Abstract
This review (with 139 refs.) gives a fundamental introduction into the sensors and logic gates based on G-quadruplexes (G4s). G4 characterizes vibrant binding activities and topology diversity, which contribute to the multiple signal output modes (including labeled moieties based on distance changes, label-free outputs by employing fluorescent ligands, G4/hemin DNAzyme with catalyzing activity and colorimetric readout using gold nanoparticles) and versatile design strategies (including target-induced G4 formation/disruption, liberation of blocked G4, split G4 probes, polymerase-assisted amplification and G4/hemin enrichment on sensor surface) of G4s-based methods. Following two important trends in logic gates (application of intelligent detection schemes and logic circuit constructions), specific sections review logic sensors and logic circuits based on G4s with several representative examples. We close this review with conclusions and give a perspective that employment of DNA technologies (such as aptamers, DNA junctions/origami, toehold-mediated strand displacement, enzyme-assisted amplification, metal ion-dependent DNAzymes) and various nanomaterials in G4s-based methods results in quite a large potential in terms of logic detection and construction of logic circuits.

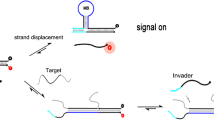
The vibrant binding activities, different structural topologies, multiple signal output modes and a variety of designing strategies contribute to the versatile roles of G-quadruplexes in the application and development of DNA logic gates and logic circuits.













Similar content being viewed by others
References
Adleman LM (1994) Molecular computation of solutions to combinatorial problems. Science 266:1021–1024
Wu G, Seeman NC (2006) Multiplying with DNA. Nat Comput 5:427–441
Ma DL, He HZ, Ma VPY, Chan DSH, Leung KH, Zhong HJ, Lu LH, Mergny JL, Leung CH (2012) Label-free sensing of pH and silver nanoparticles using an “OR” logic gate. Anal Chim Acta 733:78–83
Li W, Yang Y, Yan H, Liu Y (2013) Three-input majority logic gate and multiple input logic circuit based on DNA strand displacement. Nano Lett 13:2980–2988
Liu D, Chen W, Sun K, Deng K, Zhang W, Wang Z, Jiang X (2011) Resettable, multi-readout logic gates based on controllably reversible aggregation of gold nanoparticles. Angew Chem Int Ed 50:4103–4107
Liu Y, Dong B, Wu Z, Fang W, Zhou G, Shen A, Zhou X, Hu J (2014) Toehold-mediated DNA logic gates based on host-guest DNA-GNPs. Chem Commun 50:12026–12029
Xia F, Zuo X, Yang R, White RJ, Xiao Y, Kang D, Gong X, Lubin AA, Vallee-Belisle A, Yuen JD, Hsu BY, Plaxco KW (2010) Label-free, dual-analyte electrochemical biosensors: a new class of molecular-electronic logic gates. J Am Chem Soc 132:8557–8559
Li X, Sun L, Ding T (2011) Multiplexed sensing of mercury(II) and silver(I) ions: a new class of DNA electrochemiluminescent-molecular logic gates. Biosens Bioelectron 26:3570–3576
Ouyang Q, Kaplan PD, Liu S, Libchaber A (1997) DNA solution of the maximal clique problem. Science 278:446–449
Sakamoto K, Gouzu H, Komiya K, Kiga D, Yokoyama S, Yokomori T, Hagiya M (2000) Molecular computation by DNA hairpin formation. Science 288:1223–1226
Ma DL, He HZ, Chan DSH, Leung CH (2013) Simple DNA-based logic gates responding to biomolecules and metal ions. Chem Sci 4:3366–3380
