Abstract
Childhood maltreatment increases lifetime vulnerability for psychopathology. One proposed mechanism for this association is that early maltreatment increases vigilance for and attention to subtle threat cues, persisting outside of the environment in which maltreatment occurs. To test this possibility, the present study examined neural responses to ambiguous and nonambiguous threatening facial expressions in a sample of 25 adults reporting a history of low-to-moderate levels of abuse in childhood and 46 reporting no or low levels of childhood maltreatment. The measure of neural response used was the late positive potential (LPP), a neural marker of sustained attention to motivationally salient information that is sensitive to subtle variation in emotional content. Participants passively viewed angry–neutral and fearful–neutral face blends and rated emotional intensity for each face. In the maltreated group, as fearful faces increased in emotional intensity, the LPP similarly increased, suggesting increased sensitivity to subtle variation in threatening content. Moreover, the LPP at each level of emotional intensity was not related to current symptoms of anxiety and depression. However, contrary to our hypotheses, adults with a history of abuse did not rate angry or fearful faces as more threatening, nor did they exhibit a larger LPP to angry faces, compared to controls. These findings suggest that childhood maltreatment may be associated with increased sensitivity to ambiguous threatening information in adulthood.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Childhood maltreatment (CM) is a costly (Fang, Brown, Florence, & Mercy, 2012) and pervasive problem. Between 16% and 36% of children worldwide are victims of physical, emotional, or sexual abuse (Stoltenborgh, Bakermans-Kranenburg, Alink, & IJzendoorn, 2015). Children who experience maltreatment are more likely to develop physical health problems, perform poorly at school, and experience social difficulties than children who have not experienced maltreatment (Gilbert et al., 2009; Kim & Cicchetti, 2010). Moreover, CM is among the most potent risk factors for psychopathology and confers this risk across development (Cohen, Brown, & Smailes, 2001; McLaughlin et al., 2012). Not only is CM associated with adverse mental health outcomes in childhood and adolescence (McLaughlin et al., 2012), it also appears to increase the risk of first onset disorders in adulthood (Green et al., 2010; Kessler, Davis, & Kendler, 1997; Kessler et al., 2010). Despite the robust association between CM and psychopathology (Cohen et al., 2001; McLaughlin et al., 2012), the neural mechanisms underlying this association are not yet clear.
One oft-discussed neurodevelopmental explanation for the link between CM and elevated risk for psychopathology is that childhood abuse may sensitize neural networks involved in threat processing. This creates both a lower threshold for detection of threat (Pollak & Sinha, 2002) and increased attention to threat once it has been detected (McCrory et al., 2011; McCrory et al., 2013; McLaughlin, Peverill, Gold, Alves, & Sheridan, 2015; McLaughlin, 2016; Pollak & Tolley-Schell, 2003; Shackman, Shackman, & Pollak, 2007). In support of this, there is evidence that children with a history of abuse exhibit attentional biases toward threatening faces (Pollak, Klorman, Thatcher, & Cicchetti, 2001; Pine et al., 2005; Pollak & Tolley-Schell, 2003; Shackman et al., 2007), identify threat with limited perceptual information (Pollak, Messner, Kistler, & Cohn, 2009; Pollak & Sinha, 2002), and experience difficulties disengaging attention from facial expressions of threat (Pollak & Tolley-Schell, 2003). And in fact, increased attention to threat may play an important role in both the development and maintenance of affective disorders (Gotlib, Krasnoperova, Yue, & Joormann, 2004; Mathews & MacLeod, 2002; Van Bockstaele et al., 2014).
Despite evidence that CM is associated with both increased attention to threatening content and anxiety and depression, important gaps remain. First, previous research has tended to focus exclusively on attention to threat among physically and/or sexually abused children (Pollak et al., 2001; Pollak & Tolley-Schell, 2003; Pollak et al., 2005; Shackman et al., 2007). However, childhood emotional abuse has also been associated with enhanced neural response to threatening facial expressions among adults (van Harmelen et al., 2013), and is more likely to contribute to cognitive vulnerabilities that place individuals at risk for psychopathology (Alloy, Abramson, Walshaw, & Neeren, 2006; Gibb & Abela, 2008; Gibb et al., 2006; Rose & Abramson, 1992). Furthermore, emotional abuse is a more robust predictor than other forms of abuse for onset and severity of depression and anxiety (Bernet & Stein, 1999; Chapman et al., 2004; Gibb, Chelminzski, and Zimmerman, 2007; Simon et al., 2009). Yet few studies have included emotional abuse when examining the association between CM and attention to threat.
Additionally, previous studies have tended to employ prototypical facial expressions or emotional extremes of anger and fear to serve as threatening cues (Curtis & Cicchetti, 2011; McCrory et al., 2013; Pollak et al., 2001; Pollak & Tolley-Schell, 2003). However, facial expressions processed in real-life social situations are often ambiguous and dynamic. In hostile environments, it may be adaptive for maltreated children to attend to threat at lower thresholds, as this could facilitate attempts to avoid abuse (Cicchetti, Toth, & Maughan, 2000; Pollak, 2003). Outside of these environments, there is evidence that maltreated individuals are more likely to perceive and interpret threat at levels that others may not find threatening (Gibb, Schofield, & Coles, 2008; Pollak & Kistler, 2002). However, neither of these studies included a measure of neural response to differing levels of threat.
The aim of the present study, therefore, was to examine the associations between childhood experience of abuse and neural response to ambiguous fear and anger cues in adulthood. Event-related potentials (ERPs) have been particularly useful for examining dynamic allocation of attention to emotion, due to their precise temporal resolution. Additionally, a number of ERP components are sensitive to emotional content (Foti, Hajcak, & Dien, 2009; Hajcak, Weinberg, MacNamara, & Foti, 2012), and reflect different stages of emotional processing (Olofsson, Nordin, Sequeira, & Polich, 2008; Schupp, Flaisch, Stockburger, & Junghöfer, 2006; Weinberg & Hajcak, 2011). For example, earlier components, such as the P300, appear to index obligatory allocation of attention to emotional stimuli (Weinberg & Hajcak, 2011). Larger P300 amplitudes to threatening faces have consistently been observed among maltreated children compared to nonmaltreated children (Pollak et al., 2001; Pollak & Tolley-Schell, 2003; Shackman et al., 2007). However, abnormalities in sustained attention may be particularly salient for individuals exposed to CM (Pollak et al., 2005).
The late positive potential (LPP) is a positive deflection in the ERP waveform that is thought to reflect dynamic and sustained allocation of attentional resources to motivationally salient stimuli (Hajcak, MacNamara, & Olvet, 2010; Schupp et al., 2000; Weinberg & Hajcak, 2010, 2011). The LPP is typically maximal over the central-parietal region of the scalp between 250 and 1,000 ms following stimulus onset (Codispoti, Ferrari, & Bradley, 2006; Foti et al., 2009; Hajcak et al., 2010; Schupp et al., 2000). Variation in the magnitude of the LPP has been related to depression and anxiety (Foti, Olvet, Klein, & Hajcak, 2010; Kujawa, MacNamara, Fitzgerald, Monk, & Phan, 2015; Weinberg, Perlman, Kotov, & Hajcak, 2016) and has been implicated in risk for these disorders (Kujawa, Hajcak, Torpey, Kim, & Klein, 2012; Nelson, Perlman, Hajcak, Klein, & Kotov, 2015). The LPP is of particular interest in the current study as it reflects sustained and flexible attention to salient stimuli and is sensitive to subtle variation in emotional content (Briggs & Martin, 2009; Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000; Duval, Moser, Huppert, & Simons, 2013; Weinberg & Hajcak, 2010). The enhanced LPP persists throughout, and even beyond, the duration of stimulus presentation (Hajcak & Olvet, 2008), suggesting ongoing attention after stimulus offset. Furthermore, the amplitude of the LPP varies as a function of the level of emotion expressed in threat faces (Duval et al., 2013) as well other emotional expressions (Olofsson et al., 2008). Together, these data suggest that the LPP may be a sensitive tool for investigations of the ways in which CM is associated with sustained attention to ambiguous threat in adulthood.
