https://doi.org/10.3390/ma12040617
Teja AS, Koh P-Y (2009) Synthesis, properties, and applications of magnetic iron oxide nanoparticles. Prog Cryst Growth Charact Mater 55:22–45. https://doi.org/10.1016/j.pcrysgrow.2008.08.003
Zhao S, Yu X, Qian Y, Chen W, Shen J (2020) Multifunctional magnetic iron oxide nanoparticles: an advanced platform for cancer theranostics. Theranostics 10(14):6278–6309. https://doi.org/10.7150/thno.42564
Reddy LH, Arias JL, Nicolas J, Couvreur P (2012) Magnetic nanoparticles: design and characterization, toxicity and biocompatibility. Pharm Biomed Appl Chem Rev 112(11):5818–5878. https://doi.org/10.1021/cr300068p
Wei H, Insin N, Lee J, Han H-S, Cordero JM, Liu W, Bawendi MG (2011) Compact zwitterion-coated iron oxide nanoparticles for biological applications. Nano Lett 12(1):22–25. https://doi.org/10.1021/nl202721q
Coley WB (1891) Contribution to the knowledge of sarcoma. Ann Surg 14:199–220. https://doi.org/10.1097/00000658-189112000-00015
Chiriac H, Petreus T, Carasevici E, Labusca L, Herea D-D, Danceanu C, Lupu N (2015) In vitro cytotoxicity of Fe–Cr–Nb–B magnetic nanoparticles under high frequency electromagnetic field. J Magn Magn Mater 380:13–19. https://doi.org/10.1016/j.jmmm.2014.10.015
Hervault A, Thanh NTK (2014) Magnetic nanoparticle-based therapeutic agents for thermo-chemotherapy treatment of cancer. Nanoscale 6(20):11553–11573. https://doi.org/10.1039/c4nr03482a
Vilas-Boas V, Carvalho F, Espiña B (2020) Magnetic hyperthermia for cancer treatment: main parameters affecting the outcome of in vitro and in vivo studies. Molecules 25(12):2874. https://doi.org/10.3390/molecules25122874
Ling W, Wang M, Xiong C, Xie D, Chen Q, Chu X, Xiao X (2019) Synthesis, surface modification, and applications of magnetic iron oxide nanoparticles. J Mater Res 34(11):1828–1844. https://doi.org/10.1557/jmr.2019.129
Gilchrist RK, Medal R, Shorey WD, Hanselman RC, Parrott JC, Taylor CB (1957) Selective inductive heating of lymph nodes. Ann Surg 146(4):596–606. https://doi.org/10.1097/00000658-195710000-00007
Jordan A, Wust P, Fählin H, John W, Hinz A, Felix R (1993) Inductive heating of ferrimagnetic particles and magnetic fluids: physical evaluation of their potential for hyperthermia. Int J Hyperth 9(1):51–68. https://doi.org/10.3109/02656739309061478
Jordan A, Scholz R, Wust P, Fähling H, Felix R (1999) Magnetic fluid hyperthermia (MFH): cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J Magn Magn Mater 201(1–3):413–419. https://doi.org/10.1016/s0304-8853(99)00088-8
Chang D, Lim M, Goos JACM, Qiao R, Ng YY, Mansfeld FM, Kavallaris M (2018) Biologically targeted magnetic hyperthermia: potential and limitations. Front Pharmacol. https://doi.org/10.3389/fphar.2018.00831
Johannsen M, Gneveckow U, Eckelt L, Feussner A, WaldÖFner N, Scholz R, Jordan A (2005) Clinical hyperthermia of prostate cancer using magnetic nanoparticles: presentation of a new interstitial technique. Int J Hyperth 21(7):637–647. https://doi.org/10.1080/02656730500158360
Johannsen M, Gneveckow U, Taymoorian K, Thiesen B, Waldöfner N, Scholz R, Loening SA (2007) Morbidity and quality of life during thermotherapy using magnetic nanoparticles in locally recurrent prostate cancer: results of a prospective phase I trial. Int J Hyperth 23(3):315–323. https://doi.org/10.1080/02656730601175479
Johannsen M, Gneveckow U, Thiesen B, Taymoorian K, Cho CH, Waldöfner N, Wust P (2007) Thermotherapy of prostate cancer using magnetic nanoparticles: feasibility, imaging, and three-dimensional temperature distribution. Eur Urol 52(6):1653–1662. https://doi.org/10.1016/j.eururo.2006.11.023
Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, Jordan A (2010) Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol 103(2):317–324. https://doi.org/10.1007/s11060-010-0389-0
Spirou S, Basini M, Lascialfari A, Sangregorio C, Innocenti C (2018) Magnetic hyperthermia and radiation therapy: radiobiological principles and current practice †. Nanomaterials 8(6):401. https://doi.org/10.3390/nano8060401
Liu X, Zhang Y, Wang Y, Zhu W, Li G, Ma X, Liang X-J (2020) Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics 10(8):3793–3815. https://doi.org/10.7150/thno.40805
Raouf I, Khalid S, Khan A, Lee J, Kim HS, Kim M-H (2020) A review on numerical modeling for magnetic nanoparticle hyperthermia: Progress and challenges. J Therm Biol 91:102644. https://doi.org/10.1016/j.jtherbio.2020.102644
Alumutairi L, Yu B, Filka M, Nayfach J, Kim M-H (2020) Mild magnetic nanoparticle hyperthermia enhances the susceptibility of Staphylococcus aureus biofilm to antibiotics. Int J Hyperth 37(1):66–75. https://doi.org/10.1080/02656736.2019.1707886
Maier-Hauff K, Rothe R, Scholz R, Gneveckow U, Wust P, Thiesen B, Jordan A (2006) Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: results of a feasibility study on patients with glioblastoma multiforme. J Neurooncol 81(1):53–60. https://doi.org/10.1007/s11060-006-9195-0
Lepock JR (2004) Role of nuclear protein denaturation and aggregation in thermal radiosensitization. Int J Hyperth 20(2):115–130. https://doi.org/10.1080/02656730310001637334
Raaphorst GP (1999) Thermal radiosensitization and repair inhibition in human melanoma cells: a comparison of survival and DNA double strand breaks. Int J Hyperth 15(1):17–27. https://doi.org/10.1080/026567399285828
Coss RA, Linnemans WAM (1996) The effects of hyperthermia on the cytoskeleton: a review. Int J Hyperth 12(2):173–196. https://doi.org/10.3109/02656739609022507
Evans SS, Repasky EA, Fisher DT (2015) Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol 15(6):335–349. https://doi.org/10.1038/nri3843
Yagawa Y, Tanigawa K, Kobayashi Y, Yamamoto M (2017) Cancer immunity and therapy using hyperthermia with immunotherapy, radiotherapy, chemotherapy, and surgery. J Cancer Metastasis Treatment 3(10):218. https://doi.org/10.20517/2394-4722.2017.35