Zhang M, Le HN, Wang P, Ye BC (2012) A versatile molecular beacon-like probe for multiplexed detection based on fluorescence polarization and its application for a resettable logic gate. Chem Commun 48:10004–10006
You M, Peng L, Shao N, Zhang L, Qiu L, Cui C, Tan W (2014) DNA “nano-claw”: logic-based autonomous cancer targeting and therapy. J Am Chem Soc 136:1256–1259
Wright WE, Tesmer VM, Huffman KE, Levene SD, Shay JW (1997) Normal human chromosomes have long G-rich telomeric overhangs at one end. Genes Dev 11:2801–2809
Jing N, Li Y, Xiong W, Sha W, Jing L, Tweardy DJ (2004) G-quartet oligonucleotides: A new class of signal transducer and activator of transcription 3 inhibitors that suppresses growth of prostate and breast tumors through induction of apoptosis. Cancer Res 64:6603–6609
Qi H, Lin C, Fu X, Wood LM, Liu A, Tsai YC, Chen Y, Barbieri CM, Pilch DS, Liu L (2006) G-quadruplexes induce apoptosis in tumor cells. Cancer Res 66:11808–11816
Verdun RE, Karlseder J (2007) Replication and protection of telomeres. Nature 447:924–931
Siddiqui-Jain A, Grand CL, Bearss DJ, Hurley LH (2002) Direct evidence for a G-quadruplex in a promoter region and its targeting with a small molecule to repress c-MYC transcription. Proc Natl Acad Sci U S A 99:11593–11598
Kumari S, Bugaut A, Balasubramanian S (2008) Position and stability are determining factors for translation repression by an RNA G-quadruplex-forming sequence within the 5′ UTR of the NRAS proto-oncogene. Biochemistry 47:12664–12669
Drygin D, Siddiqui-Jain A, O’brien S, Schwaebe M, Lin A, Bliesath J, Ho CB, Proffitt C, Trent K, Whitten JP, Lim JKC, Von Hoff D, Anderes K, Rice WG (2009) Anticancer activity of CX-3543: a direct inhibitor of rRNA biogenesis. Cancer Res 69:7653–7661
Sen D, Gilbert W (1988) Formation of parallel four-stranded complexes by guanine-rich motifs in DNA and its implications for meiosis. Nature 334:364–366
Creze C, Rinaldi B, Haser R, Bouvet P, Gouet P (2007) Structure of a d(TGGGGT) quadruplex crystallized in the presence of Li+ ions. Acta Crystallogr D Biol Crystallogr 63:682–688
Ida R, Wu G (2005) Solid-state 87Rb NMR signatures for rubidium cations bound to a G-quadruplex. Chem Commun 14:4294–4296
Marincola FC, Virno A, Randazzo A, Lai A (2007) Effect of rubidium and cesium ions on the dimeric quaduplex formed by the Oxytricha nova telomeric repeat oligonucleotide d(GGGGTTTTGGGG). Nucleosides Nucleotides Nucleic Acids 26:1129–1132
Nagesh N, Chatterji D (1995) Ammonium ion at low concentration stabilizes the G-quadruplex formation by telomeric sequence. J Biochem Biophys Methods 30:1–8
Smirnov I, Shafer RH (2000) Lead is unusually effective in sequence-specific folding of DNA. J Mol Biol 296:1–5
Miyoshi D, Nakao A, Sugimoto N (2003) Structural transition from antiparallel to parallel G-quadruplex of d(G4T4G4) induced by Ca2+. Nucleic Acids Res 31:1156–1163
Wei C, Tang Q, Li C (2008) Structural transition from the random coil to quadruplex of AG(3)(T(2)AG(3))(3) induced by Zn2+. Biophys Chem 132:110–113
Zhang DN, Huang T, Lukeman PS, Paukstelis PJ (2014) Crystal structure of a DNA/Ba2+ G-quadruplex containing a water-mediated C-tetrad. Nucleic Acids Res 42:13422–13429
Chen FM (1992) Sr2+ facilitates intermolecular G-quadruplex formation of telomeric sequences. Biochemistry 31:3769–3776
Nagesh N, Bhargava P, Chatterji D (1992) Terbium(III)-induced fluorescence of four-stranded G4-DNA. Biopolymers 32:1421–1424