To that end, in this study we investigated the magnitude of the LPP elicited by facial displays of threat in adults who reported CM. To extend previous studies, we focused on young adults who reported diverse forms of abuse (e.g., physical, emotional, and sexual abuse) during childhood, and we examined LPP modulation by both ambiguous and nonambiguous facial expressions of anger and fear. Based on previous investigations of maltreated individuals (Gibb et al., 2008; Pollak & Kistler, 2002; Pollak et al., 2001; Pollak & Tolley-Schell, 2003; Shackman et al., 2007), we predicted that adults who reported a history of abuse would rate faces as more threatening across all levels of emotional intensity. We also predicted that the gradient from neutral to 100% threatening for both self-reported ratings and neural response would be steeper for individuals who had experienced CM compared to those who reported subthreshold levels of abuse, reflecting increased sensitivity to subtle variation in threatening content (Gibb et al., 2008; Pollak & Kistler, 2002).
Method
Participants
Eighty-six participants were recruited from the McGill University psychology human participant pool or from flyers posted around the campus of McGill University. Participants received either course credit or $25 compensation for their time. All participants provided written informed consent after review of the protocol, and all procedures were approved by the Research Ethics Board at McGill University.
Data from seven participants were excluded due to excessive noise in the EEG data. An additional eight participants were excluded because they did not follow instructions during the questionnaire portion, leaving 71 participants in the final sample. The mean age of the final sample was 20.77 (SD = 2.75) years; 60 (84.5%) were female; 62.0% were Caucasian, 4.20% were Chinese, 8.50% were Arab/West Asian, 5.60% were South Asian, 4.20% were Korean, 7.0% were Hispanic, and 8.50% indicated they were another ethnicity.
Measures
Abuse was assessed using the Childhood Trauma Questionnaire–Short Form (CTQ-SF), a 28-item retrospective self-report form (Bernstein et al., 2003). The CTQ-SF consists of five clinical scales designed to capture different forms of childhood trauma: physical abuse (α = .94), physical neglect (α = .24), emotional abuse (α = .75), emotional neglect (α = .86), and sexual abuse (α = .94). Items are rated on a 5-point scale, with responses ranging from never true to very often true. The CTQ-SF is widely used and has demonstrated good reliability and validity (Bernstein et al., 2003) and is designed to produce both dimensional and categorical assessments of maltreatment. In the current sample, the total and subscale scores were significantly skewed. Therefore, to maximize statistical power, we used the published cutoff scores for each abuse and neglect subscale (i.e., 8 for physical abuse, 9 for emotional abuse, and 6 for sexual abuse, 8 physical neglect, 10 emotional neglect) to create dichotomized CTQ-SF variables (Bernstein & Fink, 1998). These cutoff scores have been shown to maximize accurate identification of low-to-moderate abuse cases while also keeping the false positive rate below 20%. This yielded 25 individuals from the sample who met criteria for abuse; 24 of these met criteria for emotional abuse, four met criteria for physical abuse, and nine for sexual abuse, with two individuals meeting criteria for all three forms of abuse. Of the 25 adults who met criteria for abuse, 15 and eight individuals also met criteria for emotional neglect and physical neglect, respectively. In this sample, 46 individuals did not meet cutoff criteria for any form of abuse or neglect; these participants were the comparison group (“controls”) in the analyses that follow.
Demographic information was collected from participants, including age, gender, ethnicity, and approximate annual family income. Response options for annual family income were represented by 14 categories from “Less than $10,000” to “$250,000 or more” with incremental increases of $9,999 between. Demographic information for these two groups is presented in Table 1.
All participants completed the Inventory of Depression and Anxiety Symptoms (IDAS-II; Watson et al., 2012) to assess current depression and anxiety symptoms. The IDAS-II is a 99-item factor-analytically derived self-report inventory of empirically distinct dimensions of depression and anxiety symptoms. Each item assesses symptoms over the past 2 weeks on a 5-point Likert scale ranging from 1 (not at all) to 5 (extremely). The IDAS-II has demonstrated good internal consistency, test–retest reliability, and convergent and discriminant validity with diagnoses and self-report measures in similar populations (Watson et al., 2007; Watson et al., 2012). We include here the following seven subscales, which have been previously associated with variation in the amplitude of the LPP (e.g., Nelson et al., 2015; Weinberg & Sandre, 2017), and which demonstrated good internal reliability in the current sample: general depression (20 items; α = .84), dysphoria (10 items; α = .87), lassitude (six items; α = .74), suicidality (six items; α = .71), social anxiety (six items; α = .79), panic (eight items; α = .84), and well-being (eight items; α = .88). Examination of these subscales allowed us to examine the extent to which groups differed in terms of symptomology and well-being, as well as to determine whether neural responses to threat were uniquely associated with CM, independent of current symptoms of anxiety and depression.
Visual stimuli
Forty-five photographs of 15 actors (eight male and seven female) were selected from the Karolinska Directed Emotional Faces database (KDEF; Lundqvist, Flykt, & Öhman, 1998). For each actor, the prototypical neutral, angry, and fearful expressions were selected. Within each actor, two prototype images (either neutral and angry or neutral and fearful) were combined in different proportions (e.g., 100% angry, 75% angry, 50% angry, 25% angry, and 100% neutral) using Fantamorph software (Abrosoft; http://www.abrosoft.com/). In order to morph the images, control points on the face were identified on each of the two prototype photographs (e.g., corners of the eyes, pupils, eyebrows, nostrils, outline of the mouth, circumference of the face). Each morphed image represents the distance between the identified points in the two images proportional to the percentage of emotion expressed (e.g., in a 75% angry photograph, the points are located 75% closer to the points on the prototypical angry face than on the neutral face); pixel intensity values are also blended according to the proportion of the emotion represented. Faces were then cropped such that only facial expressions were visible. Specific images used in the study are listed in the Appendix. These facial blends allowed for examinations of attentional specificity to varying degrees of emotional expression, ranging from ambiguous (low levels of emotional intensity) to unambiguous expression (high levels of emotional intensity).
All stimuli were presented on a Pentium class computer using Presentation software (Version 18.1, Neurobehavioural Systems, Inc.). Participants were seated approximately 60.96 cm from the screen.
