Vine-Shoots as Enological Additives. A Study of Acute Toxicity and Cytotoxicity
Abstract
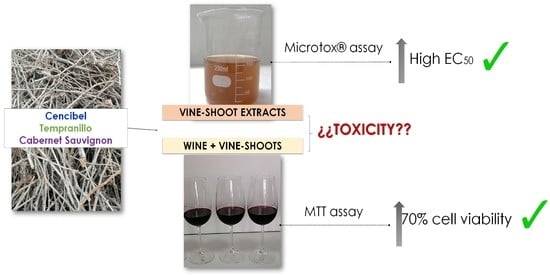
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Samples: Vine-Shoot Extracts and Wines
2.2.1. Vine-Shoot Extracts
2.2.2. Wines
2.3. Chemical Analysis for Vine-Shoot Characterization
2.3.1. Vine-Shoot Extraction
2.3.2. Phenolic Composition
2.3.3. Furan Composition
2.3.4. Mineral Composition
2.4. Toxicity Tests
2.4.1. Microtox® Assay for Vine-Shoot Extracts
2.4.2. MTT Assay for Vine-Shoot Extracts and Wines
2.5. Statistical Analysis
3. Results and Discussion
3.1. Vine-Shoot Composition
3.2. Toxicity Results
3.2.1. Vine−Shoot Acute Toxicity Evaluated toward V. fischeri
3.2.2. Cytotoxicity of Vine−Shoots and Wines Macerated with Them, Evaluated through 3T3−L1 Cell Viability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sánchez−Gómez, R.; Alonso, G.L.; Salinas, M.R.; Zalacain, A. Reuse of Vine−Shoots Wastes for Agricultural Purposes. In Handbook of Grape Processing By-Products; Galanakis, C.M., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2017; pp. 79–104. ISBN 9780128098707. [Google Scholar]
- OIV International Organisation of Vine and Wine. Guide of the Oiv for a Sustainable Vitiviniculture: Production, Transformation and Conditioning of Products; OIV International Organisation of Vine and Wine: Paris, France, 2020. [Google Scholar]
- Briones, R.; Torres, L.; Saravia, Y.; Serrano, L.; Labidi, J. Liquefied agricultural residues for film elaboration. Ind. Crops Prod. 2015, 78, 19–28. [Google Scholar] [CrossRef]
- Çetin, E.S.; Altinöz, D.; Tarçan, E.; Göktürk Baydar, N. Chemical composition of grape canes. Ind. Crops Prod. 2011, 34, 994–998. [Google Scholar] [CrossRef]
- Cebrián, C.; Sánchez−Gómez, R.; Salinas, M.R.; Alonso, G.L.; Zalacain, A. Effect of post−pruning vine−shoots storage on the evolution of high−value compounds. Ind. Crops Prod. 2017, 109, 730–736. [Google Scholar] [CrossRef]
- Cebrián−Tarancón, C.; Sánchez−Gómez, R.; Salinas, M.R.; Alonso, G.L.; Oliva, J.; Zalacain, A. Toasted vine−shoot chips as enological additive. Food Chem. 2018, 263, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Cebrián−Tarancón, C. Evaluation of Vine−Shoots as a New Source of Enological Additives. Ph.D. Thesis, Universidad de Castilla−La Mancha, Ciudad Real, Spain, 2019. [Google Scholar]
- Teissedre, P.L. Wine and health. In The Biochemistry of the Grape; Gerós, H., Chaves, M., Delrot, S., Eds.; Bentham Science: Shaqah, United Arab Emirates, 2012; pp. 269–285. [Google Scholar]
- Pezzuto, J.M. Grapes and human health: A perspective. J. Agric. Food Chem. 2008, 56, 6777–6784. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Council Regulation (EC) No 2165/2005 of 20 December 2005 Amending Regulation (EC) No 1493/1999 on the Common Organization of the Market in Wine; European Commission: Brussels, Belgium, 2005; pp. 1–4. [Google Scholar]
