Identification of key gene modules and hub genes of human mantle cell lymphoma by coexpression network analysis
- Published
- Accepted
- Received
- Academic Editor
- Ugo Bastolla
- Subject Areas
- Bioinformatics, Hematology, Oncology
- Keywords
- Mantle cell lymphoma, Coexpression network analysis, Modules, Hub genes, Survival
- Copyright
- © 2020 Guo et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2020. Identification of key gene modules and hub genes of human mantle cell lymphoma by coexpression network analysis. PeerJ 8:e8843 https://doi.org/10.7717/peerj.8843
Abstract
Purpose
Mantle cell lymphoma (MCL) is a rare and aggressive subtype of non-Hodgkin lymphoma that is incurable with standard therapies. The use of gene expression analysis has been of interest, recently, to detect biomarkers for cancer. There is a great need for systemic coexpression network analysis of MCL and this study aims to establish a gene coexpression network to forecast key genes related to the pathogenesis and prognosis of MCL.
Methods
The microarray dataset GSE93291 was downloaded from the Gene Expression Omnibus database. We systematically identified coexpression modules using the weighted gene coexpression network analysis method (WGCNA). Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) functional enrichment analysis were performed on the modules deemed important. The protein–protein interaction networks were constructed and visualized using Cytoscape software on the basis of the STRING website; the hub genes in the top weighted network were identified. Survival data were analyzed using the Kaplan–Meier method and were compared using the log-rank test.
Results
Seven coexpression modules consisting of different genes were applied to 5,000 genes in the 121 human MCL samples using WGCNA software. GO and KEGG enrichment analysis identified the blue module as one of the most important modules; the most critical pathways identified were the ribosome, oxidative phosphorylation and proteasome pathways. The hub genes in the top weighted network were regarded as real hub genes (IL2RB, CD3D, RPL26L1, POLR2K, KIF11, CDC20, CCNB1, CCNA2, PUF60, SNRNP70, AKT1 and PRPF40A). Survival analysis revealed that seven genes (KIF11, CDC20, CCNB1, CCNA2, PRPF40A, CD3D and PUF60) were associated with overall survival time (p < 0.05).
Conclusions
The blue module may play a vital role in the pathogenesis of MCL. Five real hub genes (KIF11, CDC20, CCNB1, CCNA2 and PUF60) were identified as potential prognostic biomarkers as well as therapeutic targets with clinical utility for MCL.
Introduction
Mantle cell lymphoma (MCL) is a rare, aggressive malignancy with a low survival rate comprising approximately 6% of non-Hodgkin lymphoma (NHL) cases. MCL patients are typically male with a median age over 60 (Vose, 2017). MCL is largely incurable, although traditional chemotherapy can induce a high rate of remission in previously untreated patients. However, relapse is common within a few years. An intense first-line treatment can improve a patient’s progression-free survival, however, there is still no curative regimen. Therefore, additional insights into the pathology and genetic etiology of MCL may offer new treatment solutions for MCL.
Genetic variations are reportedly related to the occurrence of MCL. MCL is characterized by the presence of t(11;14)(q13;q32), which is closely correlated with cyclin D1 overexpression (Ladha et al., 2019). The activation of cell survival pathways and alterations in the DNA damage response contribute to the constitutive dysregulation of the cell cycle and are incorporated to promote the pathogenesis of MCL. There are two different molecular subtypes of MCL, namely the classical MCL and the leukemic nonnodal subtype (Quintanilla-Martinez, 2017). However, the molecular biomarkers of MCL remain unclear. The molecular biomarkers for MCL must be identified to provide targets with which to identify its pathogenesis and to develop personalized treatment strategies. Bomben et al. (2018) have reported that six representative genes (AKT3, BCL2, BTK, CD79B, PIK3CD and SYK) through the analysis of a prediction model were correlated with a poor clinical response in MCL. Ferrero et al. (2019) confirmed mutations of KMT2D and disruption of TP53 had a significantly increased risk of progression and death by target resequencing and DNA profiling in MCL samples. The pathogenesis of MCL is a result of complex molecular mechanisms involving genetic factors, which have not yet been elucidated.
The majority of studies to date have focused on the differential expression of genes associated with MCL but have ignored their high degree of interconnectivity. Expression profiling analysis based on microarrays contributes to measuring gene expression at the genome-wide level. The weighted gene coexpression network analysis (WGCNA) is used to analyze a biological system and can detect an array of genes with similar expression levels as well as their relevant biological functions in diverse physical processes. These analyses can be defined as one gene module. WGCNA can identify the gene modules related to clinical diagnoses and has been used comprehensively in cancer-related research, including for cancers of gastric region and soft tissue (Gong et al., 2019; Zhu et al., 2019).