Mondal S, Manivasagan P, Bharathiraja S, Santha Moorthy M, Nguyen V, Kim H, Oh J (2017) Hydroxyapatite coated iron oxide nanoparticles: a promising nanomaterial for magnetic hyperthermia cancer treatment. Nanomaterials 7(12):426. https://doi.org/10.3390/nano7120426
Kirschning A, Kupracz L, Hartwig J (2012) New synthetic opportunities in miniaturized flow reactors with inductive heating. Chem Lett 41(6):562–570. https://doi.org/10.1246/cl.2012.562
Houlding TK, Rebrov EV (2012) Application of alternative energy forms in catalytic reactor engineering. Green Process Synthesis. https://doi.org/10.1515/greenps-2011-0502
Torres TE, Lima E, Calatayud MP, Sanz B, Ibarra A, Fernández-Pacheco R, Goya GF (2019) The relevance of Brownian relaxation as power absorption mechanism in Magnetic Hyperthermia. Scientif Rep. https://doi.org/10.1038/s41598-019-40341-y
Suto M, Hirota Y, Mamiya H, Fujita A, Kasuya R, Tohji K, Jeyadevan B (2009) Heat dissipation mechanism of magnetite nanoparticles in magnetic fluid hyperthermia. J Magn Magn Mater 321(10):1493–1496. https://doi.org/10.1016/j.jmmm.2009.02.070
Suriyanto NG, Kumar SD (2017) Physical mechanism and modeling of heat generation and transfer in magnetic fluid hyperthermia through Néelian and Brownian relaxation: a review. BioMed Eng Online. https://doi.org/10.1186/s12938-017-0327-x
Andrade ÂL, Cavalcante LCD, Fabris JD, Pereira MC, Fernandez-Outon LE, Pedersoli DC, Ferreira JMF (2020) Magnetically induced heating by iron oxide nanoparticles dispersed in liquids of different viscosities. Ceram Int 46(13):21496–21504. https://doi.org/10.1016/j.ceramint.2020.05.249
CHENG, F. (2005) Characterization of aqueous dispersions of Fe3O4 nanoparticles and their biomedical applications. Biomaterials 26(7):729–738. https://doi.org/10.1016/j.biomaterials.2004.03.016
Gawali SL, Shelar SB, Gupta J, Barick KC, Hassan PA (2021) Immobilization of protein on Fe3O4 nanoparticles for magnetic hyperthermia application. Int J Biol Macromol 166:851–860. https://doi.org/10.1016/j.ijbiomac.2020.10.241
Tansi FL, Fröbel F, Maduabuchi WO, Steiniger F, Westermann M, Quaas R, Hilger I (2021) Effect of matrix-modulating enzymes on the cellular uptake of magnetic nanoparticles and on magnetic hyperthermia treatment of pancreatic cancer models in vivo. Nanomaterials 11(2):438. https://doi.org/10.3390/nano11020438
Johannsen M, Thiesen B, Wust P, Jordan A (2010) Magnetic nanoparticle hyperthermia for prostate cancer. Int J Hyperth 26(8):790–795. https://doi.org/10.3109/02656731003745740
Olson B, Li Y, Lin Y, Liu ET, Patnaik A (2018) Mouse models for cancer immunotherapy research. Cancer Discov 8(11):1358–1365. https://doi.org/10.1158/2159-8290.cd-18-0044
Che Rose L, Bear JC, McNaughter PD, Southern P, Piggott RB, Parkin IP, Mayes AG (2016) A SPION-eicosane protective coating for water soluble capsules: Evidence for on-demand drug release triggered by magnetic hyperthermia. Scientif Rep. https://doi.org/10.1038/srep20271
Sengupta S, Balla VK (2018) A review on the use of magnetic fields and ultrasound for non-invasive cancer treatment. J Adv Res 14:97–111. https://doi.org/10.1016/j.jare.2018.06.003
Bhardwaj A, Parekh K, Jain N (2020) In vitro hyperthermic effect of magnetic fluid on cervical and breast cancer cells. Scientif Rep 10:1. https://doi.org/10.1038/s41598-020-71552-3
Parekh K, Bhardwaj A, Jain N (2020) Preliminary in-vitro investigation of magnetic fluid hyperthermia in cervical cancer cells. J Magn Magn Mater 497:166057. https://doi.org/10.1016/j.jmmm.2019.166057
Skandalakis GP, Rivera DR, Rizea CD, Bouras A, Jesu Raj JG, Bozec D, Hadjipanayis CG (2020) Hyperthermia treatment advances for brain tumors. Int J Hyperth 37(2):3–19. https://doi.org/10.1080/02656736.2020.1772512
Sadhasivam J, Sugumaran A (2020) Magnetic nanocarriers: emerging tool for the effective targeted treatment of lung cancer. J Drug Deliv Sci Technol 55:101493. https://doi.org/10.1016/j.jddst.2019.101493
Verma NK, Crosbie-Staunton K, Satti A, Gallagher S, Ryan KB, Doody T, Gun’ko YK (2013) Magnetic core-shell nanoparticles for drug delivery by nebulization. J Nanobiotechnol 11(1):1. https://doi.org/10.1186/1477-3155-11-1
Sadhukha T, Wiedmann TS, Panyam J (2013) Inhalable magnetic nanoparticles for targeted hyperthermia in lung cancer therapy. Biomaterials 34(21):5163–5171. https://doi.org/10.1016/j.biomaterials.2013.03.061
Kandasamy G, Sudame A, Luthra T, Saini K, Maity D (2018) Functionalized hydrophilic superparamagnetic iron oxide nanoparticles for magnetic fluid hyperthermia application in liver cancer treatment. ACS Omega 3(4):3991–4005. https://doi.org/10.1021/acsomega.8b00207
Pazos-Perez N, Pazos E, Catala C, Mir-Simon B, Gómez-de Pedro S, Sagales J, Alvarez-Puebla RA (2016) Ultrasensitive multiplex optical quantification of bacteria in large samples of biofluids. Scientif Rep. https://doi.org/10.1038/srep29014
Liao H, Hafner JH (2005) Gold nanorod bioconjugates. Chem Mater 17(18):4636–4641. https://doi.org/10.1021/cm050935k
Rahme K, Nolan MT, Doody T, McGlacken GP, Morris MA, O’Driscoll C, Holmes JD (2013) Highly stable PEGylated gold nanoparticles in water: applications in biology and catalysis. RSC Adv 3(43):21016. https://doi.org/10.1039/c3ra41873a
Xie J, Xu C, Kohler N, Hou Y, Sun S (2007) Controlled PEGylation of monodisperse Fe3O4 nanoparticles for reduced non-specific uptake by macrophage cells. Adv Mater 19(20):3163–3166. https://doi.org/10.1002/adma.200701975
Duan H, Nie S (2007) Cell-penetrating quantum dots based on multivalent and endosome-disrupting surface coatings. J Am Chem Soc 129(11):3333–3338. https://doi.org/10.1021/ja068158s
Voliani V, Luin S, Ricci F, Beltram F (2010) Single-step bifunctional coating for selectively conjugable nanoparticles. Nanoscale 2(12):2783. https://doi.org/10.1039/c0nr00350f
