Abstract
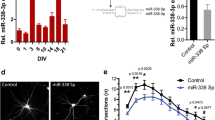
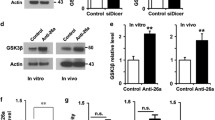
Proteins and microRNAs (miRNAs) within the axon locally regulate axonal development. However, protein profiles of distal axons of cortical neurons have not been fully investigated. In particular, networks of genes encoding axonal proteins and their related miRNAs in sub compartments of neurons such as axons remain unknown. Using embryonic cortical neurons cultured in a microfluidic device and proteomic approaches, we found that distal axons contain 883 proteins. Bioinformatics analysis revealed that 94 out of these 883 proteins are related to regulating axonal growth. Of the 94 genes encoding these proteins, there were 56 candidate genes that can be putatively targeted by axon-enriched 62 miRNAs with 8mer sites that exactly match these target genes. Among them, we validated 11 proteins and 11 miRNAs, by means of western blot and RT-PCR, respectively. Treatment of distal axons with chondroitin sulfate proteoglycans (CSPGs) that inhibit axonal growth elevated miR-133b, -203a, -29a, and -92a, which were associated with reduced protein level of AKT, MTOR, PI3K, DPYSL2, MAP1B, and PPP2CA. In contrast, reduction of miR-128, -15b, -195, -26b, -34b, -376b, and -381 by CSPGs was accompanied by increased EZR, KIF5A, DCX, GSK3B, and ROCK2 proteins. In silico pathway analysis revealed an interconnected network of these miRNAs and protein coding genes that is highly related to regulating axonal growth. Our data provide new insights into networks of miRNAs and their related proteins in distal axons in mediating axonal growth.




Similar content being viewed by others
References
Jung H, Yoon BC, Holt CE (2012) Axonal mRNA localization and local protein synthesis in nervous system assembly, maintenance and repair. Nat Rev Neurosci 13(5):308–324. https://doi.org/10.1038/nrn3210
Gomes C, Merianda TT, Lee SJ, Yoo S, Twiss JL (2014) Molecular determinants of the axonal mRNA transcriptome. Dev Neurobiol 74(3):218–232. https://doi.org/10.1002/dneu.22123
Estrada-Bernal ASS, Sosa LJ, Simon GC, Hansen KC et al (2012) Functional complexity of the axonal growth cone: a proteomic analysis. PLoS One 7(2):e31858
Igarashi M (2014) Proteomic identification of the molecular basis of mammalian CNS growth cones. Neurosci Res 88:1–15. https://doi.org/10.1016/j.neures.2014.07.005
van Niekerk EA, Tuszynski MH, Lu P, Dulin JN (2016) Molecular and cellular mechanisms of axonal regeneration after spinal cord injury. Mol Cell Proteomics 15(2):394–408. https://doi.org/10.1074/mcp.R115.053751
Natera-Naranjo O, Aschrafi A, Gioio AE, Kaplan BB (2010) Identification and quantitative analyses of microRNAs located in the distal axons of sympathetic neurons. RNA 16(8):1516–1529. https://doi.org/10.1261/rna.1833310
Aschrafi A, Schwechter AD, Mameza MG, Natera-Naranjo O, Gioio AE, Kaplan BB (2008) MicroRNA-338 regulates local cytochrome c oxidase IV mRNA levels and oxidative phosphorylation in the axons of sympathetic neurons. J Neurosci 28(47):12581–12590. https://doi.org/10.1523/JNEUROSCI.3338-08.2008
Zhang Y, Ueno Y, Liu XS, Buller B, Wang X, Chopp M, Zhang ZG (2013) The microRNA-17-92 cluster enhances axonal outgrowth in embryonic cortical neurons. J Neurosci 33(16):6885–6894. https://doi.org/10.1523/JNEUROSCI.5180-12.2013
Kaplan BB, Kar AN, Gioio AE, Aschrafi A (2013) MicroRNAs in the axon and presynaptic nerve terminal. Front Cell Neurosci 7:126. https://doi.org/10.3389/fncel.2013.00126
Liu B, Li J, Cairns MJ (2014) Identifying miRNAs, targets and functions. Brief Bioinform 15(1):1–19. https://doi.org/10.1093/bib/bbs075
Zhang Y, Chopp M, Liu XS, Kassis H, Wang X, Li C, An G, Zhang ZG (2015) MicroRNAs in the axon locally mediate the effects of chondroitin sulfate proteoglycans and cGMP on axonal growth. Dev Neurobiol 75:1402–1419. https://doi.org/10.1002/dneu.22292
Lee D-C, Hassan SS, Romero R, Tarca AL, Bhatti G, Gervasi MT, Caruso JA, Stemmer PM et al (2011) Protein profiling underscores immunological functions of uterine cervical mucus plug in human pregnancy. J Proteome 74(6):817–828. https://doi.org/10.1016/j.jprot.2011.02.025
Taylor AM, Blurton-Jones M, Rhee SW, Cribbs DH, Cotman CW, Jeon NL (2005) A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat Methods 2(8):599–605. https://doi.org/10.1038/nmeth777