We constructed a coexpression network from a dataset consisting of 20,822 genes of 123 human MCL samples. Enrichment analysis of Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) were conducted to analyze the gene functions in the six constructed coexpression modules. The protein–protein interaction (PPI) networks were constructed and visualized using the Cytoscape software and the hub genes in the top weighted network were identified. Survival analysis revealed that seven genes (KIF11, CDC20, CCNB1, CCNA2, PRPF40A, CD3D and PUF60) were associated with overall survival time. The informative genes identified in this research may contribute to the future clinical treatment of MCL.
Materials and Methods
Microarray data analysis
Analysis was performed on the raw gene expressions of the MCL datasets and the corresponding clinical follow-up obtained from the GEO data repository (http://www.ncbi.nlm.nih.gov/geo). GSE93291, a much larger and newer microarray dataset of MCL, included a total of 123 samples. Another dataset of GSE132929 was downloaded to verify the stability of the real hub genes. This dataset included 43 MCL samples. GPL570 (Affymetrix Human Genome U133 Plus 2.0 Array) was selected for the microarray. Gene IDs were mapped to the microarray probes using the annotated information offered by the record. Probes corresponding to more than one gene were excluded from the dataset. The average expression values of the genes was obtained using measurements from a number of probes. A suitable threshold value was selected based on the number of probes with different thresholds of expression. The WGCNA algorithm (Langfelder & Horvath, 2008) was applied to build the coexpression network. Samples cluster analysis was performed using the hclust tool (R package, https://www.rdocumentation.org/packages/stats/versions/3.6.1/topics/hclust) with a threshold value of 67 in GSE93291.
Coexpression modules construction
The power value was screened out during the construction of the modules using the WGCNA package in R (https://cran.r-project.org/web/packages/WGCNA/). The mean connectivity and scale independence of network modules were analyzed using the gradient test under different power values, which ranged from 1 to 20. The soft threshold power of 8 was selected according to the scale-free topology criterion. The WGCNA algorithm further identified coexpression modules under these conditions. The minimum size of the gene group was set at 50 to ensure the reliability of the results for this module.
Interaction analysis of coexpression modules
The interactive relationship among the coexpression modules was studied using the WGCNA algorithm. The WGCNA R software package can be used to determine network construction, the calculation of topological properties, gene selection, module detection, differential network analysis, and network statistics. We chose a height cut of 0.25 (red line), which corresponds with a correlation of 0.75, to merge similar modules. A heat map was drawn to display the intensity of the interaction among the modules.
Analysis of functional and pathway enrichment
Functional enrichment analysis was carried out in coexpression modules. The genetic information of the respective modules was mapped to the associated GO terms and KEGG pathways using the DAVID tool (version 6.8; http://david.abcc.ncifcrf.gov/) (Huang, Sherman & Lempicki, 2009). The top five records with p-value < 0.05 were retained for analysis.
Hub gene analysis and identification
The genes in the coexpression modules were uploaded to the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) online database (version 11.0) to evaluate PPI information and construct a functional protein association network (Szklarczyk et al., 2017). Interactions with combined scores above 0.9 were considered significant. The PPI networks were constructed and visualized using Cytoscape software (version 3.7.1; http://www.cytoscape.org/) (Su et al., 2014). PPI hub genes were detected using the cytoHubba plugin with the degree of connectivity set to the top 5%. The weighted networks of the top genes were also ranked by weighted degree in each coexpression module and were visualized using Cytoscape software. The PPI hub genes in the top weighted network were considered to be real hub genes. The validation was performed in the GSE132929 dataset. We used the same approach to detect the real hub genes.
Survival analysis
Survival analysis was performed for the real hub genes using the R package of survival (https://CRAN.R-project.org/package=survival). The samples were categorized into high and low groups according to the median expression value of each real hub gene. Kaplan–Meier analysis and the log-rank test were used to analyze the correlation between the expression of the real hub gene and its corresponding prognostic information. Significance was considered to be p < 0.05.
