Abstract
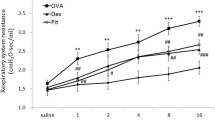
Sitagliptin, a new oral glucose lowering medication, is used for treatment of type 2 diabetes mellitus. The anti-inflammatory property of sitagliptin is reported, yet no studies have been done on asthma. In the present study, the effect of sitagliptin on allergic asthma was investigated using ovalbumin (OVA)-induced asthma model in mice. Swiss male albino mice sensitized and challenged to ovalbumin were treated with sitagliptin (8 mg/kg administered orally twice a day). Drug treatment was done on each day from days 16 to 23, 1 h before the challenge on the days of challenge. Sitagliptin treatment markedly decreased inflammatory cell accumulation in bronchoalveolar lavage (BAL) fluid and in the lungs, as revealed by histopathological examination. Furthermore, the levels of interleukin (IL)-13 in BAL fluid, total and OVA specific immunoglobulins (Ig)-E in serum, were significantly reduced as compared to the OVA group. In addition, sitagliptin significantly increased superoxidase dismutase (SOD) and reduced glutathione (GSH) activities with significant decrease in malondialdehyde (MDA) content in the lung. Importantly, sitagliptin decreased mRNA expression of the inflammatory cytokines tumor necrosis factor-α (TNF-α) and transforming growth factor-β1 (TGF-β1) in lung tissues as compared to the OVA group. Moreover, nitric oxide content as well as the mRNA expression of inducible nitric oxide synthase (iNOS) was remarkably decreased by sitagliptin treatment. Sitagliptin attenuates the allergic airway inflammation suggesting that sitagliptin may have applications in the treatment of bronchial asthma.







Similar content being viewed by others
Abbreviations
- BAL:
-
Bronchoalveolar lavage
- DPP-4:
-
Dipeptidylpeptidase-4
- IgE:
-
Immunoglobulin E
- IL:
-
Interleukin
- OVA:
-
Ovalbumin
- MDA:
-
Malondialdehyde
- GSH:
-
Reduced glutathione
- SOD:
-
Superoxide dismutase
- TNF-α:
-
Tumor necrosis factor-α
- TGF-β1 :
-
Transforming growth factor-β1
- iNOS:
-
Inducible nitric oxide synthase
References
Beckman JS, Koppenol WH (1996) Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and the ugly. Am J Physiol 271:C1424–C1437
Berger P, Girodet PO, Tunon-de-Lara JM (2005) Mast cell myositis: a new feature of allergic asthma. Allergy 60:1238–1240
Boldogh I, Bacsi A, Choudhury BK, Dharajiya N, Alam R, Hazra TK, Mitra S, Goldblum RM, Surs S (2005) ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J Clin Invest 115:2169–2179
Brightling CE, Symon FA, Birring SS, Bradding P, Pavord ID, Wardlaw AJ (2002) Th2 cytokine expression in bronchoalveolar lavage fluid T lymphocytes and bronchial submucosa is a feature of asthma and eosinophilic bronchitis. J Allergy Clin Immunol 110:899–905
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem 162:156–159
Ciencewicki J, Trivedi S, Kleeberger SR (2008) Oxidants and the pathogenesis of lung diseases. J Allergy Clin Immunol 122:456–468
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Hamelmann E, Tadeda K, Oshiba A, Gelfand EW (1999) Role of IgE in the development of allergic airway inflammation and airway hyperresponsiveness—a murine model. Allergy 54:297–305
Holgate ST (2008) The airway epithelium is central to the pathogenesis of asthma. Allergol Int 57:1–10
Ishii S, Nagase T, Shindou H, Takizawa H, Ouchi Y, Shimizu T (2004) Platelet-activating factor receptor develops airway hyperresponsiveness independently of airway inflammation in a murine asthma model. J Immunol 172:7095–7102
Kharitonov SA, Yates D, Robbins RA, Logan-Sinclair R, Shinebourne EA, Barnes PJ (1994) Increased nitric oxide in exhaled air of asthmatic patients. Lancet 343:133–135
Kruschinski C, Skripuletz T, Bedoui S, Tschernig T, Pabst R, Nassenstein C, Braun A, von Hörsten S (2005) CD26 (dipeptidyl-peptidase IV)-dependent recruitment of T cells in a rat asthma model. Clin Exp Immunol 139:17–24
Lee MY, Seo CS, Lee NH, Ha H, Lee JA, Lee H, Lee KY, Shin HK (2010) Anti-asthmatic effect of schizandrin on OVA-induced airway inflammation in a murine asthma model. Int Immunopharmacol 10:1374–1379
Mest HJ, Mentlein R (2005) Dipeptidyl peptidase inhibitors as new drugs for the treatment of type 2 diabetes. Diabetologia 48:616–620