Galezowska E, Gluszynska A, Juskowiak B (2007) Luminescence study of G-quadruplex formation in the presence of Tb3+ ion. J Inorg Biochem 101:678–685
Creacy SD, Routh ED, Iwamoto F, Nagamine Y, Akman SA, Vaughn JP (2008) G4 Resolvase 1 binds both DNA and RNA tetramolecular quadruplex with high affinity and is the major source of tetramolecular quadruplex G4-DNA and G4-RNA resolving activity in HeLa cell lysates. J Biol Chem 283:34626–34634
Fang G, Cech TR (1993) The beta subunit of Oxytricha telomere-binding protein promotes G-quartet formation by telomeric DNA. Cell 74:875–885
Giraldo R, Rhodes D (1994) The yeast telomere-binding protein RAP1 binds to and promotes the formation of DNA quadruplexes in telomeric DNA. EMBO J 13:2411–2420
Bock LC, Griffin LC, Latham JA, Vermaas EH, Toole JJ (1992) Selection of single-stranded-DNA molecules that bind and inhibit human thrombin. Nature 355:564–566
Nonaka Y, Sode K, Ikebukuro K (2010) Screening and improvement of an anti-VEGF DNA aptamer. Molecules 15:215–225
Ishikawa F, Matunis MJ, Dreyfuss G, Cech TR (1993) Nuclear proteins that bind the premessenger RNA 3′ splice-site sequence R(Uuag/G) and the human telomeric DNA-sequence D(Ttaggg)N. Mol Cell Biol 13:4301–4310
Dickinson LA, Kohwi-Shigematsu T (1995) Nucleolin is a matrix attachment region DNA-binding protein that specifically recognizes a region with high base-unpairing potential. Mol Cell Biol 15:456–465
Neidle S (2009) The structures of quadruplex nucleic acids and their drug complexes. Curr Opin Struct Biol 19:239–250
Simonsson T (2001) G-quadruplex DNA structures--variations on a theme. Biol Chem 382:621–628
Webba Da Silva M (2007) Geometric formalism for DNA quadruplex folding. Chemistry 13:9738–9745
Li T, Wang E, Dong S (2010) Parallel G-quadruplex-specific fluorescent probe for monitoring DNA structural changes and label-free detection of potassium ion. Anal Chem 82:7576–7580
He HZ, Wang M, Chan DS, Leung CH, Lin X, Lin JM, Ma DL (2013) A parallel G-quadruplex-selective luminescent probe for the detection of nanomolar calcium(II) ion. Methods 64:212–217
Credi A, Balzani V, Langford SJ, Stoddart JF (1997) Logic operations at the molecular level. An XOR gate based on a molecular machine. J Am Chem Soc 119:2679–2681
Han D, Kang H, Zhang T, Wu C, Zhou C, You M, Chen Z, Zhang X, Tan W (2014) Nucleic acid based logical systems. Chemistry 20:5866–5873
Lai YH, Sun SC, Chuang MC (2014) Biosensors with built-in biomolecular logic gates for practical applications. Biosens (Basel) 4:273–300
Zhao H, Dong J, Zhou F, Li B (2015) G-quadruplex-based homogenous fluorescence biosensing platform for ultrasensitive DNA detection through isothermal cycling and cascade signal amplification. Microchim Acta. doi:10.1007/s00604-015-1608-3
He HZ, Chan DS, Leung CH, Ma DL (2013) G-quadruplexes for luminescent sensing and logic gates. Nucleic Acids Res 41:4345–4359
Radi AE, Acero Sanchez JL, Baldrich E, O’sullivan CK (2006) Reagentless, reusable, ultrasensitive electrochemical molecular beacon aptasensor. J Am Chem Soc 128:117–124
Li X, Wang G, Ding X, Chen Y, Gou Y, Lu Y (2013) A “turn-on” fluorescent sensor for detection of Pb2+ based on graphene oxide and G-quadruplex DNA. Phys Chem Chem Phys 15:12800–12804
Li M, Zhou X, Guo S, Wu N (2013) Detection of lead (II) with a “turn-on” fluorescent biosensor based on energy transfer from CdSe/ZnS quantum dots to graphene oxide. Biosens Bioelectron 43:69–74