Results
MCL dataset pre-processing
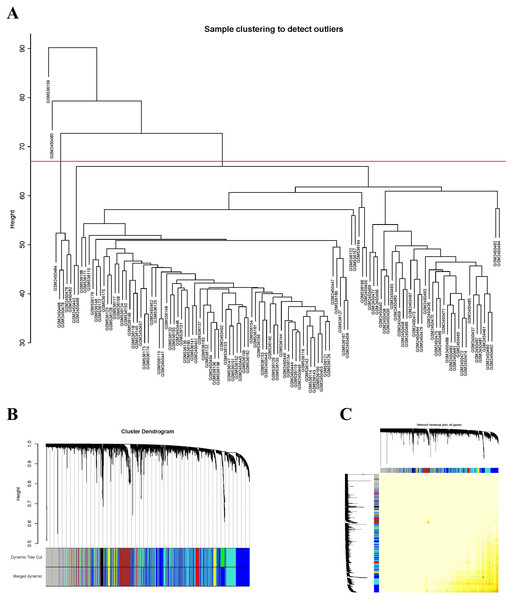
A total of 20,822 gene expression values were derived from the raw file. A total of 5,000 genes with the greatest average expression values were selected for cluster analysis (Fig. 1A). A total of 121 samples remained for subsequent analysis after two outlier samples were removed (GSM536139 and GSM2450480).
Figure 1: Sample clustering to detect outliers and construction of coexpression modules.
(A) Hierarchical clustering of the top 5,000 genes with the highest average expression values of MCL samples in the clustering analysis. There were two outlier samples in the total 123 samples, that are GSM536139 and GSM2450480, when the threshold value was determined as 67 (red line). (B) The constructed coexpression modules of MCL genes by WGCNA software. (C) Interaction analysis between gene coexpression modules. The heatmap showed the Topological Overlap Matrix (TOM) among genes in the analysis. Different colors on the x-axis and y-axis represented different modules. The yellow brightness of the middle part represented the strength of connections between modules.Identification of coexpression modules of MCL genes
The expression values of 5,000 genes in the 121 MCL samples were analyzed to identify the modules of highly correlated genes. The soft threshold power was set at 8 (scale-free R2 = 0.85) to guarantee a scale-free network (Fig. S1). A total of seven modules, including turquoise (1,571 genes), gray (883 genes), blue (1,606 genes), brown (381 genes), yellow (241 genes), red (195 genes) and black (123 genes) were identified (Fig. 1B). The genes in gray were not included in any module, so no further analysis was conducted for these genes.
Correlation analysis of coexpression modules
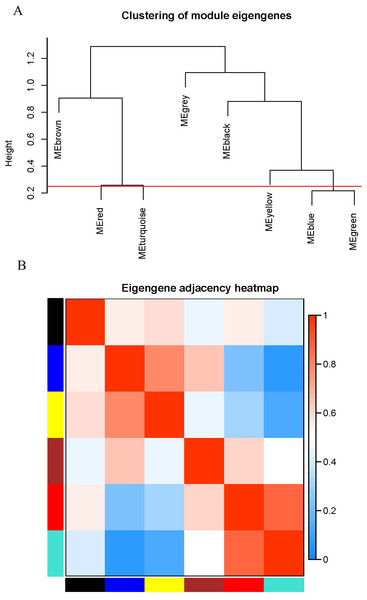
The WGCNA package analyzed the interactive relationships underlying the six coexpression modules (Fig. 1C). Gene expression among the six identified modules was relatively independent as illustrated by the topological overlap matrix (TOM) plot of 5,000 genes, suggesting that each module was independently validated. The connectivity degree of eigengenes was analyzed to further quantify the similarity of coexpression. The six modules yielded two main clusters, with two sets of three modules each (brown, red and turquoise modules, and black, blue and yellow modules), followed by cluster analysis (Fig. 2A). The blue and yellow modules, and red and turquoise modules were found to have higher adjacency values based on the heatmap plot of the adjacencies (Fig. 2B).
Figure 2: Connectivity analysis between different modules.