Teunissen CE, Dijkstra C, Polman C (2005) Biological markers in CSF and blood for axonal degeneration in multiple sclerosis. Lancet Neurol 4(1):32–41. https://doi.org/10.1016/S1474-4422(04)00964-0
López-Bendito G, Flames N, Ma L, Fouquet C, Di Meglio T, Chedotal A, Tessier-Lavigne M, Marín O (2007) Robo1 and Robo2 cooperate to control the guidance of major axonal tracts in the mammalian forebrain. J Neurosci 27(13):3395–3407
Martínez–Yélamos A, Saiz A, Sanchez-Valle R, Casado V, Ramón JM, Graus F, Arbizu T (2001) 14-3-3 protein in the CSF as prognostic marker in early multiple sclerosis. Neurology 57(4):722–724
Skene JHP, Willard M (1981) Axonally transported proteins associated with axon growth in rabbit central and peripheral nervous systems. J Cell Biol 89(1):96–103
De Vos KJ, Grierson AJ, Ackerley S, Miller CCJ (2008) Role of axonal transport in neurodegenerative diseases. Annu Rev Neurosci 31(1):151–173. https://doi.org/10.1146/annurev.neuro.31.061307.090711
Elvira G, Wasiak S, Blandford V, Tong X-K, Serrano A, Fan X, del Rayo Sánchez-Carbente M, Servant F et al (2006) Characterization of an RNA granule from developing brain. Mol Cell Proteomics 5:635–651
Hörnberg H, Holt C (2013) RNA-binding proteins and translational regulation in axons and growth cones. Front Neurosci 7:81. https://doi.org/10.3389/fnins.2013.00081
Tripathi VB, Baskaran P, Shaw CE, Guthrie S (2014) Tar DNA-binding protein-43 (TDP-43) regulates axon growth in vitro and in vivo. Neurobiol Dis 65(100):25–34. https://doi.org/10.1016/j.nbd.2014.01.004
Sotelo-Silveira JR, Calliari A, Kun A, Koenig E, Sotelo JR (2006) RNA trafficking in axons. Traffic 7(5):508–515. https://doi.org/10.1111/j.1600-0854.2006.00405.x
Read D, Gorman A (2009) Involvement of Akt in neurite outgrowth. Cell Mol Life Sci 66(18):2975–2984. https://doi.org/10.1007/s00018-009-0057-8
Tohda C, Kuboyama T, Komatsu K (2005) Search for natural products related to regeneration of the neuronal network. Neurosignals 14(1–2):34–45
More SV, Koppula S, Kim I-S, Kumar H, Kim B-W, Choi D-K (2012) The role of bioactive compounds on the promotion of neurite outgrowth. Molecules 17(6):6728–6753
Miyaguchi K (2004) Localization of selenium-binding protein at the tips of rapidly extending protrusions. Histochem Cell Biol 121(5):371–376. https://doi.org/10.1007/s00418-004-0623-y
Lu W-c, Y-x Z, Qiao P, Zheng J, Wu Q, Shen Q (2018) The protocadherin alpha cluster is required for axon extension and myelination in the developing central nervous system. Neural Regen Res 13(3):427–433. https://doi.org/10.4103/1673-5374.228724
Mosca TJ, Luginbuhl DJ, Wang IE, Luo L (2017) Presynaptic LRP4 promotes synapse number and function of excitatory CNS neurons. eLife 6:e27347. https://doi.org/10.7554/eLife.27347
Terada K, Kojima Y, Watanabe T, Izumo N, Chiba K, Karube Y (2014) Inhibition of nerve growth factor-induced neurite outgrowth from PC12 cells by dexamethasone: signaling pathways through the glucocorticoid receptor and phosphorylated Akt and ERK1/2. PLoS One 9(3):e93223. https://doi.org/10.1371/journal.pone.0093223
Ketschek A, Jones S, Spillane M, Korobova F, Svitkina T, Gallo G (2015) Nerve growth factor promotes reorganization of the axonal microtubule array at sites of axon collateral branching. Dev Neurobiol 75(12):1441–1461. https://doi.org/10.1002/dneu.22294
Higuero AM, Sánchez-Ruiloba L, Doglio LE, Portillo F, Abad-Rodríguez J, Dotti CG, Iglesias T (2010) Kidins220/ARMS modulates the activity of microtubule-regulating proteins and controls neuronal polarity and development. J Biol Chem 285(2):1343–1357. https://doi.org/10.1074/jbc.M109.024703
Liz MA, Mar FM, Santos TE, Pimentel HI, Marques AM, Morgado MM, Vieira S, Sousa VF et al (2014) Neuronal deletion of GSK3β increases microtubule speed in the growth cone and enhances axon regeneration via CRMP-2 and independently of MAP1B and CLASP2. BMC Biol 12:47–47. https://doi.org/10.1186/1741-7007-12-47
Williams RR, Venkatesh I, Pearse DD, Udvadia AJ, Bunge MB (2015) MASH1/Ascl1a leads to GAP43 expression and axon regeneration in the adult CNS. PLoS One 10(3):e0118918. https://doi.org/10.1371/journal.pone.0118918