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Moon DO, Kim MO, Lee HJ, Choi YH, Park YM, Heo MS, Kim GY (2008) Curcumin attenuates ovalbumin-induced airway inflammation by regulating nitric oxide. Biochem Biophys Res Commun 375:275–279
Morimoto C, Schlossman SF (1998) The structure and function of CD26 in the T-cell immune response. Immunol Rev 161:55–70
Myou S, Leff AR, Myo S, Boetticher E, Tong J, Meliton AY, Liu J, Munoz NM, Zhu X (2003) Blockade of inflammation and airway hyperresponsiveness in immunesensitized mice by dominant-negative phosphoinositide 3-kinase-TAT. J Exp Med 198:1573–1582
Nader MA (2011) Inhibition of anaphylaxis like reaction and mast cell activation by sitagliptin. Int Immunopharmacol 11:1052–1056
Nakajima H, Takatsu K (2007) Role of cytokines in allergic airway inflammation. Int Arch Allergy Immunol 142:265–273
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Pope SM, Brandt EB, Mishra A, Hogan SP, Zimmermann N, Matthaei KI, Foster PS, Rothenberg ME (2001) IL-13 induces eosinophil recruitment into the lung by an IL-5- and eotaxin-dependent mechanism. J Allergy Clin Immunol 108:594–601
Ricciardolo FLM (2003) Multiple roles of nitric oxide in the airways. Thorax 58:175–182
Roh SS, Kim SH, Lee YC, Seo YB (2008) Effects of radix adenophorae and cyclosporine A on an OVA-induced murine model of asthma by suppressing to T cells activity, eosinophilia, and bronchial hyper-responsiveness. Mediat Inflamm 781425:1–11
Sadeghi-Hashjin G, Folkerts G, Henricks PA, Verheyen AK, van der Linde HJ, van Ark I, Coene A, Nijkamp FP (1996) Peroxynitrite induces airway hyperresponsiveness in guinea pigs in vitro and in vivo. Am J Respir Crit Care Med 153:1697–1701
Secor ER Jr, Carson WF, Cloutier MM, Guernsey LA, Schramm CM, Wu CA, Thrall RS (2005) Bromelain exerts anti-inflammatory effects in an ovalbumin-induced murine model of allergic airway disease. Cell Immunol 237:68–75
Skripuletz T, Schmiedl A, Schade J, Bedoui S, Glaab T, Pabst R, von Hörsten S, Stephan M (2007) Dose-dependent recruitment of CD251 and CD261 T cells in a novel F344 rat model of asthma. Am J Physiol Lung Cell Mol Physiol 292:L1564–L1571
Teramoto S, Shu CY, Ouchi Y, Fukuchi Y (1996) Increased spontaneous production and generation of superoxide anion by blood neutrophils in patients with asthma. J Asthma 33:149–155
Thomas PS (2001) Tumour necrosis factor-alpha: the role of this multifunctional cytokine in asthma. Immunol Cell Biol 79:132–140
van der Velden VH, Wierenga-Wolf AF, Adriaansen-Soeting PW, Overbeek SE, Moller GM, Hoogsteden HC, Versnel MA (1998) Expression of aminopeptidase N and dipeptidyl peptidase IV in the healthy and asthmatic bronchus. Clin Exp Allergy 28:110–120
van der Velden VH, Naber BA, van Hal PT, Overbeek SE, Hoogsteden HC, Versnel MA (1999) Peptidase activities in serum and bronchoalveolar lavage fluid from allergic asthmatics—comparison with healthy non-smokers and smokers and effects of inhaled glucocorticoids. Clin Exp Allergy 29:813–823
Williams YN, Baba H, Hayashi S, Ikai H, Sugita T, Tanaka S, Miyasaka N, Kubota T (2003) Dipeptidyl peptidase IV on activated T cells as a target molecule for therapy of rheumatoid arthritis. Clin Exp Immunol 131:68–74
Wills-Karp M (1999) Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol 17:255–281
Xie S, Sukkar MB, Issa R, Khorasani NM, Chung KF (2007) Mechanisms of induction of airway smooth muscle hyperplasia by transforming growth factor-beta. Am J Physiol Lung Cell Mol Physiol 293:245–253
Yuk JE, Woo JS, Yun CY, Lee JS, Kim JH, Song GY, Yang EJ, Hur IK, Kim IS (2007) Effects of lactose-beta-sitosterol and beta-sitosterol on ovalbumin induced lung inflammation in actively sensitized mice. Int Immunopharmacol 7:1517–1527
Conflict of interest statement
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nader, M.A., El-Awady, M.S., Shalaby, A.A. et al. Sitagliptin exerts anti-inflammatory and anti-allergic effects in ovalbumin-induced murine model of allergic airway disease. Naunyn-Schmiedeberg's Arch Pharmacol 385, 909–919 (2012). https://doi.org/10.1007/s00210-012-0772-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-012-0772-9