Ueyama H, Takagi M, Takenaka S (2002) A novel potassium sensing in aqueous media with a synthetic oligonucleotide derivative. Fluorescence resonance energy transfer associated with Guanine quartet-potassium ion complex formation. J Am Chem Soc 124:14286–14287
Yue Q, Shen T, Wang C, Wang L, Li H, Xu S, Wang H, Liu J (2013) Construction of a controllable Forster resonance energy transfer system based on G-quadruplex for DNA sensing. Biosens Bioelectron 40:75–81
Qian Z, Shan X, Chai L, Chen J, Feng H (2015) A fluorescent nanosensor based on graphene quantum dots-aptamer probe and graphene oxide platform for detection of lead (II) ion. Biosens Bioelectron 68:225–231
Sun D, Thompson B, Cathers BE, Salazar M, Kerwin SM, Trent JO, Jenkins TC, Neidle S, Hurley LH (1997) Inhibition of human telomerase by a G-quadruplex-interactive compound. J Med Chem 40:2113–2116
Largy E, Granzhan A, Hamon F, Verga D, Teulade-Fichou MP (2013) Visualizing the quadruplex: from fluorescent ligands to light-up probes. Top Curr Chem 330:111–177
Monchaud D, Allain C, Teulade-Fichou MP (2006) Development of a fluorescent intercalator displacement assay (G4-FID) for establishing quadruplex-DNA affinity and selectivity of putative ligands. Bioorg Med Chem Lett 16:4842–4845
Allain C, Monchaud D, Teulade-Fichou MP (2006) FRET templated by G-quadruplex DNA: a specific ternary interaction using an original pair of donor/acceptor partners. J Am Chem Soc 128:11890–11893
Granotier C, Pennarun G, Riou L, Hoffschir F, Gauthier LR, De Cian A, Gomez D, Mandine E, Riou JF, Mergny JL, Mailliet P, Dutrillaux B, Boussin FD (2005) Preferential binding of a G-quadruplex ligand to human chromosome ends. Nucleic Acids Res 33:4182–4190
Xu H, Gao SL, Yang Q, Pan D, Wang LH, Fan CH (2010) Amplified fluorescent recognition of G-Quadruplex folding with a cationic conjugated polymer and DNA intercalator. ACS Appl Mater Interfaces 2:3211–3216
Paramasivan S, Bolton PH (2008) Mix and measure fluorescence screening for selective quadruplex binders. Nucleic Acids Res 36, e106
Yang Q, Xiang J, Yang S, Zhou Q, Li Q, Tang Y, Xu G (2009) Verification of specific G-quadruplex structure by using a novel cyanine dye supramolecular assembly: I. Recognizing mixed G-quadruplex in human telomeres. Chem Commun:1103–1105
Maiti S, Chaudhury NK, Chowdhury S (2003) Hoechst 33258 binds to G-quadruplex in the promoter region of human c-myc. Biochem Biophys Res Commun 310:505–512
Jain AK, Bhattacharya S (2011) Interaction of G-quadruplexes with nonintercalating duplex-DNA minor groove binding ligands. Bioconjug Chem 22:2355–2368
Guo Q, Lu M, Marky LA, Kallenbach NR (1992) Interaction of the dye ethidium bromide with DNA containing guanine repeats. Biochemistry 31:2451–2455
Bhasikuttan AC, Mohanty J, Pal H (2007) Interaction of malachite green with guanine-rich single-stranded DNA: preferential binding to a G-quadruplex. Angew Chem Int Ed 46:9305–9307
Kong DM, Ma YE, Wu J, Shen HX (2009) Discrimination of G-quadruplexes from duplex and single-stranded DNAs with fluorescence and energy-transfer fluorescence spectra of crystal violet. Chemistry 15:901–909
Chang CC, Wu JY, Chien CW, Wu WS, Liu H, Kang CC, Yu LJ, Chang TC (2003) A fluorescent carbazole derivative: high sensitivity for quadruplex DNA. Anal Chem 75:6177–6183
Dumat B, Bordeau G, Faurel-Paul E, Mahuteau-Betzer F, Saettel N, Bombled M, Metge G, Charra F, Fiorini-Debuisschert C, Teulade-Fichou MP (2011) N-phenyl-carbazole-based two-photon fluorescent probes: strong sequence dependence of the duplex vs quadruplex selectivity. Biochimie 93:1209–1218