Procedure
Subsequent to verbal instructions indicating that they would be passively viewing faces of varying emotional quality, participants were seated, and electroencephalograph sensors were attached. All participants performed multiple tasks during the experiment; results from other tasks will be reported elsewhere. The order of the tasks was counterbalanced across subjects. In the current study, each trial consisted of a fixation point (random interval from 3,000 to 3,500 ms), followed by a centrally presented face (300 ms), followed by a fixation cross (random interval from 1,000 to 1,500 ms), followed by two sequentially presented rating scales. These 9-point rating scales asked participants to rate the intensity of expressions for fear and anger ranging from 1 (not at all fearful) to 9 (extremely fearful) and from 1 (not at all angry) to 9 (extremely angry). Participants were asked to use the keyboard to indicate the intensity of the facial expression. Participants completed six practice trials (all using prototypical facial expressions modeled by actors who were not included in the experimental phase). In total, there were 135 trials, each presented once in a randomized order.
Electroencephalographic recording and data processing
Continuous EEG was recorded using a 32-electrode cap and a BrainVision actiCHamp system (Brain Products, Munich, Germany) based on the standard 10–20 layout and a ground electrode at Fpz. The electrooculogram (EOG) generated from eye movements and blinks was recorded using facial electrodes placed approximately 1cm above and below the left eye and referenced to an electrode on the back of the neck. All electrode impedances were below 5 kΩ, and data were recorded with a sampling rate of 1000 Hz using a 60-Hz low-pass filter.
Offline analysis was conducted using BrainVision Analyzer software (Brain Products, Munich, Germany). Data were referenced to an average of TP9 and TP10 and band-pass filtered with low and high cutoffs of 0.01 and 30 Hz. Eye blink and ocular corrections were conducted using EOG per a modification of the original algorithm published in Gratton, Coles, and Donchin (1983). A semiautomatic procedure was employed to detect and reject artifacts. The criteria applied were a voltage step of more than 50.0 μV between sample points, a voltage difference of 300.0 μV within a trial, and a maximum voltage difference of less than 0.50 μV within 100-ms intervals. These intervals were rejected from individual channels in each trial. Visual inspection of the data was then conducted to detect and reject any remaining artifacts.
The EEG was segmented for each trial beginning 200 ms prior to stimulus onset and continuing for 1,000 ms after stimulus onset (i.e., a time window of 1,200 ms). For each trial, a baseline of the average activity in a 200-ms window prior to stimulus onset was subtracted from every data point. ERPs were constructed by separately averaging each of the nine stimulus types (i.e., 100% fearful, 75% fearful, 50% fearful, 25% fearful, neutral, 25% angry, 50% angry, 75% angry, 100% angry). Visual inspection of grand averages across all participants confirmed that the LPP was maximal at site Pz, consistent with previous research (Baskin-Sommers, Curtin, & Newman, 2013; Schupp et al., 2000). Therefore, the LPP was scored by averaging amplitudes at electrode Pz, where the LPP was maximal, from 400–1,000 ms after picture onset (MacNamara & Proudfit, 2014; Moser, Hajcak, Bukay, & Simons, 2006; Weinberg, Venables, Proudfit, & Patrick, 2014).
Data analysis
All statistical analyses were conducted using SPSS General Linear Model Software (Version 23). Pearson’s chi-square and independent t tests were used to compare the groups on demographic variables. Group differences in symptoms of anxiety and depression were evaluated using a one-way ANOVA. To evaluate differences in neural response to faces in the CM and control groups, data were analyzed using two 2 (group: CM or controls) × 5 (emotional intensity) mixed-model ANOVAs. Therefore, we examined group differences separately for fearful (100% fearful, 75% fearful, 50% fearful, 25% fearful, neutral) and angry (100% angry, 75% angry, 50% angry, 25% angry, neutral) faces. To examine whether the magnitude of the LPP to fearful and angry faces increased in a linear fashion among abused and control groups, we subsequently examined modulation of the LPP by emotional intensity separately within each group and face type using two five-level repeated-measures ANOVAs. Finally, group differences in ratings for each face type were analyzed using two 2 (group: CM or controls) × 5 (emotional intensity: e.g., 100% fearful, 75% fearful, 50% fearful, 25% fearful, neutral) mixed-model ANOVAs. Whenever assumptions of sphericity in repeated-measures analyses were violated (Mauchly, 1940), the Huynh–Feldt statistic (Huynh & Feldt, 1976) was used to adjust the degrees of freedom (using estimated epsilon, ε). Significant interactions were decomposed using linear polynomial contrasts, and effect sizes are reported in partial eta squared (ηp2). (For data and scripts for generating stimuli and performing data analyses, please contact the corresponding author.)
Results
The CM and control groups did not significantly differ on any demographic variables, including age, gender, ethnicity, and annual family income. Table 1 summarizes the demographic and clinical characteristics of the subjects. As shown in Table 1, the group reporting CM exhibited higher symptoms of general depression, dysphoria, lassitude, insomnia, suicidality, panic, social anxiety, and traumatic intrusions, as well as lower well-being than the control group. Table 2 displays the means and standard deviations for self-reported emotional intensity ratings and LPP for fearful and angry faces.
Fear and anger ratings
Ratings of fearful faces showed a significant main effect of emotional intensity, F(2.40, 160.2) = 591.5, p < .001, ηp2 = .90. Significant linear trends were found for the effect of intensity for fearful faces, Flin(1, 67) = 967.63, p < .001, ηp2 = .93. The degree of emotional intensity also significantly influenced the ratings of anger faces, F(2.10, 140.3) = 402.81, p < .001, ηp2 = .86. A significant linear trend for the main effect of anger ratings was found, Flin(1, 67) = 602.3, p < .001, ηp2 = .90. Contrary to our hypotheses, there was no main effect of group for emotional intensity ratings of fearful, F(1, 67) = 1.22, p = .27, ηp2 = .02, or angry faces, F(1, 67) = .11, p = .74, ηp2 = .002. Furthermore, there was no interaction between group and emotional intensity ratings for fearful, F(2.39, 160.2) = .09, p = .94, ηp2 = .001, or angry faces, F(2.09, 140.3) = .55, p = .59, ηp2 = .008. These effects are depicted in Fig. 1a–b.
LPP
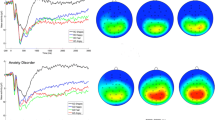
Response-locked ERP waveforms at electrode site Pz for abused and control groups are shown in Fig. 2. To evaluate differences in neural response to fearful faces in the abuse and control groups, a 2 (group: CM or controls) × 5 (emotional intensity) mixed model ANOVA was conducted. There was a main effect of emotional intensity such that the magnitude of the LPP differed across fearful face types, F(2.56, 176.4) = 3.41, p = .03, ηp2 = .05, but there was no main effect of group, F(1, 69) = .30, p = .59, ηp2 = .004. The main effect of emotional intensity of fearful faces was qualified by a group x emotional intensity interaction, F(2.56, 176.4) = 2.77, p = .05, ηp2 = .04.
To decompose this interaction, two five-level repeated-measures ANOVAs were conducted, one within each group. In the CM group, a significant main effect of emotional intensity was found for fearful faces, F(2.61, 62.55) = 5.30, p < .01, ηp2 = .18. This reflected a significant linear effect, such that, as faces increased in emotional intensity from neutral and approached fearful prototypes, the LPP elicited in abused participants similarly increased, Flin(1, 24) = 8.40, p = .008, ηp2 = .26. In contrast, in the control group, no significant effect of emotional intensity of fearful faces on the LPP was observed, F(2.21, 99.5) = 1.09, p = .35, ηp2 = .02; moreover, the linear effect was small, and nonsignificant Flin(1, 45) = 1.74, p = .19, ηp2 = .04.