(A) Hierarchical cluster analysis of the genes in different modules; (B) connectivity level analysis of the genes in different modules. Within the heatmap, red represents a positive correlation and blue represents a negative correlation. Squares of red color along the diagonal are the meta-modules.Functional and pathway enrichment analysis
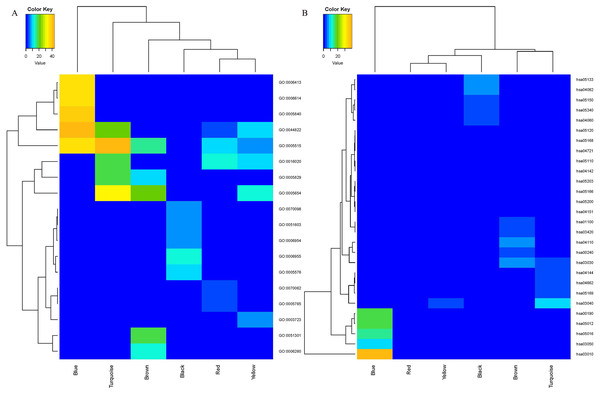
Enrichment analyses of GO and KEGG were conducted to assess the functions of the genes in the six identified modules. The top five enriched GO and KEGG terms with p value < 0.05 were selected for further analysis. The heatmap plots for GO (Fig. 3A) and KEGG (Fig. 3B) analysis revealed a large difference in the enriched degree and terms of the coexpression modules. The blue module was determined to be the most important module in enrichment degree after heatmap analysis was conducted (Figs. 3A and 3B). Each module was determined to be significantly different from each other based on analysis of the GO biological process (Table 1). The genes in the black module were primarily enriched in GO:0006955 (immune response), GO:0006954 (inflammatory response), GO:0070098 (chemokine-mediated signaling pathway) and GO:0051603 (proteolysis associated with cellular protein catabolic process). The genes in the blue module were primarily enriched in GO:0006413 (translational initiation) and GO:0006614 (SRP-dependent cotranslational protein aiming at membrane). The genes in the brown module were primarily enriched in GO:0051301 (cell division) and GO:0006260 (DNA replication). The genes in the other three modules were primarily enriched in GO molecular function and cellular components. The results of KEGG pathway analysis are shown in Table 2. The black module was mainly enriched in immune pathways hsa04060: Cytokine–cytokine receptor interaction, and hsa05340: Primary immunodeficiency. The blue module was primarily enriched in the pathways hsa03010: Ribosome, hsa00190: Oxidative phosphorylation (OXPHOS) and hsa03050: Proteasome. The brown module was primarily enriched in cellular processes hsa04110: Cell cycle, hsa03030: DNA replication, hsa00240: Pyrimidine metabolism, hsa01100: Metabolic pathways and hsa03420: Nucleotide excision repair. The red module was enriched in pathways hsa04142: Lysosome and hsa04721: Synaptic vesicle cycle. The turquoise module was principally enriched in Genetic Information Processing hsa03040: Spliceosome and hsa03030: DNA replication. Nineteen genes from the turquoise module were primarily enriched in hsa04662: B cell receptor signaling pathway. The yellow module was primarily enriched in the pathways hsa03040: Spliceosome, hsa05203: Viral carcinogenesis, hsa05166: HTLV-I infection, hsa05200: Pathways in cancer and hsa04151: PI3K–Akt signaling pathway.
Figure 3: The heatmap for GO (A) and KEGG (B) enrichment analysis of MCL genes in coexpression modules.
Rows and columns represent the terms and modules, respectively.| Term | Count | Percentage | p Value | |
|---|---|---|---|---|
| Module black | GO:0006955~immune response | 22 | 18.18181818 | 2.44E−13 |
| GO:0005576~extracellular region | 36 | 29.75206612 | 5.60E−11 | |
| GO:0006954~inflammatory response | 17 | 14.04958678 | 2.55E−09 | |
| GO:0070098~chemokine-mediated signaling pathway | 9 | 7.438016529 | 1.71E−08 | |
| GO:0051603~proteolysis involved in cellular protein catabolic process | 8 | 6.611570248 | 2.22E−08 | |
| Module blue | GO:0044822~poly(A) RNA binding | 240 | 14.99063086 | 3.72E−44 |
| GO:0005840~ribosome | 79 | 4.93441599 | 8.92E−41 | |
| GO:0005515~protein binding | 963 | 60.14990631 | 2.32E−37 | |
| GO:0006413~translational initiation | 69 | 4.309806371 | 6.67E−37 | |
| GO:0006614~SRP-dependent cotranslational protein targeting to membrane | 57 | 3.560274828 | 2.80E−36 | |
| Module brown | GO:0005654~nucleoplasm | 130 | 34.21052632 | 5.42E−22 |
| GO:0051301~cell division | 43 | 11.31578947 | 1.13E−20 | |
| GO:0005515~protein binding | 251 | 66.05263158 | 2.41E−16 | |
| GO:0006260~DNA replication | 25 | 6.578947368 | 1.00E−14 | |
| GO:0005829~cytosol | 121 | 31.84210526 | 6.12E−12 | |
| Module red | GO:0016020~membrane | 61 | 31.28205128 | 4.66E−13 |
| GO:0005515~protein binding | 137 | 70.25641026 | 8.21E−10 | |
| GO:0044822~poly(A) RNA binding | 31 | 15.8974359 | 4.86E−06 | |
| GO:0070062~extracellular exosome | 52 | 26.66666667 | 2.53E−05 | |
| GO:0005765~lysosomal membrane | 13 | 6.666666667 | 2.86E−05 | |
| Module turquoise | GO:0005515~protein binding | 964 | 61.51882578 | 6.97E−44 |
| GO:0005654~nucleoplasm | 389 | 24.82450542 | 8.96E−33 | |
| GO:0044822~poly(A) RNA binding | 188 | 11.99744735 | 6.88E−22 | |
| GO:0016020~membrane | 291 | 18.57051691 | 4.03E−20 | |
| GO:0005829~cytosol | 394 | 25.14358647 | 3.53E−19 | |
| Module yellow | GO:0005654~nucleoplasm | 81 | 33.75 | 3.95E−14 |
| GO:0044822~poly(A) RNA binding | 45 | 18.75 | 3.59E−11 | |
| GO:0016020~membrane | 61 | 25.41666667 | 1.62E−09 | |
| GO:0005515~protein binding | 156 | 65 | 1.01E−08 | |
| GO:0003723~RNA binding | 25 | 10.41666667 | 1.96E−07 |
Note:
GO, Gene Ontology; SRP, signal recognition particle.