Guerrini L, Alvarez-Puebla R, Pazos-Perez N (2018) Surface Modifications of nanoparticles for stability in biological fluids. Materials 11(7):1154. https://doi.org/10.3390/ma11071154
Boles MA, Ling D, Hyeon T, Talapin DV (2016) The surface science of nanocrystals. Nat Mater 15(2):141–153. https://doi.org/10.1038/nmat4526
Patil RM, Thorat ND, Shete PB, Otari SV, Tiwale BM, Pawar SH (2016) In vitro hyperthermia with improved colloidal stability and enhanced SAR of magnetic core/shell nanostructures. Mater Sci Eng, C 59:702–709. https://doi.org/10.1016/j.msec.2015.10.064
Salunkhe AB, Khot VM, Ruso JM, Patil SI (2016) Water dispersible superparamagnetic Cobalt iron oxide nanoparticles for magnetic fluid hyperthermia. J Magn Magn Mater 419:533–542. https://doi.org/10.1016/j.jmmm.2016.06.057
Goswami MM, Dey C, Bandyopadhyay A, Sarkar D, Ahir M (2016) Micelles driven magnetite (Fe3O4) hollow spheres and a study on AC magnetic properties for hyperthermia application. J Magn Magn Mater 417:376–381. https://doi.org/10.1016/j.jmmm.2016.05.069
Reyes-Ortega F, Checa Fernández BL, Delgado AV, Iglesias GR (2019) Hyperthermia-triggered doxorubicin release from polymer-coated magnetic nanorods. Pharmaceutics 11(10):517. https://doi.org/10.3390/pharmaceutics11100517
Shete PB, Patil RM, Thorat ND, Prasad A, Ningthoujam RS, Ghosh SJ, Pawar SH (2014) Magnetic chitosan nanocomposite for hyperthermia therapy application: preparation, characterization and in vitro experiments. Appl Surf Sci 288:149–157. https://doi.org/10.1016/j.apsusc.2013.09.169
Oh Y, Lee N, Kang HW, Oh J (2016) In vitrostudy on apoptotic cell death by effective magnetic hyperthermia with chitosan-coated MnFe2O4. Nanotechnology 27(11):115101. https://doi.org/10.1088/0957-4484/27/11/115101
Prakash KASSP, Nishad KV, Komath M, Nair BN (2020) Amino acid inspired tunable superparamagnetic iron oxide (SPION) nanostructures with high magnetic hyperthermia potential for biofunctional applications. New J Chem 44(5):1962–1970. https://doi.org/10.1039/c9nj05343c
Salimi M, Sarkar S, Saber R, Delavari H, Alizadeh AM, Mulder HT (2018) Magnetic hyperthermia of breast cancer cells and MRI relaxometry with dendrimer-coated iron-oxide nanoparticles. Cancer Nanotechnol. https://doi.org/10.1186/s12645-018-0042-8
Sarma L, Borah JP, Srinivasan A, Sarma S (2019) Synthesis and characterization of tea polyphenol-coated magnetite nanoparticles for hyperthermia application. J Supercond Novel Magn 33(6):1637–1644. https://doi.org/10.1007/s10948-019-05189-3
Hatamie S, Parseh B, Ahadian MM, Naghdabadi F, Saber R, Soleimani M (2018) Heat transfer of PEGylated cobalt ferrite nanofluids for magnetic fluid hyperthermia therapy: In vitro cellular study. J Magn Magn Mater 462:185–194. https://doi.org/10.1016/j.jmmm.2018.05.020
Coffey WT, Crothers DSF, Kalmykov YP, Waldron JT (1995) Constant-magnetic-field effect in Néel relaxation of single-domain ferromagnetic particles. Phys Rev B 51(22):15947–15956. https://doi.org/10.1103/physrevb.51.15947
Attar MM, Haghpanahi M, Shahverdi H, Imam A (2016) Thermo-mechanical analysis of soft tissue in local hyperthermia treatment. J Mech Sci Technol 30(3):1459–1469. https://doi.org/10.1007/s12206-015-1053-6
Thorat ND, Lemine OM, Bohara RA, Omri K, El Mir L, Tofail SAM (2016) Superparamagnetic iron oxide nanocargoes for combined cancer thermotherapy and MRI applications. Phys Chem Chem Phys 18(31):21331–21339. https://doi.org/10.1039/c6cp03430f
Ghosh R, Pradhan L, Devi YP, Meena SS, Tewari R, Kumar A, Ningthoujam RS (2011) Induction heating studies of Fe3O4 magnetic nanoparticles capped with oleic acid and polyethylene glycol for hyperthermia. J Mater Chem 21(35):13388. https://doi.org/10.1039/c1jm10092k
Yallapu MM, Othman SF, Curtis ET, Gupta BK, Jaggi M, Chauhan SC (2011) Multi-functional magnetic nanoparticles for magnetic resonance imaging and cancer therapy. Biomaterials 32(7):1890–1905. https://doi.org/10.1016/j.biomaterials.2010.11.028
Zargar T, Kermanpur A, Labbaf S, Houreh AB, Esfahani MHN (2018) PEG coated Zn0.3Fe2.7O4 nanoparticles in the presence of <alpha>Fe2O3 phase synthesized by citric acid assisted hydrothermal reduction process for magnetic hyperthermia applications. Mater Chem Phys 212:432–439. https://doi.org/10.1016/j.matchemphys.2018.03.054
Wydra RJ, Kruse AM, Bae Y, Anderson KW, Hilt JZ (2013) Synthesis and characterization of PEG-iron oxide core-shell composite nanoparticles for thermal therapy. Mater Sci Eng, C 33(8):4660–4666. https://doi.org/10.1016/j.msec.2013.07.019
Von Rybinski W (1996) Alkyl glycosides and polyglycosides. Curr Opin Colloid Interface Sci 1(5):587–597. https://doi.org/10.1016/s1359-0294(96)80096-3
Söderberg I, Drummond CJ, Neil Furlong D, Godkin S, Matthews B (1995) Non-ionic sugar-based surfactants: Self assembly and air/water interfacial activity. Colloids Surf, A 102:91–97. https://doi.org/10.1016/0927-7757(95)03250-h
Linh PH, Anh NTN, Nam PH, Bach TN, Lam VD, Manh DH (2018) A facile ultrasound assisted synthesis of dextran-stabilized Co0.2Fe0.8Fe2O4 nanoparticles for hyperthermia application. IEEE Trans Magn 54(6):1–4. https://doi.org/10.1109/tmag.2018.2815080
Predescu AM, Matei E, Berbecaru AC, Pantilimon C, Drăgan C, Vidu R, Kuncser V (2018) Synthesis and characterization of dextran-coated iron oxide nanoparticles. R Soc Open Sci 5(3):171525. https://doi.org/10.1098/rsos.171525
El-Boubbou K, Zhu DC, Vasileiou C, Borhan B, Prosperi D, Li W, Huang X (2010) Magnetic glyco-nanoparticles: a tool to detect, differentiate, and unlock the glyco-codes of cancer via magnetic resonance imaging. J Am Chem Soc 132(12):4490–4499. https://doi.org/10.1021/ja100455c
Marradi M, García I, Penadés S (2011) Carbohydrate-based nanoparticles for potential applications in medicine. Nanoparticles Transl Sci Med. https://doi.org/10.1016/b978-0-12-416020-0.00004-8