Subsequently, we examined differences in neural response to angry faces in the abuse and control group using a 2 (group: CM or controls) × 5 (emotional intensity) mixed-model ANOVA. There was a main effect of emotional intensity such that the magnitude of the LPP differed across angry face types, F(4, 276) = 6.25, p = .000, ηp2 = .08. However, there was no main effect of group, F(1, 69) = 0.00, p = .98, ηp2 = .00, nor was there a Group × Emotional Intensity interaction, F(4, 276) = 1.59, p = .18, ηp2 = .02. See Fig. 3 for scalp distributions of 100% emotional minus neutral faces for each face type and group.
Associations among LPP and symptoms of depression and anxiety
Lastly, correlations between LPP magnitude to each face type and symptoms of anxiety and depression were examined for abused and control groups. There were no significant associations between the magnitude of the LPP at each level of emotional intensity to any of the IDAS scales across the abused and control groups (ps > .05).
Discussion
The aim of the present study was to investigate the neural correlates of attention to ambiguous threatening faces in a sample of young adults who had experienced CM. Consistent with our hypotheses, the LPP was modulated by the intensity of fearful faces in a linear fashion among maltreated individuals, and not among individuals exposed to no CM or subthreshold CM. Specifically, as faces increased in emotional intensity and approached fearful prototypes, the LPP elicited in maltreated individuals similarly increased, consistent with previous behavioral studies (Gibb et al., 2008; Pollak & Kistler, 2002). Moreover, this linear tracking of attention to progressively more intense or unambiguous fearful faces was observed in a sample of adults who endorsed low-to-moderate levels of any type of abuse. These data indicate that exposure to even relatively low levels of abuse may sensitize neural networks associated with threat detection, leading some individuals to exhibit increased neural sensitivity to ambiguous threat. Together, these results suggest that exposure to CM may contribute to long-term changes in the way that the brain processes threat. Our results add to the growing body of evidence indicating that cognitive dysfunctions associated with early experiences of CM persist beyond childhood and into adulthood (Gibb et al., 2008; van Harmelen et al., 2013).
Among maltreated individuals, we observed enhanced LPP amplitudes as fearful faces increased in emotional intensity, suggesting ongoing processing even in the absence of threat. This is consistent with evidence that maltreated individuals experience difficulties diverting attention away from threatening cues (Pollak & Tolley-Schell, 2003). Attending to mild or less overt threatening social signals may be adaptive as it protects maltreated individuals from potential harm in unpredictable and threatening situations (Cicchetti et al., 2000; Pollak, 2003). However, this ability may come at a cost—sustained threat processing may limit the encoding of other relevent emotional cues in the environment and thereby interfere with adequate emotional responding and regulation (Dodge, Pettit, Bates, and Valente, 1995; Pollak & Tolley-Schell, 2003), potentially leading to aggressive behavior, social withdrawal, and difficulty with peer relations (Dodge et al., 1995; Humphreys, Kircanski, Colich, & Gotlib, 2016; Kim & Cicchetti, 2010; Price & Glad, 2003; Teisl & Cicchetti, 2008). Futhermore, sustained attention toward threat may play an important role in the development (Kujawa et al., 2012; Nelson et al., 2015) and maintenance of anxiety and depression (Foti et al., 2010; Kujawa et al., 2015; Weinberg et al., 2016).
We would note, however, that these effects were specific to fear faces, though our study used both angry and fearful stimuli. Contrary to our hypotheses, the LPP to angry faces was not modulated by emotional intensity to a greater extent in adults who reported a history of CM compared to control individuals. The pattern of results we observed here may highlight important distinctions between these displays of threat. Though anger and fear are comparable with respect to self-reported ratings of emotional intensity and arousal (Goeleven, De Raedt, Leyman & Verschuere, 2008), these two expressions substantially differ in terms of the information they convey and the responses they elicit. Unlike angry faces, fearful facial expressions may act as more ambiguous warning signs that threat may be present in the immediate environment (Adams, Gordon, Baird, Ambady, & Kleck, 2003; Whalen, 1998). Consistent with this, there is evidence for different patterns of attention, neural activity, and behavioral responses associated with these two facial expressions (Marsh, Ambady, & Kleck, 2005; Williams et al., 2005). Given that fear signals are ambiguous about the source or location of danger (Whalen, 1998; Whalen et al., 2001), the results of the present study suggest that CM may be associated with increased recruitment of neural resources when decoding whether subtle signs of threat are present in the immediate environment (Hein & Monk, 2017; McLaughlin et al., 2015).
Results from participant self-report also indicated that participants were able to differentiate ambiguous and nonambiguous face stimuli across neutral–fearful and neutral–angry continuums. However, contrary to our hypothesis, the maltreated and control groups did not differ in their ratings of emotion expressed. The two previous studies that indicated associations between CM and increased perceptions of threat to ambiguous faces included facial stimuli that increased in 10% increments of emotion (Gibb et al., 2008; Pollak & Kistler, 2002). In the current study, however, the degree of emotion in the faces changed in 25% increments, which may have reduced the degree of ambiguity. Despite this, adults who reported a history of CM did display a steeper increase in neural responses to threatening content in the absence of any observable emotion perception deficits. These results may therefore highlight the usefulness of ERPs in future investigations of CM and disruptions in threat processing, in that ERPs may be more sensitive to subtle variation in affect than self-report.
Interestingly, the magnitude of the LPP to threat in adults with histories of abuse was not related to current symptoms of anxiety and depression, suggesting that sustained attention to threat following CM is not the result of ongoing emotional dysfunction. Rather, this increased attention to threat may constitute a latent vulnerability factor for future symptoms (McCrory & Viding, 2015; McCrory, Gerin, & Viding, 2017). Consistent with this, hypersensitivity to threat can increase negative affect in response to stressors (Eldar, Ricon, & Bar-Haim, 2008; MacLeod, Rutherford, Campbell, Ebsworthy, & Holker, 2002), and may play a role in the maladaptive coping strategies that characterize affective disorders (e.g., worry and rumination; Burkhouse, Woody, Owens, & Gibb, 2015; Siegle, Steinhauer, Thase, Stenger, & Carter, 2002). Furthermore, evidence suggests that the magnitude of the LPP may be a useful endophenotype for internalizing disorders, indicating that enhanced neural responses to threat may reflect increased risk for first onset or recurrence of symptoms (Kujawa et al., 2016; Speed, Nelson, Auerbach, Klein, & Hajcak, 2016; Nelson et al., 2015; Weinberg et al., 2014). Thus, it is possible that an enhanced LPP associated with CM may be a neural marker of risk that impairs adaptation to stressful life events and predisposes individuals to psychopathology.