| Term | Count | Percentage | p Value | |
|---|---|---|---|---|
| Module black | hsa05133: Pertussis | 9 | 7.438016529 | 2.87759E−07 |
| hsa04062: Chemokine signaling pathway | 12 | 9.917355372 | 6.4214E−07 | |
| hsa04060: Cytokine–cytokine receptor interaction | 12 | 9.917355372 | 8.78906E−06 | |
| hsa05150: Staphylococcus aureus infection | 6 | 4.958677686 | 0.00011374 | |
| hsa05340: Primary immunodeficiency | 5 | 4.132231405 | 0.00022661 | |
| Module blue | hsa03010: Ribosome | 74 | 4.622111181 | 2.2086E−40 |
| hsa00190: Oxidative phosphorylation | 49 | 3.060587133 | 1.25281E−17 | |
| hsa05012: Parkinson’s disease | 50 | 3.123048095 | 4.75678E−17 | |
| hsa05016: Huntington’s disease | 56 | 3.497813866 | 7.26902E−15 | |
| hsa03050: Proteasome | 21 | 1.3116802 | 3.86976E−10 | |
| Module brown | hsa04110: Cell cycle | 17 | 4.473684211 | 5.56038E−08 |
| hsa03030: DNA replication | 10 | 2.631578947 | 1.55272E−07 | |
| hsa00240: Pyrimidine metabolism | 13 | 3.421052632 | 6.60428E−06 | |
| hsa01100: Metabolic pathways | 48 | 12.63157895 | 0.000894963 | |
| hsa03420: Nucleotide excision repair | 7 | 1.842105263 | 0.000996474 | |
| Module red | hsa05110: Vibrio cholerae infection | 5 | 2.564102564 | 0.006215548 |
| hsa04142: Lysosome | 7 | 3.58974359 | 0.007386343 | |
| hsa04721: Synaptic vesicle cycle | 5 | 2.564102564 | 0.012146872 | |
| hsa05120: Epithelial cell signaling in Helicobacter pylori infection | 5 | 2.564102564 | 0.014972709 | |
| hsa05168: Herpes simplex infection | 8 | 4.102564103 | 0.01521917 | |
| Module turquoise | hsa03040: Spliceosome | 36 | 2.297383535 | 1.93556E−09 |
| hsa05169: Epstein–Barr virus infection | 28 | 1.786853861 | 5.54839E−06 | |
| hsa04144: Endocytosis | 43 | 2.744097001 | 1.19036E−05 | |
| hsa04662: B cell receptor signaling pathway | 19 | 1.212507977 | 1.97499E−5 | |
| hsa03030: DNA replication | 13 | 0.829610721 | 3.50627E−05 | |
| Module yellow | hsa03040: Spliceosome | 9 | 3.75 | 0.00053903 |
| hsa05203: Viral carcinogenesis | 9 | 3.75 | 0.008102343 | |
| hsa05166: HTLV-I infection | 10 | 4.166666667 | 0.009303826 | |
| hsa05200: Pathways in cancer | 12 | 5 | 0.021866894 | |
| hsa04151: PI3K–Akt signaling pathway | 11 | 4.583333333 | 0.022950705 |
Note:
KEGG, Kyoto Encyclopedia of Genes and Genomes; HTLV-I, Human T-cell leukemia virus type 1; PI3K, Phosphatidylinositol-3-kinase; Akt, protein kinase B.