Sosa LJ, Bergman J, Estrada-Bernal A, Glorioso TJ, Kittelson JM, Pfenninger KH (2013) Amyloid precursor protein is an autonomous growth cone adhesion molecule engaged in contact guidance. PLoS One 8(5):e64521. https://doi.org/10.1371/journal.pone.0064521
Thelen K, Jaehrling S, Spatz JP, Pollerberg GE (2012) Depending on its nano-spacing, ALCAM promotes cell attachment and axon growth. PLoS One 7(12):e40493. https://doi.org/10.1371/journal.pone.0040493
Hur E-M, Zhou F-Q (2010) GSK3 signaling in neural development. Nat Rev Neurosci 11(8):539–551. https://doi.org/10.1038/nrn2870
Hur E-M, Saijilafu LBD, Kim S-J, Xu W-L, Zhou F-Q (2011) GSK3 controls axon growth via CLASP-mediated regulation of growth cone microtubules. Genes Dev 25(18):1968–1981. https://doi.org/10.1101/gad.17015911
Goldie BJ, Cairns MJ (2012) Post-transcriptional trafficking and regulation of neuronal gene expression. Mol Neurobiol 45(1):99–108. https://doi.org/10.1007/s12035-011-8222-0
Sasaki Y, Gross C, Xing L, Goshima Y, Bassell GJ (2014) Identification of axon-enriched microRNAs localized to growth cones of cortical neurons. Dev Neurobiol 74(3):397–406. https://doi.org/10.1002/dneu.22113
Nam J-W, Rissland OS, Koppstein D, Abreu-Goodger C, Jan CH, Agarwal V, Yildirim MA, Rodriguez A et al (2014) Global analyses of the effect of different cellular contexts on microRNA targeting. Mol Cell 53(6):1031–1043. https://doi.org/10.1016/j.molcel.2014.02.013
Silver J, Miller JH (2004) Regeneration beyond the glial scar. Nat Rev Neurosci 5(2):146–156
Twiss JL, Kalinski AL, Sachdeva R, Houle JD (2016) Intra-axonal protein synthesis – a new target for neural repair? Neural Regen Res 11(9):1365–1367. https://doi.org/10.4103/1673-5374.191193
Irena Ivanovska MAC (2008) Combinatorial microRNAs: working together to make a difference. Cell Cycle 7(20):3137–3142
Tarang S, Weston MD (2014) Macros in microRNA target identification: a comparative analysis of in silico, in vitro, and in vivo approaches to microRNA target identification. RNA Biol 11(4):324–333. https://doi.org/10.4161/rna.28649
Dajas-Bailador F, Bonev B, Garcez P, Stanley P, Guillemot F, Papalopulu N (2012) MicroRNA-9 regulates axon extension and branching by targeting Map1b in mouse cortical neurons. Nat Neurosci 15(5):697–699 http://www.nature.com/neuro/journal/v15/n5/abs/nn.3082.html#supplementary-information
Lai Y-W, Chu S-Y, Wei J-Y, Cheng C-Y, Li J-C, Chen P-L, Chen C-H, Yu H-H (2016) Drosophila microRNA-34 impairs axon pruning of mushroom body γ neurons by downregulating the expression of ecdysone receptor. Sci Rep 6:39141. https://doi.org/10.1038/srep39141
Sun X, Zhou Z, Fink DJ, Mata M (2013) HspB1 silences translation of PDZ-RhoGEF by enhancing miR-20a and miR-128 expression to promote neurite extension. Mol Cell Neurosci 57:111–119. https://doi.org/10.1016/j.mcn.2013.10.006
Funding
This work was supported by the National Institutes of Health (RO1 NS088656 and RO1 NS75156) and American Heart Association (16SDG29860003).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: C.L. and Z.Z. Performed the experiments: C.L., Y.Z., M.G., H.T., and B.F. Analyzed the data: C.L., A.L., and Z.Z. Prepared all the figures: C.L., Y.Z., and Z.Z. Wrote the manuscript: C.L., A.L., M.C., and Z.Z.
Corresponding author
Ethics declarations
The study was carried out in accordance with the NIH Guide for the Care and Use of Laboratory. Animals were approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Significance
Axonal proteins locally regulate axonal development. However, the protein profile in the distal axon has not been investigated. Using proteomic and quantitative RT-PCR approaches in combination with bioinformatics analysis, the present study identified a network of miRNAs and their target proteins in distal axons, which mediate axonal growth. This finding provides molecular basis for further investigating the roles of proteins and miRNAs within the distal axon in mediating axonal function.
Rights and permissions
About this article
Cite this article
Li, C., Zhang, Y., Levin, A.M. et al. Distal Axonal Proteins and Their Related MiRNAs in Cultured Cortical Neurons. Mol Neurobiol 56, 2703–2713 (2019). https://doi.org/10.1007/s12035-018-1266-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1266-7