Arthanari H, Basu S, Kawano TL, Bolton PH (1998) Fluorescent dyes specific for quadruplex DNA. Nucleic Acids Res 26:3724–3728
Han FXG, Wheelhouse RT, Hurley LH (1999) Interactions of TMPyP4 and TMPyP2 with quadruplex DNA. Structural basis for the differential effects on telomerase inhibition. J Am Chem Soc 121:3561–3570
Wei C, Han G, Jia G, Zhou J, Li C (2008) Study on the interaction of porphyrin with G-quadruplex DNAs. Biophys Chem 137:19–23
Hong Y, Haussler M, Lam JW, Li Z, Sin KK, Dong Y, Tong H, Liu J, Qin A, Renneberg R, Tang BZ (2008) Label-free fluorescent probing of G-quadruplex formation and real-time monitoring of DNA folding by a quaternized tetraphenylethene salt with aggregation-induced emission characteristics. Chemistry 14:6428–6437
Luo J, Xie Z, Lam JW, Cheng L, Chen H, Qiu C, Kwok HS, Zhan X, Liu Y, Zhu D, Tang B (2001) Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem Commun:1740–1741
Arora A, Balasubramanian C, Kumar N, Agrawal S, Ojha RP, Maiti S (2008) Binding of berberine to human telomeric quadruplex - spectroscopic, calorimetric and molecular modeling studies. Febs J 275:3971–3983
Sun H, Tang Y, Xiang J, Xu G, Zhang Y, Zhang H, Xu L (2006) Spectroscopic studies of the interaction between quercetin and G-quadruplex DNA. Bioorg Med Chem Lett 16:3586–3589
Tan J, Ou T, Hou J, Lu Y, Huang S, Luo H, Wu J, Huang Z, Wong K, Gu L (2009) Isaindigotone derivatives: a new class of highly selective ligands for telomeric G-quadruplex DNA. J Med Chem 52:2825–2835
Ma D, Che C, Yan S (2009) Platinum(II) complexes with dipyridophenazine ligands as human telomerase inhibitors and luminescent probes for G-quadruplex DNA. J Am Chem Soc 131:1835–1846
Shi S, Geng X, Zhao J, Yao T, Wang C, Yang D, Zheng L, Ji L (2010) Interaction of [Ru(bpy)(2)(dppz)](2+) with human telomeric DNA: preferential binding to G-quadruplexes over i-motif. Biochimie 92:370–377
Alzeer J, Vummidi BR, Roth PJC, Luedtke NW (2009) Guanidinium-modified phthalocyanines as high-affinity G-quadruplex fluorescent probes and transcriptional regulators. Angew Chem Int Ed 48:9362–9365
Mergny JL, Lacroix L, Teulade-Fichou MP, Hounsou C, Guittat L, Hoarau M, Arimondo PB, Vigneron JP, Lehn JM, Riou JF, Garestier T, Helene C (2001) Telomerase inhibitors based on quadruplex ligands selected by a fluorescence assay. Proc Natl Acad Sci U S A 98:3062–3067
Teulade-Fichou MP, Carrasco C, Guittat L, Bailly C, Alberti P, Mergny JL, David A, Lehn JM, Wilson WD (2003) Selective recognition of G-qQuadruplex telomeric DNA by a bis(quinacridine) macrocycle. J Am Chem Soc 125:4732–4740
Manet I, Manoli F, Zambelli B, Andreano G, Masi A, Cellai L, Monti S (2011) Affinity of the anthracycline antitumor drugs Doxorubicin and Sabarubicin for human telomeric G-quadruplex structures. Phys Chem Chem Phys 13:540–551
Manet I, Manoli F, Zambelli B, Andreano G, Masi A, Cellai L, Ottani S, Marconi G, Monti S (2011) Complexes of the antitumoral drugs Doxorubicin and Sabarubicin with telomeric G-quadruplex in basket conformation: ground and excited state properties. Photochem Photobiol Sci 10:1326–1337
Vummidi BR, Alzeer J, Luedtke NW (2013) Fluorescent probes for G-quadruplex structures. Chembiochem 14:540–558
Travascio P, Li Y, Sen D (1998) DNA-enhanced peroxidase activity of a DNA-aptamer-hemin complex. Chem Biol 5:505–517