- Cebrián−Tarancón, C.; Sánchez−Gómez, R.; Cabrita, M.J.; García, R.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Winemaking with vine−shoots. Modulating the composition of wines by using their own resources. Food Res. Int. 2019, 121, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Oliva, J.; Cámara, M.A.; Cebrían, C.; Sánchez−Gómez, R.; Zalacain, A.; Salinas, M.R. Dissapearance study of fungicide residues on treated vine−shoots. In Proceedings of the Vino Analytica Scientia Symposium, Salamanca, Spain, 17–20 July 2017. [Google Scholar]
- Cebrián−Tarancón, C.; Sánchez−Gómez, R.; Oliva, J.; Cámara, M.A.; Zalacain, A.; Salinas, M.R. Evolution of fungicide residues in pruned vine−shoots. Oeno One 2021, 55, 145–152. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Basu, A. Phenolic Compounds: Potential Health Benefits and Toxicity. In Utilisation of Bioactive Compounds from Agricultural and Food Production Waste; Vuong, Q.V., Ed.; CRC Press, Taylor & Francis Group: Milton Park, UK, 2017; pp. 27–59. [Google Scholar]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H. Bin Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef] [PubMed]
- Medrano−Padial, C.; Puerto, M.; Moreno, F.J.; Richard, T.; Cantos−Villar, E.; Pichardo, S. In vitro toxicity assessment of stilbene extract for its potential use as antioxidant in the wine industry. Antioxidants 2019, 8, 467. [Google Scholar] [CrossRef] [Green Version]
- Pang, R. −L.; Wang, S.−Y.; Wang, R.−P.; Dang, Q.; Guo, L.−L.; Xie, H.−X.; Fang, J.−B. Study on the enrichment and migration characteristics of heavy metals in soil−grapevine system. J. Ecol. Rural Environ. 2019, 35, 515–521. [Google Scholar]
- Sánchez−Gómez, R.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Vine−shoot waste aqueous extracts for re−use in agriculture obtained by different extraction techniques: Phenolic, volatile, and mineral compounds. J. Agric. Food Chem. 2014, 62, 10861–10872. [Google Scholar] [CrossRef]
- Delgado−Torre, M.P.; Ferreiro−Vera, C.; Priego−Capote, F.; Pérez−Juan, P.M.; De Castro, M.D.L. Comparison of accelerated methods for the extraction of phenolic compounds from different vine−shoot cultivars. J. Agric. Food Chem. 2012, 60, 3051–3060. [Google Scholar] [CrossRef]
- Skotti, E.; Anastasaki, E.; Kanellou, G.; Polissiou, M.; Tarantilis, P.A. Total phenolic content, antioxidant activity and toxicity of aqueous extracts from selected Greek medicinal and aromatic plants. Ind. Crops Prod. 2014, 53, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Sotiropoulou, N.S.; Megremi, S.F.; Tarantilis, P. Evaluation of antioxidant activity, toxicity, and phenolic profile of aqueous extracts of chamomile (Matricaria chamomilla L.) and sage (Salvia ocinalis L.) prepared at different temperatures. Appl. Sci. 2020, 10, 2270. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Vinken, M.; Blaauboer, B.J. In vitro testing of basal cytotoxicity: Establishment of an adverse outcome pathway from chemical insult to cell death. Toxicol. Vitr. 2017, 39, 104–110. [Google Scholar] [CrossRef] [Green Version]