While the current study focused on associations between neural response to threat and CM, as well as heightened depressive and anxious symptoms in a group reporting CM, emerging evidence suggests that early life exposure to abuse may be a causal risk factor for virtually all commonly occurring forms of psychopathology (Keyes et al., 2012; Vachon, Krueger, Rogosch, & Cicchetti, 2015). Heightened threat sensitivity may operate as a specific mechanism of risk for future internalizing psychopathology in those who have experienced CM; it will be important for future studies to consider associations with externalizing psychopathology as well (McLaughlin, 2016; McLaughlin & Lambert, 2017). The current study was not sufficiently powered to examine associations between specific types of abuse and variation in attention to threat. Given that the various forms of abuse and neglect frequently co-occur (Green et al., 2010), future investigations with larger sample sizes will need to carefully consider links between specific forms of CM, neural responses to threat, and diverse forms of psychopathology.
Limitations of the study suggest directions for future research. First, reports of CM were measured retrospectively, and may have been subject to recall or response biases (Hardt & Rutter, 2004). However, a potential advantage of self-report measures is that they may identify CM experiences that were never formally reported or documented (London, Bruck, Ceci, & Shuman, 2005). Furthermore, the CTQ focuses on specific behavioral experiences or events of abuse, which may be more accurate than global beliefs of perceived abuse (Brewin, Andrews, & Gotlib, 1993). Second, our sample consisted of a predominately female university student population, which may limit the generalizability of the current findings. Future research will be needed to replicate the current study’s findings in a more diverse sample of young adults. Third, our sample consisted primarily of individuals who endorsed experiences of emotional abuse; thus, we could not examine the extent to which specific forms of abuse might be more strongly linked to threat sensitivity. However, the current study’s results complement previous research (Pollak et al., 2001; Pollak & Tolley-Schell, 2003; Pollak et al., 2005; Shackman et al., 2007) by demonstrating that emotional abuse in childhood may also have long-term implications for threat sensitivity in adulthood. Fourth, our study was also underpowered to compare the magnitude of the LPP elicited by each level of emotional intensity between abused and control groups. Nonetheless, we observed a significant and large linear effect of emotional intensity on the magnitude of the LPP elicited by fearful faces in the CM group (ηp2 = .26; Cohen, 1992; Miles & Shevlin, 2001), but not in the control group (ηp2 = .04). Future studies with larger samples might consider examining whether individuals exposed to CM demonstrate enhanced neural responses to threatening faces of different emotional intensities compared to individuals with no CM history.
In sum, the results of this study suggest that CM is associated with increased sensitivity to subtle variation in fearful content. Future prospective research is needed to examine the extent to which CM leads to functional variation in orienting and sustained attention to threat, and whether increased attention to threat remains a relatively stable and trait-like risk factor for social deficits and psychopathology. Furthermore, neural markers of sustained attention to threat, such as the LPP, may be helpful in future studies seeking to clarify causal relationships between CM and the onset of psychopathology. Understanding the extent to which CM influences cognitive and affective functioning will be important in generating empirically-informed interventions that can ameliorate the long-term effects of abuse on development.
References
Adams, R. B., Gordon, H. L., Baird, A. A., Ambady, N., & Kleck, R. E. (2003). Effects of gaze on amygdala sensitivity to anger and fear faces. Science, 300(5625), 1536–1536. doi:https://doi.org/10.1126/science.1082244
Alloy, L. B., Abramson, L. Y., Walshaw, P. D., & Neeren, A. M. (2006). Cognitive vulnerability to unipolar and bipolar mood disorders. Journal of Social and Clinical Psychology, 25(7),726–754. doi:https://doi.org/10.1521/jscp.2006.25.7.726
Baskin-Sommers, A. R., Curtin, J. J., & Newman, J. P. (2013). Emotion-modulated startle in psychopathy: Clarifying familiar effects. Journal of Abnormal Psychology, 122(2), 458. doi:https://doi.org/10.1037/a0030958
Bernet, C. Z., & Stein, M. B. (1999). Relationship of childhood maltreatment to the onset and course of major depression in adulthood. Depression and Anxiety, 9(4), 169–174. doi:https://doi.org/10.1002/(SICI)1520-6394(1999)9:4<169::AID-DA4>3.0.CO;2-2
Bernstein, D. P., & Fink, L. (1998). Childhood trauma questionnaire: A retrospective self-report: Manual. New York: Harcourt Brace.
Bernstein, D. P., Stein, J. A., Newcomb, M. D., Walker, E., Pogge, D., Ahluvalia, T., & Zule, W. (2003). Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse and Neglect, 27(2), 169–190. doi:https://doi.org/10.1016/s0145-2134(02)00541-0
Brewin, C. R., Andrews, B., & Gotlib, I. H. (1993). Psychopathology and early experience: A reappraisal of retrospective reports. Psychological Bulletin, 113(1), 82. doi:https://doi.org/10.1037/0033-2909.113.1.82
Briggs, K. E., & Martin, F. H. (2009). Affective picture processing and motivational relevance: Arousal and valence effects on ERPs in an oddball task. International Journal of Psychophysiology, 72(3), 299–306. doi:https://doi.org/10.1016/j.ijpsycho.2009.01.009
Burkhouse, K. L., Woody, M. L., Owens, M., & Gibb, B. E. (2015). Influence of worry on sustained attention to emotional stimuli: Evidence from the late positive potential. Neuroscience Letters, 588, 57–61. doi:https://doi.org/10.1016/j.neulet.2014.11.006
Chapman, D. P., Whitfield, C. L., Felitti, V. J., Dube, S. R., Edwards, V. J., & Anda, R. F. (2004). Adverse childhood experiences and the risk of depressive disorders in adulthood. Journal of Affective Disorders, 82(2), 217–225. doi:https://doi.org/10.1016/j.jad.2003.12.013
Cicchetti, D., Toth, S. L., & Maughan, A. (2000). An ecological-transactional model of child maltreatment. In A. J. Sameroff, M. Lewis, & S. M. Miller (Eds.), Handbook of developmental psychopathology (pp. 689–722). New York: Springer.