Li T, Dong S, Wang E (2009) Label-free colorimetric detection of aqueous mercury ion (Hg2+) using Hg2+-modulated G-quadruplex-based DNAzymes. Anal Chem 81:2144–2149
Hao Y, Guo Q, Wu H, Guo L, Zhong L, Wang J, Lin T, Fu F, Chen G (2014) Amplified colorimetric detection of mercuric ions through autonomous assembly of G-quadruplex DNAzyme nanowires. Biosens Bioelectron 52:261–264
Zhou WJ, Su J, Chai YQ, Yuan R, Xiang Y (2014) Naked eye detection of trace cancer biomarkers based on biobarcode and enzyme-assisted DNA recycling hybrid amplifications. Biosens Bioelectron 53:494–498
Li D, Shlyahovsky B, Elbaz J, Willner I (2007) Amplified analysis of low-molecular-weight substrates or proteins by the self-assembly of DNAzyme-aptamer conjugates. J Am Chem Soc 129:5804–5805
Li T, Wang E, Dong S (2008) G-quadruplex-based DNAzyme for facile colorimetric detection of thrombin. Chem Commun:3654–3656
Jiang X, Zhang H, Wu J, Yang X, Shao J, Lu Y, Qiu B, Lin Z, Chen G (2014) G-quadruplex DNA biosensor for sensitive visible detection of genetically modified food. Talanta 128:445–449
He K, Li W, Nie Z, Huang Y, Liu Z, Nie L, Yao S (2012) Enzyme-regulated activation of DNAzyme: a novel strategy for a label-free colorimetric DNA ligase assay and ligase-based biosensing. Chemistry 18:3992–3999
Kolpashchikov DM (2008) Split DNA enzyme for visual single nucleotide polymorphism typing. J Am Chem Soc 130:2934–2935
Yin B, Ye B, Tan W, Wang H, Xie C (2009) An allosteric dual-DNAzyme unimolecular probe for colorimetric detection of copper(II). J Am Chem Soc 131:14624–14625
Xiao Y, Pavlov V, Gill R, Bourenko T, Willner I (2004) Lighting up biochemiluminescence by the surface self-assembly of DNA-hemin complexes. Chembiochem 5:374–379
Freeman R, Liu X, Willner I (2011) Chemiluminescent and chemiluminescence resonance energy transfer (CRET) detection of DNA, metal ions, and aptamer-substrate complexes using hemin/G-quadruplexes and CdSe/ZnS quantum dots. J Am Chem Soc 133:11597–11604
Pelossof G, Tel-Vered R, Willner I (2012) Amplified surface plasmon resonance and electrochemical detection of Pb2+ ions using the Pb2+-dependent DNAzyme and Hemin/G-Quadruplex as a label. Anal Chem 84:3703–3709
Li H, Rothberg L (2004) Colorimetric detection of DNA sequences based on electrostatic interactions with unmodified gold nanoparticles. Proc Natl Acad Sci U S A 101:14036–14039
Xia F, Zuo X, Yang R, Xiao Y, Kang D, Vallee-Belisle A, Gong X, Yuen JD, Hsu BBY, Heeger AJ, Plaxco KW (2010) Colorimetric detection of DNA, small molecules, proteins, and ions using unmodified gold nanoparticles and conjugated polyelectrolytes. Proc Natl Acad Sci U S A 107:10837–10841
Wang J, Wang Y, Xue J, Zhou B, Qian Q, Wang Y, Yin J, Zhao H, Liu H, Liu S (2014) An ultrasensitive label-free assay of 8-hydroxy-2 ′-deoxyguanosine based on the conformational switching of aptamer. Biosens Bioelectron 58:22–26
Zhang Z, Sharon E, Freeman R, Liu X, Willner I (2012) Fluorescence detection of DNA, adenosine-5′-triphosphate (ATP), and telomerase activity by zinc(II)-protoporphyrin IX/G-quadruplex labels. Anal Chem 84:4789–4797
Ge J, Li XP, Jiang JH, Yu RQ (2014) A highly sensitive label-free sensor for Mercury ion (Hg2+) by inhibiting thioflavin T as DNA G-quadruplexes fluorescent inducer. Talanta 122:85–90
Zhou Z, Zhu J, Zhang L, Du Y, Dong S, Wang E (2013) G-quadruplex-based fluorescent assay of S1 nuclease activity and K+. Anal Chem 85:2431–2435