- Serrano−Díaz, J.; Estevan, C.; Sogorb, M.Á.; Carmona, M.; Alonso, G.L.; Vilanova, E. Cytotoxic effect against 3T3 fibroblasts cells of saffron floral bio−residues extracts. Food Chem. 2014, 147, 55–59. [Google Scholar] [CrossRef]
- Fuchs, C.; Bakuradze, T.; Steinke, R.; Grewal, R.; Eckert, G.P.; Richling, E. Polyphenolic composition of extracts from winery by−products and effects on cellular cytotoxicity and mitochondrial functions in HepG2 cells. J. Funct. Foods 2020, 70, 103988. [Google Scholar] [CrossRef]
- Cebrián−Tarancón, C.; Sánchez−Gómez, R.; Carot, J.M.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Assessment of vine−shoots in a model wines as enological additives. Food Chem. 2019, 288, 86–95. [Google Scholar] [CrossRef]
- European Commission. EU Official Methods for Wine Analysis, Regulation 440/2003; European Commission: Brussels, Belgium, 2003. [Google Scholar]
- ISO 11348−1:2007/AMD 1:2018. Water Quality—Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio fischeri (Luminescent bacteria Test)—Part 1: Method using Freshly Prepared Bacteria—Amendment 1; ISO: Geneva, Switzerland, 2018. [Google Scholar]
- Microbics. Data Quality Applying Results in Microtox Manual—A Toxicity Testing Handbook; Microbics Corporation: Carlsbad, CA, USA, 1992. [Google Scholar]
- Escobar, L.; Rivera, A.; Aristizábal, F.A. Estudio comparativo de los métodos de resazurina y MTT en estudios de citotoxicidad en líneas celulares tumorales humanas. Vitae, Rev. Fac. Química Farm. 2010, 17, 67–74. [Google Scholar]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a Tetrazolium−based Semiautomated Colorimetric Assay: Assessment of Chemosensitivity Testing. Cancer Res. 1987, 47, 936–942. [Google Scholar] [PubMed]
- Chatonnet, P.; Escobessa, J. Impact of toasting oak barrels on the presence of polycyclic aromatic hydrocarbons in wine. J. Agric. Food Chem. 2007, 55, 10351–10358. [Google Scholar] [CrossRef] [PubMed]
- EC Regulation No. 1493/99. Conuncil Regulation (EC) No 1493/1999 of 17 May 1999 on the Common Organisation of the Market in Wine; ISO: Geneva, Switzerland, 1999. [Google Scholar]
- ISO 10993−5:2009. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Riddell, R.J.; Panacer, D.S.; Wilde, S.M.; Clothier, R.H.; Balls, M. The importance of exposure period and cell type in in vitro cytotoxicity tests. ATLA 1986, 14, 86–92. [Google Scholar] [CrossRef]
- Zhang, X.H.; Huang, B.; Choi, S.K.; Seo, J.S. Anti−obesity effect of resveratrol−amplified grape skin extracts on 3T3−L1 adipocytes differentiation. Nutr. Res. Pract. 2012, 6, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Medrano−Padial, C.; Puerto, M.; del Merchán−Gragero, M.; Moreno, F.J.; Richard, T.; Cantos-Villar, E.; Pichardo, S. Cytotoxicity studies of a stilbene extract and its main components intended to be used as preservative in the wine industry. Food Res. Int. 2020, 137. [Google Scholar] [CrossRef] [PubMed]




| Tempranillo | Cencibel | Cabernet Sauvignon | F 1 | |