Ban Z, Bosques CJ, Sasisekharan R (2008) A simple assay to probe disease-associated enzyme activity using glycosaminoglycan-assisted synthesized gold nanoparticles. Org Biomol Chem 6(23):4290. https://doi.org/10.1039/b813210k
Kamat M, El-Boubbou K, Zhu DC, Lansdell T, Lu X, Li W, Huang X (2010) Hyaluronic acid immobilized magnetic nanoparticles for active targeting and imaging of macrophages. Bioconjug Chem 21(11):2128–2135. https://doi.org/10.1021/bc100354m
Nath S, Kaittanis C, Tinkham A, Perez JM (2008) Dextran-coated gold nanoparticles for the assessment of antimicrobial susceptibility. Anal Chem 80(4):1033–1038. https://doi.org/10.1021/ac701969u
Mohammadi F, Moeeni M, Li C, Boukherroub R, Szunerits S (2020) Interaction of cellulose and nitrodopamine coated superparamagnetic iron oxide nanoparticles with alpha-lactalbumin. RSC Adv 10(16):9704–9716. https://doi.org/10.1039/c9ra09045b
Liu T, Bai R, Zhou H, Wang R, Liu J, Zhao Y, Chen C (2020) The effect of size and surface ligands of iron oxide nanoparticles on blood compatibility. RSC Adv 10(13):7559–7569. https://doi.org/10.1039/c9ra10969b
Dabbagh A, Hedayatnasab Z, Karimian H, Sarraf M, Yeong CH, Madaah Hosseini HR, Rahman NA (2018) Polyethylene glycol-coated porous magnetic nanoparticles for targeted delivery of chemotherapeutics under magnetic hyperthermia condition. Int J Hyperth 36(1):104–114. https://doi.org/10.1080/02656736.2018.1536809
Sherwood J, Xu Y, Lovas K, Qin Y, Bao Y (2017) Surface functionalization of dopamine coated iron oxide nanoparticles for various surface functionalities. J Magn Magn Mater 427:220–224. https://doi.org/10.1016/j.jmmm.2016.10.039
Yu B, Liu J, Liu S, Zhou F (2010) Pdop layer exhibiting zwitterionicity: a simple electrochemical interface for governing ion permeability. Chem Commun 46(32):5900. https://doi.org/10.1039/c0cc00596g
Gu X, Zhang Y, Sun H, Song X, Fu C, Dong P (2015) Mussel-inspired polydopamine coated iron oxide nanoparticles for biomedical application. J Nanomater 2015:1–12. https://doi.org/10.1155/2015/154592
Wu Y, Lu Z, Li Y, Yang J, Zhang X (2020) Surface modification of iron oxide-based magnetic nanoparticles for cerebral theranostics: application and prospection. Nanomaterials 10(8):1441. https://doi.org/10.3390/nano10081441
Patitsa M, Karathanou K, Kanaki Z, Tzioga L, Pippa N, Demetzos C, Klinakis A (2017) Magnetic nanoparticles coated with polyarabic acid demonstrate enhanced drug delivery and imaging properties for cancer theranostic applications. Scientif Rep. https://doi.org/10.1038/s41598-017-00836-y
Albarqi HA, Wong LH, Schumann C, Sabei FY, Korzun T, Li X, Taratula O (2019) Biocompatible nanoclusters with high heating efficiency for systemically delivered magnetic hyperthermia. ACS Nano 13(6):6383–6395. https://doi.org/10.1021/acsnano.8b06542
Vijayan VM, Beeran AE, Shenoy SJ, Muthu J, Thomas V (2019) New magneto-fluorescent hybrid polymer nanogel for theranostic applications. ACS Appl Bio Mater 2(2):757–768. https://doi.org/10.1021/acsabm.8b00616
Wei H, Bruns OT, Chen O, Bawendi MG (2012) Compact zwitterion-coated iron oxide nanoparticles for in vitro and in vivo imaging. Integr Biol 5(1):108–114. https://doi.org/10.1039/c2ib20142a
Mondini S, Leonzino M, Drago C, Ferretti AM, Usseglio S, Maggioni D, Ponti A (2015) Zwitterion-coated iron oxide nanoparticles: surface chemistry and intracellular uptake by hepatocarcinoma (HepG2) cells. Langmuir 31(26):7381–7390. https://doi.org/10.1021/acs.langmuir.5b01496
Xiao W, Lin J, Li M, Ma Y, Chen Y, Zhang C, Gu H (2012) Prolonged in vivo circulation time by zwitterionic modification of magnetite nanoparticles for blood pool contrast agents. Contrast Media Mol Imag 7(3):320–327. https://doi.org/10.1002/cmmi.501
Pombo-García K, Weiss S, Zarschler K, Ang C, Hübner R, Pufe J, Graham B (2016) Zwitterionic polymer-coated ultrasmall superparamagnetic iron oxide nanoparticles with low protein interaction and high biocompatibility. ChemNanoMat 2(10):959–971. https://doi.org/10.1002/cnma.201600233
Ma D, Chen J, Luo Y, Wang H, Shi X (2017) Zwitterion-coated ultrasmall iron oxide nanoparticles for enhanced T1-weighted magnetic resonance imaging applications. J Mater Chem B 5(35):7267–7273. https://doi.org/10.1039/c7tb01588g
Wei H, Bruns OT, Kaul MG, Hansen EC, Barch M, Wiśniowska A, Bawendi MG (2017) Exceedingly small iron oxide nanoparticles as positive MRI contrast agents. Proc Natl Acad Sci 114(9):2325–2330. https://doi.org/10.1073/pnas.1620145114
Iacovita C, Florea A, Scorus L, Pall E, Dudric R, Moldovan AI, Lucaciu CM (2019) Hyperthermia, cytotoxicity, and cellular uptake properties of manganese and zinc ferrite magnetic nanoparticles synthesized by a polyol-mediated process. Nanomaterials 9(10):1489. https://doi.org/10.3390/nano9101489
Barra A, Alves Z, Ferreira NM, Martins MA, Oliveira H, Ferreira LP, Ferreira P (2020) Biocompatible chitosan-based composites with properties suitable for hyperthermia therapy. J Mater Chem B 8(6):1256–1265. https://doi.org/10.1039/c9tb02067e
Hoque SM, Huang Y, Cocco E, Maritim S, Santin AD, Shapiro EM, Hyder F (2016) Improved specific loss power on cancer cells by hyperthermia and MRI contrast of hydrophilic FexCo1-xFe2O4nanoensembles. Contrast Media Mol Imag 11(6):514–526. https://doi.org/10.1002/cmmi.1713
Ferjaoui Z, Jamal Al Dine E, Kulmukhamedova A, Bezdetnaya L, Soon Chang C, Schneider R, Alem H (2019) Doxorubicin-loaded thermoresponsive superparamagnetic nanocarriers for controlled drug delivery and magnetic hyperthermia applications. ACS Appl Mater Interfaces 11(34):30610–30620. https://doi.org/10.1021/acsami.9b10444
Zhu N, Ji H, Shen C, Wu J, Niu J, Yang J, Niu X (2019) Study of alternating magnetic heating characteristics of mnzn ferrite nanoparticles for magnetic hyperthermia applications. IEEE Trans Appl Supercond 29(2):1–5. https://doi.org/10.1109/tasc.2018.2882416