Hub Gene analysis and identification
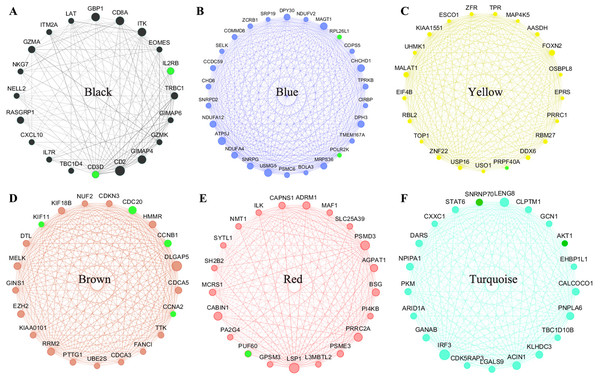
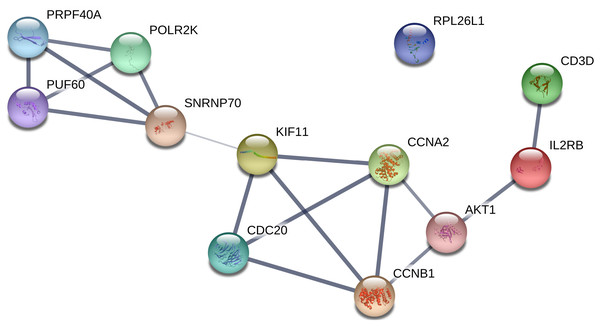
All of the genes from the six modules were uploaded to the STRING database and a PPI network was constructed using Cytoscape software. The PPI hub genes from the top weighted network were regarded as real hub genes (IL2RB, CD3D, RPL26L1, POLR2K, KIF11, CDC20, CCNB1, CCNA2, PUF60, SNRNP70, AKT1 and PRPF40A) (Fig. 4). The STRING database was used to evaluate the interrelationships of the real hub genes. There was a close correlation among five real hub genes (CDC20, CCNB1, CCNA2, KIF11 and AKT1) (Fig. 5), which indicates their importance.
Figure 4: The top genes ranked by weighted degree in each coexpression module (A–F).
The green nodes illustrate the real hub genes. Nodes are ordered and sized according to their degree and edges are sized according to their weight.Figure 5: PPI network of the real hub genes.
Survival analysis
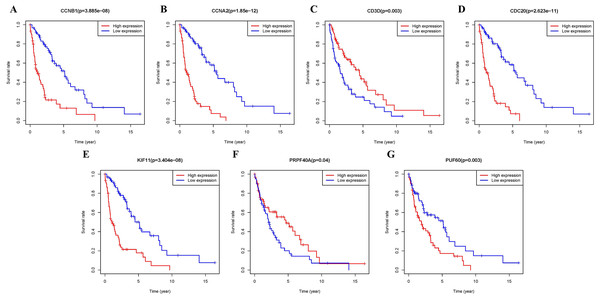
Additional survival analysis was conducted on the real hub genes to evaluate their effects on the survival of MCL. Seven genes (KIF11, CDC20, CCNB1, CCNA2, PRPF40A, CD3D and PUF60) (Fig. 6) were found to be associated with overall survival time (p < 0.05). Therefore, Kaplan–Meier survival curves indicated that seven genes (KIF11, CDC20, CCNB1, CCNA2, PRPF40A, CD3D and PUF60) may be used as prognostic biomarkers for MCL. Two real hub genes (CD3D and PRPF40A) with high expressions were correlated with longer overall survival which indicated a protective role in MCL biogenesis, while five real hub genes (CCNB1, CCNA2, CDC20, KIF11 and PUF60) were significantly associated with a reduced overall survival rate. Five real hub genes (KIF11, CDC20, CCNB1, CCNA2 and PUF60) were identified as potential prognostic biomarkers for MCL.
Figure 6: Survival analysis of seven real hub genes expression in MCL patients.