Codispoti, M., Ferrari, V., & Bradley, M. M. (2006). Repetitive picture processing: Autonomic and cortical correlates. Brain Research, 1068(1), 213–220. doi:https://doi.org/10.1016/j.brainres.2005.11.009
Cohen, J. (1992). A power primer. Psychological Bulletin, 112(1), 155. doi:https://doi.org/10.1037/0033-2909.112.1.155
Cohen, P., Brown, J., & Smailes, E. (2001). Child abuse and neglect and the development of mental disorders in the general population. Developmental Psychopathology, 13, 981–999. doi:https://doi.org/10.1017/S0954579401004126
Curtis, W. J., & Cicchetti, D. (2011). Affective facial expression processing in young children who have experienced maltreatment during the first year of life: An event-related potential study. Development and Psychopathology, 23(2), 373. doi:https://doi.org/10.1017/s0954579411000125
Cuthbert, B. N., Schupp, H. T., Bradley, M. M., Birbaumer, N., & Lang, P. J. (2000). Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology, 52(2), 95–111. doi:https://doi.org/10.1016/s03010511(99)00044-7
Dodge, K. A., Pettit, G. S., Bates, J. E., & Valente, E. (1995). Social information-processing patterns partially mediate the effect of early physical abuse on later conduct problems. Journal of Abnormal Psychology, 104(4), 632. doi:https://doi.org/10.1037/0021-843x.104.4.632
Duval, E. R., Moser, J. S., Huppert, J. D., & Simons, R. F. (2013). What’s in a Face? Journal of Psychophysiology, 27(1), 27–38. doi:https://doi.org/10.1027/0269-8803/a000083
Eldar, S., Ricon, T., & Bar-Haim, Y. (2008). Plasticity in attention: Implications for stress response in children. Behaviour Research and Therapy, 46(4), 450–461. doi:https://doi.org/10.1016/j.brat.2008.01.012
Fang, X., Brown, D. S., Florence, C. S., & Mercy, J. A. (2012). The economic burden of child maltreatment in the United States and implications for prevention. Child Abuse & Neglect, 36(2), 156–165. doi:https://doi.org/10.1016/j.chiabu.2011.10.006
Foti, D., Hajcak, G., & Dien, J. (2009). Differentiating neural responses to emotional pictures: Evidence from temporal-spatial PCA. Psychophysiology, 46(3), 521–530. doi:https://doi.org/10.1111/j.1469-8986.2009.00796.x
Foti, D., Olvet, D. M., Klein, D. N., & Hajcak, G. (2010). Reduced electrocortical response to threatening faces in major depressive disorder. Depression and Anxiety, 27(9), 813–820.doi:https://doi.org/10.1002/da.20712
Gibb, B. E., & Abela, J. R. (2008). Emotional abuse, verbal victimization, and the development of children’s negative inferential styles and depressive symptoms. Cognitive Therapy and Research, 32(2), 161–176. doi:https://doi.org/10.1007/s10608-006-9106-x
Gibb, B. E., Alloy, L. B., Walshaw, P. D., Comer, J. S., Shen, G. H., & Villari, A. G. (2006). Predictors of attributional style change in children. Journal of Abnormal Child Psychology, 34(3), 408–422. doi:https://doi.org/10.1007/s10802-006-9022-2
Gibb, B. E., Chelminzski, I., & Zimmerman, M. (2007). Childhood emotional, physical, and sexual abuse, and diagnoses of depressive and anxiety disorders in adult psychiatric outpatients. Depression and Anxiety, 24(4), 256–263. doi:10.1002/da.20238
Gibb, B. E., Schofield, C. A., & Coles, M. E. (2008). Reported history of childhood abuse and young adults’ information processing biases for facial displays of emotion. Child Maltreatment, 14(2), 148–156. doi:https://doi.org/10.1177/1077559508326358
Gilbert, R., Widom, C. S., Browne, K., Fergusson, D., Webb, E., & Janson, S. (2009). Burden and consequences of child maltreatment in high-income countries. The Lancet, 373(9657), 68–81. doi:https://doi.org/10.1016/s0140-6736(08)61706-7
Goeleven, E., De Raedt, R., Leyman, L., & Verschuere, B. (2008). The Karolinska directed emotional faces: A validation study. Cognition and Emotion, 22(6), 1094–1118. doi:https://doi.org/10.1080/02699930701626582
Gotlib, I. H., Krasnoperova, E., Yue, D. N., & Joormann, J. (2004). Attentional biases for negative interpersonal stimuli in clinical depression. Journal of Abnormal Psychology, 113(1), 127. doi:https://doi.org/10.1037/0021-843x.113.1.121
Gratton, G., Coles, M. G., & Donchin, E. (1983). A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology, 55(4), 468-484. doi:https://doi.org/10.1016/0013-4694(83)90135-9
Green, J. G., McLaughlin, K. A., Berglund, P. A., Gruber, M. J., Sampson, N. A., Zaslavsky, A.M., & Kessler, R. C. (2010). Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication: I. Associations with first onset of DSM-IV disorders. Archives of General Psychiatry, 67(2), 113–123. doi:https://doi.org/10.1001/archgenpsychiatry.2009.186
Hajcak, G., MacNamara, A., & Olvet, D. M. (2010). Event-related potentials, emotion, and emotion regulation: An integrative review. Developmental Neuropsychology, 35(2), 129–155. doi:https://doi.org/10.1080/87565640903526504
Hajcak, G., & Olvet, D. M. (2008). The persistence of attention to emotion: Brain potentials during and after picture presentation. Emotion, 8(2), 250. doi:https://doi.org/10.1037/1528-3542.8.2.250
Hajcak, G., Weinberg, A., MacNamara, A., & Foti, D. (2012). ERPs and the study of emotion (Oxford handbooks online). doi:https://doi.org/10.1093/oxfordhb/9780195374148.013.0222
Hardt, J., & Rutter, M. (2004). Validity of adult retrospective reports of adverse childhood experiences: Review of the evidence. Journal of Child Psychology and Psychiatry, 45(2), 260–273. doi:https://doi.org/10.1111/j.1469-7610.2004.00218.x
Hein, T. C., & Monk, C. S. (2017). Research review: Neural response to threat in children, adolescents, and adults after child maltreatment–a quantitative meta-analysis. Journal of Child Psychology and Psychiatry, 58(3), 222–230. doi:https://doi.org/10.1111/jcpp.12651
Humphreys, K. L., Kircanski, K., Colich, N. L., & Gotlib, I. H. (2016). Attentional avoidance of fearful facial expressions following early life stress is associated with impaired social functioning. Journal of Child Psychology and Psychiatry, 57(10), 1174–1182. doi:https://doi.org/10.1111/jcpp.12607
Huynh, H., & Feldt, L. S. (1976). Estimation of the Box correction for degrees of freedom from sample data in randomized block and split-plot designs. Journal of Educational Statistics,1(1), 69–82. doi:https://doi.org/10.2307/1164736
Kessler, R. C., Davis, C. G., & Kendler, K. S. (1997). Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychological Medicine, 27(05), 1101–1119. doi:https://doi.org/10.1017/s0033291797005588
Kessler, R. C., McLaughlin, K. A., Green, J. G., Gruber, M. J., Sampson, N. A., Zaslavsky, A. M., & Benjet, C. (2010). Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. The British Journal of Psychiatry, 197(5), 378–385.doi:https://doi.org/10.1192/bjp.bp.110.080499
Keyes, K. M., Eaton, N. R., Krueger, R. F., McLaughlin, K. A., Wall, M. M., Grant, B. F., & Hasin, D. S. (2012). Childhood maltreatment and the structure of common psychiatric disorders. The British Journal of Psychiatry, 200(2), 107–115. doi:https://doi.org/10.1192/bjp.bp.111.093062
Kim, J., & Cicchetti, D. (2010). Longitudinal pathways linking child maltreatment, emotion regulation, peer relations, and psychopathology. Journal of Child Psychology and Psychiatry, 51(6), 706–716. doi:https://doi.org/10.1111/j.1469-7610.2009.02202.x
Kujawa, A., Hajcak, G., Danzig, A. P., Black, S. R., Bromet, E. J., Carlson, G. A., … Klein, D. N. (2016). Neural reactivity to emotional stimuli prospectively predicts the impact of a natural disaster on psychiatric symptoms in children. Biological Psychiatry, 80(5), 381–389. doi:https://doi.org/10.1016/j.biopsych.2015.09.008
Kujawa, A., Hajcak, G., Torpey, D., Kim, J., & Klein, D. N. (2012). Electrocortical reactivity to emotional faces in young children and associations with maternal and paternal depression. Journal of Child Psychology and Psychiatry, 53(2), 207–215. doi:https://doi.org/10.1111/j.1469-7610.2011.02461.x
Kujawa, A., MacNamara, A., Fitzgerald, K. D., Monk, C. S., & Phan, K. L. (2015). Enhanced neural reactivity to threatening faces in anxious youth: Evidence from event-related potentials. Journal of Abnormal Child Psychology, 43(8), 1493–1501. doi:https://doi.org/10.1007/s10802-015-0029-4
London, K., Bruck, M., Ceci, S. J., & Shuman, D. W. (2005). Disclosure of child sexual abuse: What does the research tell us about the ways that children tell?. Psychology, Public Policy, and Law, 11(1), 194. doi:https://doi.org/10.1037/1076-8971.11.1.194
Lundqvist, D., Flykt, A., Öhman, A. (1998): The Karolinska directed emotional faces – KDEF [CDROM]. Department of Clinical Neuroscience, Psychology section, Karolinska Institutet, Stockholm, Sweden.