Piazza RD, Viali WR, dos Santos CC, Nunes ES, Marques RFC, Morais PC, Jafelicci M (2020) PEGlatyon-SPION surface functionalization with folic acid for magnetic hyperthermia applications. Mater Res Expr 7(1):015078. https://doi.org/10.1088/2053-1591/ab6700
Fotukian SM, Barati A, Soleymani M, Alizadeh AM (2020) Solvothermal synthesis of CuFe2O4 and Fe3O4 nanoparticles with high heating efficiency for magnetic hyperthermia application. J Alloy Compd 816:152548. https://doi.org/10.1016/j.jallcom.2019.152548
Hedayatnasab Z, Dabbagh A, Abnisa F, Wan Daud WMA (2020) Polycaprolactone-coated superparamagnetic iron oxide nanoparticles for in vitro magnetic hyperthermia therapy of cancer. Eur Polymer J 133:109789. https://doi.org/10.1016/j.eurpolymj.2020.109789
Reyes-Ortega F, Delgado Á, Schneider E, Checa Fernández B, Iglesias G (2017) Magnetic nanoparticles coated with a thermosensitive polymer with hyperthermia properties. Polymers 10(1):10. https://doi.org/10.3390/polym10010010
Zhang L, Xue H, Gao C, Carr L, Wang J, Chu B, Jiang S (2010) Imaging and cell targeting characteristics of magnetic nanoparticles modified by a functionalizable zwitterionic polymer with adhesive 3,4-dihydroxyphenyl-l-alanine linkages. Biomaterials 31(25):6582–6588. https://doi.org/10.1016/j.biomaterials.2010.05.018
Wei R, Gong X, Lin H, Zhang K, Li A, Liu K, Gao J (2019) Versatile octapod-shaped hollow porous manganese(II) oxide nanoplatform for real-time visualization of cargo delivery. Nano Lett 19(8):5394–5402. https://doi.org/10.1021/acs.nanolett.9b01900
Li H, Han J, Liang G (2020) Phase transfer of hydrophobic nanoparticles using a zwitterionic sulfobetaine siloxane generates highly biocompatible and compact surfaces. ACS Appl Nano Mater 3(2):1489–1496. https://doi.org/10.1021/acsanm.9b02306
Malisova B, Tosatti S, Textor M, Gademann K, Zürcher S (2010) Poly(ethylene glycol) adlayers immobilized to metal oxide substrates through catechol derivatives: influence of assembly conditions on formation and stability. Langmuir 26(6):4018–4026. https://doi.org/10.1021/la903486z
Dalsin JL, Messersmith PB (2005) Bioinspired antifouling polymers. Mater Today 8(9):38–46. https://doi.org/10.1016/s1369-7021(05)71079-8
Zhou M, Luo G, Zhang Z, Li S, Wang C (2017) Synthesis and properties evaluation of sulfobetaine surfactant with double hydroxyl. J Mol Struct 1144:199–205. https://doi.org/10.1016/j.molstruc.2017.05.023
Ayranci E, Duman O (2007) Removal of anionic surfactants from aqueous solutions by adsorption onto high area activated carbon cloth studied by in situ UV spectroscopy. J Hazard Mater 148(1–2):75–82. https://doi.org/10.1016/j.jhazmat.2007.02.006
Duman O, Ayranci E (2010) Adsorptive removal of cationic surfactants from aqueous solutions onto high-area activated carbon cloth monitored by in situ UV spectroscopy. J Hazard Mater 174(1–3):359–367. https://doi.org/10.1016/j.jhazmat.2009.09.058
Tunç S, Duman O (2007) Investigation of interactions between some anionic dyes and cationic surfactants by conductometric method. Fluid Phase Equilib 251(1):1–7. https://doi.org/10.1016/j.fluid.2006.10.020
Yoshimura T, Nyuta K, Esumi K (2005) Zwitterionic heterogemini surfactants containing ammonium and carboxylate headgroups 1 adsorption and micellization. Langmuir 21(7):2682–2688. https://doi.org/10.1021/la047773b
Yaseen M, Wang Y, Su TJ, Lu JR (2005) Surface adsorption of zwitterionic surfactants: n-alkyl phosphocholines characterised by surface tensiometry and neutron reflection. J Colloid Interface Sci 288(2):361–370. https://doi.org/10.1016/j.jcis.2005.03.024
Zhou M, Zhao J, Hu X (2011) Synthesis of Bis[N, N′-(alkylamideethyl)ethyl] triethylenediamine bromide surfactants and their oilfield application investigation. J Surfactants Deterg 15(3):309–315. https://doi.org/10.1007/s11743-011-1313-0
Geng XF, Hu XQ, Jia XC, Luo LJ (2013) Effects of sodium salicylate on the microstructure of a novel zwitterionic gemini surfactant and its rheological responses. Colloid Polym Sci 292(4):915–921. https://doi.org/10.1007/s00396-013-3137-0
Raviv U, Giasson S, Kampf N, Gohy J-F, Jérôme R, Klein J (2003) Lubrication by charged polymers. Nature 425(6954):163–165. https://doi.org/10.1038/nature01970
Wu P, Grainger DW (2006) Drug/device combinations for local drug therapies and infection prophylaxis. Biomaterials 27(11):2450–2467. https://doi.org/10.1016/j.biomaterials.2005.11.031
Valsesia A, Colpo P, Manso Silvan M, Meziani T, Ceccone G, Rossi F (2004) Fabrication of nanostructured polymeric surfaces for biosensing devices. Nano Lett 4(6):1047–1050. https://doi.org/10.1021/nl0496567
Chae KH, Jang YM, Kim YH, Sohn O-J, Rhee JI (2007) Anti-fouling epoxy coatings for optical biosensor application based on phosphorylcholine. Sens Actuators, B Chem 124(1):153–160. https://doi.org/10.1016/j.snb.2006.12.012
Lee JH, Ju YM, Kim DM (2000) Platelet adhesion onto segmented polyurethane film surfaces modified by addition and crosslinking of PEO-containing block copolymers. Biomaterials 21(7):683–691. https://doi.org/10.1016/s0142-9612(99)00197-0
Advincula RC (2003) Surface initiated polymerization from nanoparticle surfaces. J Dispersion Sci Technol 24(3–4):343–361. https://doi.org/10.1081/dis-120021794
Rundqvist J, Hoh JH, Haviland DB (2005) Poly(ethylene glycol) self-assembled monolayer island growth. Langmuir 21(7):2981–2987. https://doi.org/10.1021/la0471792
Senaratne W, Andruzzi L, Ober CK (2005) Self-assembled monolayers and polymer brushes in biotechnology: current applications and future perspectives. Biomacromol 6(5):2427–2448. https://doi.org/10.1021/bm050180a
Konradi R, Pidhatika B, Mühlebach A, Textor M (2008) Poly-2-methyl-2-oxazoline: a peptide-like polymer for protein-repellent surfaces. Langmuir 24(3):613–616. https://doi.org/10.1021/la702917z