Kaplan–Meier survival curves of (A) CCNB1, (B) CCNA2, (C) CD3D, (D) CDC20, (E) KIF11, (F) PRPF40A and (G) PUF60.Verification of the real hub genes
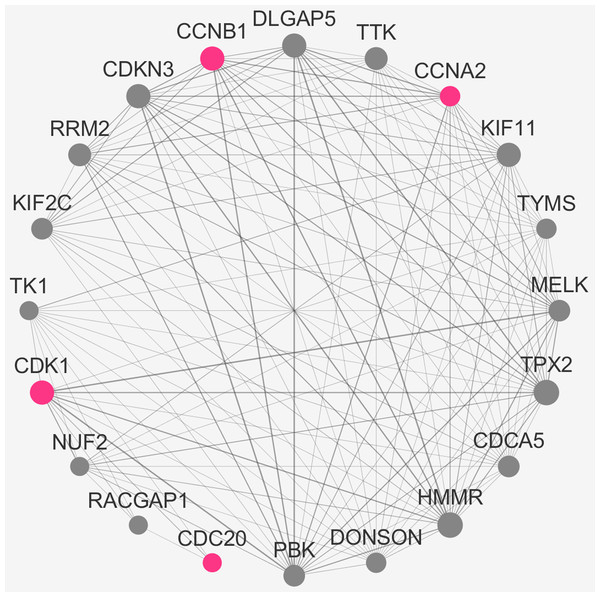
To verify the stability of five real hub genes (KIF11, CDC20, CCNB1, CCNA2 and PUF60), we constructed a coexpression network using WGCNA in the 43 GSE132929 MCL samples. The PPI network was constructed by the STRING database (Fig. S2). The PPI hub genes from the top weighted network were regarded as real hub genes (CDC20, CCNB1, CCNA2 and CDK1) (Fig. 7). We also found that KIF11 appeared in the top weighted network (Fig. 7). Therefore, CDC20, CCNB1 and CCNA2 are stable in GSE132929. And KIF11 is also important in MCL. We failed the validation of PUF60 in GSE132929.
Figure 7: The top genes ranked by weighted degree in the GSE132929.
The red nodes illustrate the real hub genes. Nodes are ordered and sized according to their degree and edges are sized according to their weight.Discussion
The goal of our research was to establish a gene coexpression network that could be used to forecast the clusters of hub genes involved in the pathogenesis of MCL. WGCNA has a distinct advantage over profiling done by conventional microarray-based expression by targeting a batch of gene modules instead of analyzing genes and their interactions separately. This approach eliminates the potential for error that can occur when taking an independent approach to genes and focuses on molecular transcriptional networks. According to a review of the existing literature, this study is the first to employ WGCNA to detect an array of hub genes as biomarkers related to the pathogenesis of MCL. A total of 123 samples were used to construct the coexpression modules and two samples were excluded as outliers. The tightly co-expressed gene modules with common functional annotations could predict the candidate gene sets underlying a certain biological process and six coexpression modules were identified on this premise. Functional enrichment analysis was carried out in the genes of the modules to identify the critical module. KEGG pathway and GO enrichment analyses were conducted to further investigate the biological functions of the genes enriched among these six modules. The blue module was identified as the most significant module during the diagnosis and progression of MCL. The most critical three KEGG pathways (including Ribosome, OXPHOS and Proteasome) were enriched.
Ribosome, OXPHOS and Proteasome pathways have been found to be closely related to MCL. PF-04691502, a novel phosphoinositide 3-kinase (PI3K)/mTOR inhibitor has potent activity in MCL cell lines, and PF-04691502 decreased phosphorylation of Akt and S6 ribosomal protein. These relationships led Chen et al. (2016) to suggest PF-04691502 as a novel therapeutic agent for MCL patients. Dihydrocelastrol induced apoptosis and cell cycle arrest in MCL cells by inhibiting the (PI3K)/mTOR-mediated phosphorylation of ribosomal protein S6 kinase and eukaryotic initiation factor 4E binding protein (Xie et al., 2018). Inhibition of OXPHOS repressed cell growth in ibrutinib-resistant MCL (Zhang et al., 2019). Targeting metabolic pathways can aid patients with highly refractory MCL. Researchers are in the process of developing an inhibitor of OXPHOS that has already shown efficacy in mouse models of ibrutinib-resistant MCL. The proteasome inhibitor bortezomib was initially approved for the treatment of patients with relapsed MCL (Robak et al., 2015). There is already sufficient evidence for the use of proteasome inhibitors in MCL and these three metabolic pathways can serve as therapeutic targets for patients with MCL.
The real hub genes (including IL2RB, CD3D, RPL26L1, POLR2K, KIF11, CDC20, CCNB1, CCNA2, PUF60, SNRNP70, AKT1 and PRPF40A) were identified by PPI and gene coexpression network analysis. There was a close correlation among five real hub genes (CDC20, CCNB1, CCNA2, KIF11 and AKT1). Survival analysis showed that seven genes (KIF11, CDC20, CCNB1, CCNA2, PRPF40A, CD3D and PUF60) were associated with overall survival. The five hub genes (CDC20, CCNB1, CCNA2, KIF11 and PUF60) were significantly correlated with a shorter overall rate of survival. The results suggest that these five genes could influence the development of MCL treatments. The stability of the hub genes (CDC20, CCNB1 and CCNA2) was confirmed with the same approach in GSE132929. WGCNA is a biology method for interaction and correlation analysis. Therefore, the more data got, the result would be more accurate. The discrepancy in results depends on plenty of reasons such as sample sizes, environmental factors, ethnic origin. Studies with larger sample size are needed in the future.