MacLeod, C., Rutherford, E., Campbell, L., Ebsworthy, G., & Holker, L. (2002). Selective attention and emotional vulnerability: Assessing the causal basis of their association through the experimental manipulation of attentional bias. Journal of Abnormal Psychology, 111(1), 107. doi:https://doi.org/10.1037/0021-843x.111.1.107
MacNamara, A., & Proudfit, G. H. (2014). Cognitive load and emotional processing in generalized anxiety disorder: Electrocortical evidence for increased distractibility. Journal of Abnormal Psychology, 123(3), 557. doi:https://doi.org/10.1037/a0036997
Marsh, A. A., Ambady, N., & Kleck, R. E. (2005). The effects of fear and anger facial expressions on approach-and avoidance-related behaviors. Emotion, 5(1), 119. doi:https://doi.org/10.1037/1528-3542.5.1.119
Mathews, A., & MacLeod, C. (2002). Induced processing biases have causal effects on anxiety. Cognition & Emotion, 16(3), 331–354. doi:https://doi.org/10.1080/02699930143000518
Mauchly, J. W. (1940). Significance test for sphericity of a normal n-variate distribution. The Annals of Mathematical Statistics, 11(2), 204–209. doi:https://doi.org/10.1214/aoms/1177731915
McCrory, E. J., De Brito, S. A., Kelly, P. A., Bird, G., Sebastian, C. L., Mechelli, A., & Viding, E. (2013). Amygdala activation in maltreated children during pre-attentive emotional processing. The British Journal of Psychiatry, 202, 269–276. doi:https://doi.org/10.1192/bjp.bp.112.116624
McCrory, E. J., De Brito, S. A., Sebastian, C. L., Mechelli, A., Bird, G., Kelly, P. A., & Viding, E. (2011). Heightened neural reactivity to threat in child victims of family violence. Current Biology, 21, 947–948. doi:https://doi.org/10.1016/j.cub.2011.10.015
McCrory, E. J., Gerin, M. I., & Viding, E. (2017). Annual research review: Childhood maltreatment, latent vulnerability and the shift to preventative psychiatry—The contribution of functional brain imaging. Journal of Child Psychology and Psychiatry, 58, 338–357. doi:https://doi.org/10.1111/jcpp.12713
McCrory, E. J., & Viding, E. (2015). The theory of latent vulnerability: Reconceptualizing the link between childhood maltreatment and psychiatric disorder. Development and Psychopathology, 27(02), 493–505. doi:https://doi.org/10.1017/s0954579415000115
McLaughlin, K. A. (2016). Future directions in childhood adversity and youth psychopathology. Journal of Clinical Child & Adolescent Psychology, 45(3), 361–382. doi:https://doi.org/10.1080/15374416.2015.1110823
McLaughlin, K. A., Green, J. G., Gruber, M. J., Sampson, N. A., Zaslavsky, A., & Kessler, R. C. (2012). Childhood adversities and first onset of psychiatric disorders in a national sample of adolescents. Archives of General Psychiatry, 69, 1151–1160. doi:https://doi.org/10.1001/archgenpsychiatry.2011.2277
McLaughlin, K. A., & Lambert, H. K. (2017). Child trauma exposure and psychopathology: Mechanisms of risk and resilience. Current Opinion in Psychology, 14, 29–34. doi:https://doi.org/10.1016/j.copsyc.2016.10.004
McLaughlin, K. A., Peverill, M., Gold, A. L., Alves, S., & Sheridan, M. A. (2015). Child maltreatment and neural systems underlying emotion regulation. Journal of the American Academy of Child & Adolescent Psychiatry, 54(9), 753–762. doi:https://doi.org/10.1111/j.1469-7610.2009.02202.x
Miles, J., & Shevlin, M. (2001). Applying regression and correlation: A guide for students and researchers. London: Sage.
Moser, J. S., Hajcak, G., Bukay, E., & Simons, R. F. (2006). Intentional modulation of emotional responding to unpleasant pictures: An ERP study. Psychophysiology, 43(3), 292–296. doi:https://doi.org/10.1111/j.1469-8986.2006.00402.x
Nelson, B. D., Perlman, G., Hajcak, G., Klein, D. N., & Kotov, R. (2015). Familial risk for distress and fear disorders and emotional reactivity in adolescence: An event-related potential investigation. Psychological Medicine, 45(12), 2545–2556. doi:https://doi.org/10.1017/s0033291715000471
Olofsson, J. K., Nordin, S., Sequeira, H., & Polich, J. (2008). Affective picture processing: An integrative review of ERP findings. Biological Psychology, 77(3), 247–265. doi:https://doi.org/10.1016/j.biopsycho.2007.11.006
Pine, D. S., Mogg, K., Bradley, B. P., Montgomery, L. A., Monk, C. S., McClure, E., … Kaufman, J. (2005). Attention bias to threat in maltreated children: Implications for vulnerability to stress-related psychopathology. American Journal of Psychiatry, 162, 291–296. doi:https://doi.org/10.1176/appi.ajp.162.2.291
Pollak, S. D. (2003). Experience-dependent affective learning and risk for psychopathology in children. Annals of the New York Academy of Sciences, 1008(1), 102–111. doi:https://doi.org/10.1196/annals.1301.011
Pollak, S. D., & Kistler, D. J. (2002). Early experience is associated with the development of categorical representations for facial expressions of emotion. Proceedings of the National Academy of Sciences, 99(13), 9072–9076. doi:https://doi.org/10.1073/pnas.142165999
Pollak, S. D., Klorman, R., Thatcher, J. E., & Cicchetti, D. (2001). P3b reflects maltreated children’s reactions to facial displays of emotion. Psychophysiology, 38(02), 267–274. doi:https://doi.org/10.1017/S0048577201990808
Pollak, S. D., Messner, M., Kistler, D. J., & Cohn, J. F. (2009). Development of perceptual expertise in emotion recognition. Cognition, 110(2), 242–247. doi:https://doi.org/10.1093/acprof:oso/9780195309607.003.0004
Pollak, S. D., & Sinha, P. (2002). Effects of early experience on children’s recognition of facial displays of emotion. Developmental Psychology, 38(5), 784. doi:https://doi.org/10.1037//0012-1649.38.5.784
Pollak, S. D., & Tolley-Schell, S. A. (2003). Selective attention to facial emotion in physically abused children. Journal of Abnormal Psychology, 112(3), 323. doi:https://doi.org/10.1037/0021-843x.112.3.323
Pollak, S. D., Vardi, S., Putzer Bechner, A. M., & Curtin, J. J. (2005). Physically abused children's regulation of attention in response to hostility. Child Development, 76(5), 968-977.