Rovira-Bru M, Giralt F, Cohen Y (2001) Protein adsorption onto zirconia modified with terminally grafted polyvinylpyrrolidone. J Colloid Interface Sci 235(1):70–79. https://doi.org/10.1006/jcis.2000.7355
Ruegsegger MA, Marchant RE (2001) Reduced protein adsorption and platelet adhesion by controlled variation of oligomaltose surfactant polymer coatings. J Biomed Mater Res 56(2):159–167. https://doi.org/10.1002/1097-4636(200108)56:2%3c159::AID-JBM1080%3e3.0.CO;2-R
Lewis AL, Tolhurst LA, Stratford PW (2002) Analysis of a phosphorylcholine-based polymer coating on a coronary stent pre- and post-implantation. Biomaterials 23(7):1697–1706. https://doi.org/10.1016/s0142-9612(01)00297-6
Statz AR, Meagher RJ, Barron AE, Messersmith PB (2005) New peptidomimetic polymers for antifouling surfaces. J Am Chem Soc 127(22):7972–7973. https://doi.org/10.1021/ja0522534
Lowe AB, McCormick CL (2002) Synthesis and solution properties of zwitterionic polymers. Chem Rev 102(11):4177–4190. https://doi.org/10.1021/cr020371t
Zhang Z, Chao T, Chen S, Jiang S (2006) Superlow fouling sulfobetaine and carboxybetaine polymers on glass slides. Langmuir 22(24):10072–10077. https://doi.org/10.1021/la062175d
Soo Choi H, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Frangioni JV (2007) Renal clearance of quantum dots. Nat Biotechnol 25(10):1165–1170. https://doi.org/10.1038/nbt1340
Radziuk D, Skirtach A, Sukhorukov G, Shchukin D, Möhwald H (2007) Stabilization of Silver nanoparticles by polyelectrolytes and poly(ethylene glycol). Macromol Rapid Commun 28(7):848–855. https://doi.org/10.1002/marc.200600895
Bakandritsos A, Psarras GC, Boukos N (2008) Some Physicochemical aspects of nanoparticulate magnetic iron oxide colloids in neat water and in the presence of Poly(vinyl alcohol). Langmuir 24(20):11489–11496. https://doi.org/10.1021/la801901j
Estephan ZG, Schlenoff PS, Schlenoff JB (2011) Zwitteration as an alternative to pegylation. Langmuir 27(11):6794–6800. https://doi.org/10.1021/la200227b
Schlenoff JB (2014) Zwitteration: coating surfaces with zwitterionic functionality to reduce nonspecific adsorption. Langmuir 30(32):9625–9636. https://doi.org/10.1021/la500057j
Walkey CD, Olsen JB, Guo H, Emili A, Chan WCW (2012) Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J Am Chem Soc 134(4):2139–2147. https://doi.org/10.1021/ja2084338
García KP, Zarschler K, Barbaro L, Barreto JA, O’Malley W, Spiccia L, Graham B (2014) Zwitterionic-coated “stealth” nanoparticles for biomedical applications: recent advances in countering biomolecular corona formation and uptake by the mononuclear phagocyte system. Small 10(13):2516–2529. https://doi.org/10.1002/smll.201303540
Kane RS, Deschatelets P, Whitesides GM (2003) Kosmotropes form the basis of protein-resistant surfaces. Langmuir 19(6):2388–2391. https://doi.org/10.1021/la020737x
Kim JC, Kim M, Jung J, Kim H, Kim IJ, Kim JR, Ree M (2012) Biocompatible characteristics of sulfobetaine-containing brush polymers. Macromol Res 20(7):746–753. https://doi.org/10.1007/s13233-012-0099-x
Schlenoff JB, Rmaile AH, Bucur CB (2008) Hydration Contributions to association in polyelectrolyte multilayers and complexes: visualizing hydrophobicity. J Am Chem Soc 130(41):13589–13597. https://doi.org/10.1021/ja802054k
Zhang Z, Vaisocherová H, Cheng G, Yang W, Xue H, Jiang S (2008) Nonfouling behavior of polycarboxybetaine-grafted surfaces: structural and environmental effects. Biomacromol 9(10):2686–2692. https://doi.org/10.1021/bm800407r
Ning J, Li G, Haraguchi K (2013) Synthesis of Highly Stretchable, mechanically tough, zwitterionic sulfobetaine nanocomposite gels with controlled thermosensitivities. Macromolecules 46(13):5317–5328. https://doi.org/10.1021/ma4009059
Aryal SKC, Bhattarai N, Kim CK, Kim HY (2006) Study of electrolyte induced aggregation of gold nanoparticles capped by amino acids. J Colloid Interface Sci 299(1):191–197. https://doi.org/10.1016/j.jcis.2006.01.045
Nosaka Y, Ohta N, Fukuyama T, Fujii N (1993) Size control of ultrasmall cds particles in aqueous solution by using various thiols. J Colloid Interface Sci 155(1):23–29. https://doi.org/10.1006/jcis.1993.1004
Bae W, Mehra RK (1998) Cysteine-capped ZnS nanocrystallites: preparation and characterization. J Inorg Biochem 70(2):125–135. https://doi.org/10.1016/s0162-0134(98)10008-9
Kolny-Olesiak J, Kloper V, Osovsky R, Sashchiuk A, Lifshitz E (2007) Synthesis and characterization of brightly photoluminescent CdTe nanocrystals. Surf Sci 601(13):2667–2670. https://doi.org/10.1016/j.susc.2006.12.013
Ahmadi R, Ranjbarnodeh E, Gu N (2012) Synthesizing cysteine-coated magnetite nanoparticles as MRI contrast agent: effect of pH and cysteine addition on particles size distribution. Mater Sci-Pol 30(4):382–389. https://doi.org/10.2478/s13536-012-0048-6
Mohammadi H, Farzinpour A, Vaziry A (2017) Reproductive performance of breeder quails fed diets supplemented with L-cysteine-coated iron oxide nanoparticles. Reprod Domest Anim 52(2):298–304. https://doi.org/10.1111/rda.12902
Iwasaki Y, Ishihara K (2012) Cell membrane-inspired phospholipid polymers for developing medical devices with excellent biointerfaces. Sci Technol Adv Mater 13(6):064101. https://doi.org/10.1088/1468-6996/13/6/064101
Hess M, Jones RG, Kahovec J, Kitayama T, Kratochvíl P, Kubisa P, Wilks ES (2006) Terminology of polymers containing ionizable or ionic groups and of polymers containing ions (IUPAC Recommendations 2006). Pure Appl Chem 78(11):2067–2074. https://doi.org/10.1351/pac200678112067
Laschewsky A (2014) Structures and synthesis of zwitterionic polymers. Polymers 6(5):1544–1601. https://doi.org/10.3390/polym6051544
Kudaibergenov S, Jaeger W, Laschewsky A (2006) Polymeric betaines: synthesis, characterization, and application. Adv Polym Sci. https://doi.org/10.1007/12_078