Emerging evidence reveals that CDC20 plays an important role in tumorigenesis. CDC20 is an oncogene whose overexpression has been observed in numerous cancers (Paul et al., 2017; Sun et al., 2019a) and which plays a pivotal role in mitotic progression. The suppression of the activity of CDC20 can regulate the cell cycle and promote apoptosis (Doğan Şiğva et al., 2019). CDC20 is highly expressed in hepatocellular carcinoma, which is recognized as a predictor of adverse clinical outcomes and an independent prognostic factor (Zhuang, Yang & Meng, 2018). The suppression of CDC20 could mediate the tumor suppressing function of p53 and that CDC20 could be negatively regulated by p53. These findings are consistent with the p53 inactivation observed in various cancer tissues, including acute myeloid leukemia and lung cancer (Hu & Chen, 2015; Simonetti et al., 2019); this effect may be ascribed to CDC20 up-regulation. The anaphase-promoting complex CDC20 can suppress apoptosis by targeting Bim for destruction and ubiquitination. CDC20 has been shown to directly degrade the MLL (KMT2D) protein to assure the progression of the cell cycle during the late M phase. CDC20 fails to interact with MLL fusions in human MLL leukemia cells, which continue to promote the progression of the cell cycle. The deregulated expression of MLL produces the biological change responsible for the constant development of MLL leukemias (Rao & Dou, 2015). Ferrero et al. (2019) recently published the poor prognostic role of MLL (KMT2D) in MCL. The interaction of CDC20 and MLL (KMT2D) in MCL requires further study. CDC20 can predict the overall survival in Diffuse large B-cell lymphoma based on The Cancer Genome Atlas data (Sun et al., 2019a). As a result, the development of specific CDC20 inhibitors may provide a new approach to treat human cancers by virtue of elevated CDC20 expression.
Cancer is characterized by its dysregulation of the cell cycle. CCNB1, the hub gene of the brown module, can promote the transition of cells from the G2 to M phase. However, the overexpression of CCNB1 in cancer results in unchecked cell growth due to its binding to Cdks. The binding of Cdks results in the phosphorylation of other substrates at an improper time and unchecked proliferation (Chiu et al., 2018), which can be ascribed to the inactivation of p53, the tumor suppressor protein. The wild-type p53 has been reported to suppress the expression of cyclin B1 (Lu et al., 2016). High CCNB1 expression is seen in a diverse number of cancers, including esophageal, gastric and colorectal cancers (Ding et al., 2018; Wen et al., 2017). The high expression level of cyclin B1 is also correlated to the degree of tumor invasion and metastasis (Gu et al., 2019). The down-regulation of cyclin B1 results in tumor regression, making CCNB1 an attractive target for further study.
CCNA2 is a hub gene found in the brown module. It can promote the transition of G1/S and G2/M phases in the cell cycle. The up-regulated CCNA2 expression is found in numerous types of cancer, including pancreatic ductal adenocarcinoma and colorectal cancers (Dong et al., 2019; Gan et al., 2018). An increased CCNA2 expression can promote tumorigenesis. Recent experimental data indicates that CCNA2 is a potential target for cancer therapy. The kinesin family member 11(KIF11) is one of the kinesin family motor protein members and is associated with spindle formation and tumor genesis (Jin et al., 2019). High KIF11 expression contributes to tumor progression and is associated with unfavorable prognosis (Jin et al., 2019; Pei et al., 2017; Piao et al., 2017). KIF11 inhibitors have been developed as chemotherapeutic agents to treat cancer. Poly-U binding splicing factor 60 kDa (PUF60), a factor regulating RNA splicing, is closely involved in tumor progression. The expression of PUF60 was highly correlated with TNM staging and lymph node metastasis in breast cancer (Sun et al., 2019b). Kobayashi et al. (2016) detected PUF60 auto-antibodies in the sera of early-stage colon cancer patients and concluded that it may be a candidate biomarker for the diagnosis and prognosis of colon cancer.
Conclusion
Significant key pathways and the hub genes related to the prognostic biomarkers of MCL were identified using comprehensive analysis and a bioinformatic approach. The development of the potential and targeted selective inhibitors for key pathways and the extractive real hub genes, including CDC20, CCNB1, CCNA2, KIF11 and PUF60, may provide a novel treatment opportunity for MCL therapy. However, these significant pathways and hub genes should be analyzed and validated in future clinical studies to determine the biological targets that are most effective for MCL.