Price, J. M., & Glad, K. (2003). Hostile attributional tendencies in maltreated children. Journal of Abnormal Child Psychology, 31(3), 329-343. doi:https://doi.org/10.1023/A:1023237731683
Rose, D. T., & Abramson, L. Y. (1992). Developmental predictors of depressive cognitive styles. In D. Cicchetti & S. L. Toth (Eds.), Developmental perspectives on depression (pp. 323–349). Rochester: Rochester Symposium on Developmental Psychopathology.
Schupp, H. T., Cuthbert, B. N., Bradley, M. M., Cacioppo, J. T., Ito, T., & Lang, P. J. (2000). Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology, 37, 257–261. doi:https://doi.org/10.1017/s0048577200001530
Schupp, H. T., Flaisch, T., Stockburger, J., & Junghöfer, M. (2006). Emotion and attention: Event-related brain potential studies. Progress in Brain Research, 156, 31–51. doi:https://doi.org/10.1016/s0079-6123(06)56002-9
Shackman, J. E., Shackman, A. J., & Pollak, S. D. (2007). Physical abuse amplifies attention to threat and increases anxiety in children. Emotion, 7(4), 838. doi:https://doi.org/10.1037/1528-3542.7.4.838
Siegle, G. J., Steinhauer, S. R., Thase, M. E., Stenger, V. A., & Carter, C. S. (2002). Can’t shake that feeling: Event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry, 51(9), 693–707. doi:https://doi.org/10.1016/s0006-3223(02)01314-8
Simon, N. M., Herlands, N. N., Marks, E. H., Mancini, C., Letamendi, A., Li, Z., & Stein, M. B. (2009). Childhood maltreatment linked to greater symptom severity and poorer quality of life and function in social anxiety disorder. Depression and Anxiety, 26(11), 1027–1032. doi:https://doi.org/10.1002/da.20604
Speed, B. C., Nelson, B. D., Auerbach, R. P., Klein, D. N., & Hajcak, G. (2016). Depression risk and electrocortical reactivity during self-referential emotional processing in 8- to 14-year old girls. Journal of Abnormal Psychology, 125(5), 607. doi:https://doi.org/10.1037/abn0000173
Stoltenborgh, M., Bakermans-Kranenburg, M. J., Alink, L. R., & IJzendoorn, M. H. (2015). The prevalence of child maltreatment across the globe: Review of a series of meta analyses. Child Abuse Review, 24(1), 37–50. doi:https://doi.org/10.1002/car.2353
Teisl, M., & Cicchetti, D. (2008). Physical abuse, cognitive and emotional processes, and aggressive/disruptive behavior problems. Social Development, 17(1), 1–23. doi:https://doi.org/10.1111/j.1467-9507.2007.00412.x
Vachon, D. D., Krueger, R. F., Rogosch, F. A., & Cicchetti, D. (2015). Assessment of the harmful psychiatric and behavioral effects of different forms of child maltreatment. Journal of the American Medical Association of Psychiatry, 72(11), 1135–1142. doi:https://doi.org/10.1001/jamapsychiatry.2015.1792
Van Bockstaele, B., Verschuere, B., Tibboel, H., De Houwer, J., Crombez, G., & Koster, E. H. (2014). A review of current evidence for the causal impact of attentional bias on fear and anxiety. Psychological Bulletin, 140(3), 682. doi:https://doi.org/10.1037/a0034834
van Harmelen, A. L., van Tol, M. J., Demenescu, L. R., van der Wee, N. J., Veltman, D. J., Aleman, A., … & Elzinga, B. M. (2013). Enhanced amygdala reactivity to emotional faces in adults reporting childhood emotional maltreatment. Social Cognitive and Affective Neuroscience, 8(4), 362–369. doi:https://doi.org/10.1093/scan/nss007
Watson, D., O’Hara, M. W., Naragon-Gainey, K., Koffel, E., Chmielewski, M., Kotov, R., … Ruggero, C. J. (2012). Development and validation of new Anxiety and Bipolar Symptom scales for an expanded version of the IDAS (the IDAS-II). Assessment, 19(4), 399–420. doi:https://doi.org/10.1177/1073191112449857
Watson, D., O’hara, M. W., Simms, L. J., Kotov, R., Chmielewski, M., McDade-Montez, E. A., … Stuart, S. (2007). Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS). Psychological Assessment, 19(3), 253. doi:https://doi.org/10.1037/1040-3590.19.3.253
Weinberg, A., & Hajcak, G. (2010). Beyond good and evil: The time-course of neural activity elicited by specific picture content. Emotion, 10(6), 767. doi:https://doi.org/10.1037/a0020242
Weinberg, A., & Hajcak, G. (2011). The late positive potential predicts subsequent interference with target processing. Journal of Cognitive Neuroscience, 23(10), 2994-3007. doi:https://doi.org/10.1162/jocn.2011.21630
Weinberg, A., Perlman, G., Kotov, R., & Hajcak, G. (2016). Depression and reduced neural response to emotional images: Distinction from anxiety, and importance of symptom dimensions and age of onset. Journal of Abnormal Psychology, 125(1), 26. doi:https://doi.org/10.1037/abn0000118
Weinberg, A., & Sandre, A. (2017). Distinct associations between low positive affect, panic, and neural responses to reward and threat during late stages of affective picture processing. Manuscript submitted for publication.
Weinberg, A., Venables, N. C., Proudfit, G. H., & Patrick, C. J. (2014). Heritability of the neural response to emotional pictures: Evidence from ERPs in an adult twin sample. Social Cognitive and Affective Neuroscience, 10(3), 424–434. doi:https://doi.org/10.1093/scan/nsu059
Whalen, P.J. (1998). Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science, 7, 177–188. doi:https://doi.org/10.1111/1467-8721.ep10836912
Whalen, P. J., Shin, L. M., McInerney, S. C., Fischer, H., Wright, C. I., & Rauch, S. L. (2001). Afunctional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion, 1(1), 70. doi:https://doi.org/10.1037/1528-3542.1.1.70
Williams, L. M., Das, P., Liddell, B., Olivieri, G., Peduto, A., Brammer, M. J., & Gordon, E. (2005). BOLD, sweat and fears: fMRI and skin conductance distinguish facial fear signals. Neuro Report, 16(1), 49–52. doi:https://doi.org/10.1097/00001756-200501190-00012
Acknowledgements
This study was supported by Canada Research Chair in Clinical Neuroscience awarded to A.W.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Appendix: Faces used in the study
Appendix: Faces used in the study
01M, 03M, 06M, 09M, 13M, 14M, 25M, 30M, 03F, 06F, 08F, 09F, 11F, 12F, 14F
Rights and permissions
About this article
Cite this article
Sandre, A., Ethridge, P., Kim, I. et al. Childhood maltreatment is associated with increased neural response to ambiguous threatening facial expressions in adulthood: Evidence from the late positive potential. Cogn Affect Behav Neurosci 18, 143–154 (2018). https://doi.org/10.3758/s13415-017-0559-z
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-017-0559-z