Laughlin RG (1991) Fundamentals of the zwitterionic hydrophilic group. Langmuir 7(5):842–847. https://doi.org/10.1021/la00053a006
Vaisocherová H, Yang W, Zhang Z, Cao Z, Cheng G, Piliarik M, Jiang S (2008) Ultralow fouling and functionalizable surface chemistry based on a zwitterionic polymer enabling sensitive and specific protein detection in undiluted blood plasma. Anal Chem 80(20):7894–7901. https://doi.org/10.1021/ac8015888
Yang W, Chen S, Cheng G, Vaisocherová H, Xue H, Li W, Jiang S (2008) Film thickness dependence of protein adsorption from blood serum and plasma onto poly(sulfobetaine)-grafted surfaces. Langmuir 24(17):9211–9214. https://doi.org/10.1021/la801487f
Yang W, Zhang L, Wang S, White AD, Jiang S (2009) Functionalizable and ultra stable nanoparticles coated with zwitterionic poly(carboxybetaine) in undiluted blood serum. Biomaterials 30(29):5617–5621. https://doi.org/10.1016/j.biomaterials.2009.06.036
Biehl P, von der Lühe M, Dutz S, Schacher F (2018) Synthesis, Characterization, and applications of magnetic nanoparticles featuring polyzwitterionic coatings. Polymers 10(1):91. https://doi.org/10.3390/polym10010091
Zhang X, Lin W, Chen S, Xu H, Gu H (2011) Development of a stable dual functional coating with low non-specific protein adsorption and high sensitivity for new superparamagnetic nanospheres. Langmuir 27(22):13669–13674. https://doi.org/10.1021/la202566d
Chen Y, Xiong Z, Zhang L, Zhao J, Zhang Q, Peng L, Zou H (2015) Facile synthesis of zwitterionic polymer-coated core–shell magnetic nanoparticles for highly specific capture of N-linked glycopeptides. Nanoscale 7(7):3100–3108. https://doi.org/10.1039/c4nr05955g
Von der Lühe M, Günther U, Weidner A, Gräfe C, Clement JH, Dutz S, Schacher FH (2015) SPION@polydehydroalanine hybrid particles. RSC Adv 5(40):31920–31929. https://doi.org/10.1039/c5ra01737h
Mincheva R, Stoilova O, Penchev H, Ruskov T, Spirov I, Manolova N, Rashkov I (2008) Synthesis of polymer-stabilized magnetic nanoparticles and fabrication of nanocomposite fibers thereof using electrospinning. Eur Polymer J 44(3):615–627. https://doi.org/10.1016/j.eurpolymj.2007.11.001
Robles J, Das R, Glassell M, Phan MH, Srikanth H (2018) Exchange-coupled Fe3O4/CoFe2O4 nanoparticles for advanced magnetic hyperthermia. AIP Adv 8(5):056719. https://doi.org/10.1063/1.5007249
Lee S-C, Fu C-M, Chang F-H (2013) Effects of core/shell structure on magnetic induction heating promotion in Fe3O4/γ-Fe2O3 magnetic nanoparticles for hyperthermia. Appl Phys Lett 103(16):163104. https://doi.org/10.1063/1.4825270
Nemati Z, Alonso J, Khurshid H, Phan MH, Srikanth H (2016) Core/shell iron/iron oxide nanoparticles: are they promising for magnetic hyperthermia? RSC Adv 6(45):38697–38702. https://doi.org/10.1039/c6ra05064f
Nemati Z, Alonso J, Martinez LM, Khurshid H, Garaio E, Garcia JA, Srikanth H (2016) Enhanced magnetic hyperthermia in iron oxide nano-octopods: size and anisotropy effects. J Phys Chem C 120(15):8370–8379. https://doi.org/10.1021/acs.jpcc.6b01426
Darwish MSA, Kim H, Lee H, Ryu C, Young Lee J, Yoon J (2020) Engineering core-shell structures of magnetic ferrite nanoparticles for high hyperthermia performance. Nanomaterials 10(5):991. https://doi.org/10.3390/nano10050991
Yang M, Ho C, Ruta S, Chantrell R, Krycka K, Hovorka O, Lai C (2018) Magnetic interaction of multifunctional core-shell nanoparticles for highly effective theranostics. Adv Mater 30(50):1802444. https://doi.org/10.1002/adma.201802444
Lavorato GC, Das R, Xing Y, Robles J, Litterst FJ, Baggio-Saitovitch E, Srikanth H (2020) Origin and shell-driven optimization of the heating power in core/shell bimagnetic nanoparticles. ACS Appl Nano Mater 3(2):1755–1765. https://doi.org/10.1021/acsanm.9b02449
Coşkun M, Çitoğlu S, Korkmaz M, Firat T (2017) The magnetic anisotropy effectiveness on NiFe2O4 and NiFe2O4@SiO2 nanoparticles for hyperthermia applications. Cumhuriyet Sci J. https://doi.org/10.17776/csj.363654
Xie J, Zhang Y, Yan C, Song L, Wen S, Zang F, Gu N (2014) High-performance PEGylated Mn–Zn ferrite nanocrystals as a passive-targeted agent for magnetically induced cancer theranostics. Biomaterials 35(33):9126–9136. https://doi.org/10.1016/j.biomaterials.2014.07.019
Yang Q, Gong M, Cai S, Zhang T, Douglas JT, Chikan V, Forrest ML (2015) Combining hard and soft magnetism into a single core-shell nanoparticle to achieve both hyperthermia and image contrast. Ther Deliv 6(10):1195–1210. https://doi.org/10.4155/tde.15.68
Fabris F, Lima E, De Biasi E, Troiani HE, Vásquez Mansilla M, Torres TE, Winkler EL (2019) Controlling the dominant magnetic relaxation mechanisms for magnetic hyperthermia in bimagnetic core–shell nanoparticles. Nanoscale 11(7):3164–3172. https://doi.org/10.1039/c8nr07834c
Nica V, Caro C, Páez-Muñoz JM, Leal MP, Garcia-Martin ML (2020) Bi-Magnetic core-shell CoFe2O4@MnFe2O4 nanoparticles for in vivo theranostics. Nanomaterials 10(5):907. https://doi.org/10.3390/nano10050907
Demillo VG, Zhu X (2015) Zwitterionic amphiphile coated magnetofluorescent nanoparticles – synthesis, characterization and tumor cell targeting. J Mater Chem B 3(42):8328–8336. https://doi.org/10.1039/c5tb01116g
Liu W, Zhong W, Du YW (2008) Magnetic nanoparticles with core/shell structures. J Nanosci Nanotechnol 8(6):2781–2792. https://doi.org/10.1166/jnn.2008.18307
Venkatesha N, Qurishi Y, Atreya HS, Srivastava C (2016) Effect of core–shell nanoparticle geometry on the enhancement of the proton relaxivity value in a nuclear magnetic resonance experiment. RSC Adv 6(69):64605–64610. https://doi.org/10.1039/c6ra11016a
Obaidat IM, Narayanaswamy V, Alaabed S, Sambasivam S, Muralee Gopi CVV (2019) Principles of magnetic hyperthermia: a focus on using multifunctional hybrid magnetic nanoparticles. Magnetochemistry 5(4):67. https://doi.org/10.3390/magnetochemistry5040067