Ma DL, Lu LH, Lin S, He BY, Leung CH (2015) A G-triplex luminescent switch-on probe for the detection of mung bean nuclease activity. J Mater Chem B 3:348–352
Guo Y, Xu P, Hu H, Zhou X, Hu J (2013) A label-free biosensor for DNA detection based on ligand-responsive G-quadruplex formation. Talanta 114:138–142
Hu D, Pu F, Huang Z, Ren J, Qu X (2010) A quadruplex-based, label-free, and real-time fluorescence assay for RNase H activity and inhibition. Chemistry 16:2605–2610
Zhao C, Wu L, Ren J, Qu X (2011) A label-free fluorescent turn-on enzymatic amplification assay for DNA detection using ligand-responsive G-quadruplex formation. Chem Commun 47:5461–5463
Guo Y, Wang Q, Wang Z, Chen X, Xu L, Hu J, Pei R (2015) Label-free detection of T4 DNA ligase and polynucleotide kinase activity based on toehold-mediated strand displacement and split G-quadruplex probes. Sens Actuators B-Chem 214:50–55
Jiang H, Kong D, Shen H (2014) Amplified detection of DNA ligase and polynucleotide kinase/phosphatase on the basis of enrichment of catalytic G-quadruplex DNAzyme by rolling circle amplification. Biosens Bioelectron 55:133–138
Xue Q, Wang L, Jiang W (2013) A novel label-free cascade amplification strategy based on dumbbell probe-mediated rolling circle amplification-responsive G-quadruplex formation for highly sensitive and selective detection of NAD+ or ATP. Chem Commun 49:2640–2642
Wang X, Yin B, Wang P, Ye B (2013) Highly sensitive detection of microRNAs based on isothermal exponential amplification-assisted generation of catalytic G-quadruplex DNAzyme. Biosens Bioelectron 42:131–135
Xue Q, Lv Y, Xu S, Zhang Y, Wang L, Li R, Yue Q, Li H, Gu X, Zhang S, Liu J (2015) Highly sensitive fluorescence assay of DNA methyltransferase activity by methylation-sensitive cleavage-based primer generation exponential isothermal amplification-induced G-quadruplex formation. Biosens Bioelectron 66:547–553
Zhang P, Wu X, Yuan R, Chai Y (2015) An “off-on” electrochemiluminescent biosensor based on DNAzyme-assisted target recycling and rolling circle amplifications for ultrasensitive detection of microRNA. Anal Chem 87:3202–3207
Zhou J, Lai W, Zhuang J, Tang J, Tang D (2013) Nanogold-functionalized DNAzyme concatamers with redox-active intercalators for quadruple signal amplification of electrochemical immunoassay. ACS Appl Mater Interfaces 5:2773–2781
Jing P, Xu W, Yi H, Wu Y, Bai L, Yuan R (2014) An amplified electrochemical aptasensor for thrombin detection based on pseudobienzymic Fe3O4-Au nanocomposites and electroactive hemin/G-quadruplex as signal enhancers. Analyst 139:1756–1761
Zhang H, Wang Y, Wu M, Feng Q, Shi H, Chen H, Xu J (2014) Visual electrochemiluminescence detection of telomerase activity based on multifunctional Au nanoparticles modified with G-quadruplex deoxyribozyme and luminol. Chem Commun 50:12575–12577
Li T, Wang E, Dong S (2009) Potassium-lead-switched G-quadruplexes: a new class of DNA logic gates. J Am Chem Soc 131:15082–15083
Guo JH, Kong DM, Shen HX (2010) Design of a fluorescent DNA IMPLICATION logic gate and detection of Ag+ and cysteine with triphenylmethane dye/G-quadruplex complexes. Biosens Bioelectron 26:327–332
Li T, Zhang L, Ai J, Dong S, Wang E (2011) Ion-tuned DNA/Ag fluorescent nanoclusters as versatile logic device. ACS Nano 5:6334–6338
Li T, Ackermann D, Hall AM, Famulok M (2012) Input-dependent induction of oligonucleotide structural motifs for performing molecular logic. J Am Chem Soc 134:3508–3516