Lee J-H, Jang J, Choi J, Moon SH, Noh S, Kim J, Cheon J (2011) Exchange-coupled magnetic nanoparticles for efficient heat induction. Nat Nanotechnol 6(7):418–422. https://doi.org/10.1038/nnano.2011.95
Nayek C, Manna K, Bhattacharjee G, Murugavel P, Obaidat I (2017) Investigating Size- and temperature-dependent coercivity and saturation magnetization in PEG coated Fe3O4 nanoparticles. Magnetochemistry 3(2):19. https://doi.org/10.3390/magnetochemistry3020019
Guardia P, Di Corato R, Lartigue L, Wilhelm C, Espinosa A, Garcia-Hernandez M, Pellegrino T (2012) Water-Soluble iron oxide nanocubes with high values of specific absorption rate for cancer cell hyperthermia treatment. ACS Nano 6(4):3080–3091. https://doi.org/10.1021/nn2048137
Martinez-Boubeta C, Simeonidis K, Makridis A, Angelakeris M, Iglesias O, Guardia P, Baldomir D (2013) Learning from nature to improve the heat generation of iron-oxide nanoparticles for magnetic hyperthermia applications. Scientif Rep. https://doi.org/10.1038/srep01652
Usov NA, Nesmeyanov MS, Gubanova EM, Epshtein NB (2019) Heating ability of magnetic nanoparticles with cubic and combined anisotropy. Beilstein J Nanotechnol 10:305–314. https://doi.org/10.3762/bjnano.10.29
López-Ortega A, Estrader M, Salazar-Alvarez G, Roca AG, Nogués J (2015) Applications of exchange coupled bi-magnetic hard/soft and soft/hard magnetic core/shell nanoparticles. Phys Rep 553:1–32. https://doi.org/10.1016/j.physrep.2014.09.007
Lavorato GC, Lima E, Troiani HE, Zysler RD, Winkler EL (2017) Tuning the coercivity and exchange bias by controlling the interface coupling in bimagnetic core/shell nanoparticles. Nanoscale 9(29):10240–10247. https://doi.org/10.1039/c7nr03740f
Noh S, Na W, Jang J, Lee J-H, Lee EJ, Moon SH, Cheon J (2012) Nanoscale magnetism control via surface and exchange anisotropy for optimized ferrimagnetic hysteresis. Nano Lett 12(7):3716–3721. https://doi.org/10.1021/nl301499u
Skoropata E, Desautels RD, Chi C-C, Ouyang H, Freeland JW, van Lierop J (2014) Magnetism of iron oxide based core-shell nanoparticles from interface mixing with enhanced spin-orbit coupling. Phys Rev B 89:2. https://doi.org/10.1103/physrevb.89.024410
Nogués J, Sort J, Langlais V, Skumryev V, Suriñach S, Muñoz JS, Baró MD (2005) Exchange bias in nanostructures. Phys Rep 422(3):65–117. https://doi.org/10.1016/j.physrep.2005.08.004
Phan M-H, Alonso J, Khurshid H, Lampen-Kelley P, Chandra S, Stojak Repa K, Srikanth H (2016) Exchange bias effects in iron oxide-based nanoparticle systems. Nanomaterials 6(11):221. https://doi.org/10.3390/nano6110221
Moon SH, Noh S, Lee J-H, Shin T-H, Lim Y, Cheon J (2017) Ultrathin interface regime of core-shell magnetic nanoparticles for effective magnetism tailoring. Nano Lett 17(2):800–804. https://doi.org/10.1021/acs.nanolett.6b04016
Song Q, Zhang ZJ (2012) Controlled synthesis and magnetic properties of bimagnetic spinel ferrite CoFe2O4 and MnFe2O4 nanocrystals with core-shell architecture. J Am Chem Soc 134(24):10182–10190. https://doi.org/10.1021/ja302856z
Zhang Q, Castellanos-Rubio I, Munshi R, Orue I, Pelaz B, Gries KI, Pralle A (2015) Model driven optimization of magnetic anisotropy of exchange-coupled core-shell ferrite nanoparticles for maximal hysteretic loss. Chem Mater 27(21):7380–7387. https://doi.org/10.1021/acs.chemmater.5b03261
Bordet A, Landis RF, Lee Y, Tonga GY, Asensio JM, Li C-H, Chaudret B (2019) Water-dispersible and biocompatible iron carbide nanoparticles with high specific absorption rate. ACS Nano 13(3):2870–2878. https://doi.org/10.1021/acsnano.8b05671
Kuimova MK, Botchway SW, Parker AW, Balaz M, Collins HA, Anderson HL, Ogilby PR (2009) Imaging intracellular viscosity of a single cell during photoinduced cell death. Nat Chem 1(1):69–73. https://doi.org/10.1038/nchem.120
Shaterabadi Z, Nabiyouni G, Soleymani M (2020) Correlation between effects of the particle size and magnetic field strength on the magnetic hyperthermia efficiency of dextran-coated magnetite nanoparticles. Mater Sci Eng, C 117:111274. https://doi.org/10.1016/j.msec.2020.111274
Avolio M, Guerrini A, Brero F, Innocenti C, Sangregorio C, Cobianchi M, Lascialfari A (2019) In-gel study of the effect of magnetic nanoparticles immobilization on their heating efficiency for application in magnetic fluid hyperthermia. J Magn Magn Mater 471:504–512. https://doi.org/10.1016/j.jmmm.2018.09.111
Bruvera IJ, Actis DG, Calatayud MP, Mendoza Zélis P (2019) Typical experiment vs. in-cell like conditions in magnetic hyperthermia: Effects of media viscosity and agglomeration. J Magn Magn Mater 491:165563. https://doi.org/10.1016/j.jmmm.2019.165563
De la Presa P, Luengo Y, Multigner M, Costo R, Morales MP, Rivero G, Hernando A (2012) Study of heating efficiency as a function of concentration, size, and applied field in γ-Fe2O3 nanoparticles. J Phys Chem C 116(48):25602–25610. https://doi.org/10.1021/jp310771p
Department of Inorganic Biomaterials (Kawashita Lab) http://www.tmd.ac.jp/bcr/research-e.html, website adopted date: May 2019.
Acknowledgements
Dr. Krishnamoorthi Chintagumpala thanks SERB, New Delhi, for providing EMR funding under file No.: EMR/2017/005081.
Funding
SERB, New Delhi, for providing EMR funding under file No.: EMR/2017/005081.
Author information
Authors and Affiliations
Contributions
VV contributed to writing—original draft, data curation, conceptualization;. KC designed conceptualization and helped in review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vijayakanth, V., Chintagumpala, K. A review on an effect of dispersant type and medium viscosity on magnetic hyperthermia of nanoparticles. Polym. Bull. 80, 4737–4781 (2023). https://doi.org/10.1007/s00289-022-04324-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04324-w