Wang Z, Ning L, Duan A, Zhu X, Wang H, Li G (2012) A set of logic gates fabricated with G-quadruplex assembled at an electrode surface. Chem Commun 48:7507–7509
Zhao Y, Zhang Q, Wang W, Jin Y (2013) Input-dependent induction of G-quadruplex formation for detection of lead (II) by fluorescent ion logic gate. Biosens Bioelectron 43:231–236
Zhou J, Amrane S, Korkut DN, Bourdoncle A, He H, Ma DL, Mergny JL (2013) Combination of i-motif and G-quadruplex structures within the same strand: formation and application. Angew Chem Int Ed 52:7742–7746
Zhai W, Du C, Li X (2014) A series of logic gates based on electrochemical reduction of Pb2+ in self-assembled G-quadruplex on the gold electrode. Chem Commun 50:2093–2095
Guo Y, Zhou L, Xu L, Zhou X, Hu J, Pei R (2014) Multiple types of logic gates based on a single G-quadruplex DNA strand. Sci Rep 4:7315
Li T, Lohmann F, Famulok M (2014) Interlocked DNA nanostructures controlled by a reversible logic circuit. Nat Commun 5:4940
Zhu J, Zhang L, Dong S, Wang E (2013) Four-way junction-driven DNA strand displacement and its application in building majority logic circuit. ACS Nano 7:10211–10217
Zhu J, Zhang L, Li T, Dong S, Wang E (2013) Enzyme-free unlabeled DNA logic circuits based on toehold-mediated strand displacement and split G-quadruplex enhanced fluorescence. Adv Mater 25:2440–2444
Guo Y, Cheng J, Wang J, Zhou X, Hu J, Pei R (2014) Label-free logic modules and two-layer cascade based on stem-loop probes containing a g-quadruplex domain. Chem Asian J 9:2397–2401
Chen J, Zhou S, Wen J (2015) Concatenated logic circuits based on a three-way DNA junction: a keypad-lock security system with visible readout and an automatic reset function. Angew Chem Int Ed 54:446–450
Lan T, Furuya K, Lu Y (2010) A highly selective lead sensor based on a classic lead DNAzyme. Chem Commun 46:3896–3898
Zhu J, Zhang L, Zhou Z, Dong S, Wang E (2014) Aptamer-based sensing platform using three-way DNA junction-driven strand displacement and its application in DNA logic circuit. Anal Chem 86:312–316
Seelig G, Soloveichik D, Zhang DY, Winfree E (2006) Enzyme-free nucleic acid logic circuits. Science 314:1585–1588
Park KS, Seo MW, Jung C, Lee JY, Park HG (2012) Simple and universal platform for logic gate operations based on molecular beacon probes. Small 8:2203–2212
Zhang DY, Seelig G (2011) Dynamic DNA nanotechnology using strand-displacement reactions. Nat Chem 3:103–113
Qian L, Winfree E, Bruck J (2011) Neural network computation with DNA strand displacement cascades. Nature 475:368–372
Cornett EM, Campbell EA, Gulenay G, Peterson E, Bhaskar N, Kolpashchikov DM (2012) Molecular logic gates for DNA analysis: detection of rifampin resistance in M. tuberculosis DNA. Angew Chem Int Ed 51:9075–9077
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (Nos. 21275156, 31300096, 31301495, 21305154, 81471696, 41273093, 21203076), the CAS Hundred Talents program and the CAS/SAFEA International Innovation Teams program, and National Key Technology R&D Program in the 12th Five year Plan of China (2012BAD36B02).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Guo, Y., Yao, W., Xie, Y. et al. Logic gates based on G-quadruplexes: principles and sensor applications. Microchim Acta 183, 21–34 (2016). https://doi.org/10.1007/s00604-015-1633-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1633-2