|---|---|---|---|---|
| Phenolic compounds (mg/kg) | ||||
| Flavanols | ||||
| (+)-Catechin | 964.68 ± 177.92 a | 1166.22 ± 261.08 a | 932.40 ± 38.58 a | 1.90 |
| (−)-Epicatechin | 1595.66 ± 260.68 a | 1950.65 ± 191.75 b | 2107.58 ± 66.98 b | 7.56 * |
| Acids | ||||
| Gallic acid | 17.62 ± 3.19 a | 30.63 ± 3.63 b | 15.13 ± 0.30 a | 35.57 *** |
| Ellagic acid | 790.90 ± 34.84 a | 937.03 ± 51.71 b | 795.32 ± 11.06 a | 20.67 *** |
| Protocatechuic acid | 11.03 ± 2.32 a | 19.18 ± 3.84 b | 8.19 ± 1.15 a | 18.14 *** |
| trans-Caftaric acid | 52.21 ± 14.51 a | 74.37 ± 26.26 a | 64.28 ± 1.63 a | 1.64 |
| trans-Coutaric acid | 10.13 ± 1.59 a | 14.29 ± 2.60 b | 14.77 ± 3.20 b | 4.00 * |
| Stilbens | ||||
| trans-Resveratrol | 200.43 ± 13.84 a | 248.33 ± 6.99 b | 235.49 ± 28.38 b | 7.05 * |
| Piceid-trans-resveratrol | 22.79 ± 0.04 b | 15.72 ± 0.13 a | 26.87 ± 0.78 c | 608.21 *** |
| Piceatannol | 82.67 ± 8.64 a | 80.07 ± 10.91 a | 126.46 ± 2.20 b | 41.04 *** |
| trans-ε-viniferin | 64.31 ± 0.21 a | 187.26 ± 0.66 c | 96.86 ± 0.46 b | 70,261 *** |
| Flavonols | ||||
| Quercetin | n.d. | 14.53 ± 0.12 a | 15.14 ± 0.28 b | 16.23 ** |
| Total | 3812.44 ± 6.76 a | 4738.28 ± 15.09 c | 4438.49 ± 10.64 b | 692.36 *** |
| Furan compounds (mg/kg) | ||||
| Furfural | 12.37 ± 3.72 a | 18.83 ± 3.35 b | 18.87 ± 4.54 b | 3.67 * |
| 2-Furancarboxaldehyde | 0.57 ± 0.22 a | 1.49 ± 0.33 b | 1.90 ± 0.44 b | 15.45 ** |
| 2-Furanmethanol | 146.44 ± 25.26 a | 165.03 ± 28.85 a | 164.71 ± 30.86 a | 0.56 |
| 5-Hydroxymethylfurfural | 16.94 ± 7.17 a | 17.16 ± 5.68 a | 30.46 ± 9.45 b | 4.16 * |
| Total | 176.32 ± 29.61 a | 202.51 ± 37.70 a | 215.94 ± 19.90 a | 1.81 |
| Minerals (mg/kg) | ||||
| Al | 5.12 ± 1.88 a | 25.03 ± 3.74 b | 5.16 ± 0.77 a | 65.63 *** |
| As | 1.20 ± 0.24 a | 1.59 ± 0.35 a | 2.31 ± 0.28 b | 11.07 ** |
| Bi | 4.76 ± 0.23 a | 4.33 ± 0.70 a | n.d. | 0.99 |
| B | 7.85 ± 1.74 a | 10.35 ± 1.22 a | 8.44 ± 2.06 a | 1.75 |
| Ca (g/kg) | 2.71 ± 0.06 a | 4.89 ± 0.99 b | 2.81 ± 0.23 a | 13.34 ** |
| Co | 0.08 ± 0.02 b | 0.04 ± 0.02 a | 0.12 ± 0.02 c | 16.00 ** |
| Cr | n.d. | 0.16 ± 0.02 | n.d. | - |
| Cu | 3.33 ± 0.60 a | 4.09 ± 0.08 a | 3.62 ± 0.77 a | 1.36 |
| Fe | 12.15 ± 1.58 b | 20.16 ± 1.88 c | 8.39 ± 0.81 a | 48.89 *** |
| K (g/kg) | 5.96 ± 0.06 b | 7.71 ± 0.04 c | 4.10 ± 0.37 a | 207.72 *** |
| Li | 0.26 ± 0.00 a | 0.57 ± 0.05 b | 0.32 ± 0.05 a | 49.19 *** |
| Mg (g/kg) | 0.56 ± 0.13 a | 1.03 ± 0.03 b | 0.53 ± 0.03 a | 36.92 *** |
| Mn | 13.28 ± 1.63 a | 19.73 ± 0.79 b | 24.41 ± 1.01 c | 65.32 *** |
| Na | 0.03 ± 0.01 b | 0.08 ± 0.01 c | 0.01 ± 0.01 a | 41.55 *** |
| Ni | 0.24 ± 0.05 ab | 0.41 ± 0.16 b | 0.15 ± 0.03 a | 5.55 * |
| Pb | 0.26 ± 0.05 a | 0.26 ± 0.06 a | n.d. | 0.01 |
| Rb | 0.78 ± 0.20 a | 0.82 ± 0.06 a | 0.60 ± 0.07 a | 2.41 |
| Sb | 0.35 ± 0.07 b | 0.26 ± 0.07 ab | 0.21 ± 0.04 a | 5.16 * |
| Si | 25.92 ± 5.36 b | 26.89 ± 4.70 b | 12.13 ± 0.24 a | 12.08 ** |
| Sr | 69.11 ± 9.85 a | 144.91 ± 3.46 b | 81.17 ± 11.49 a | 61.95 *** |
| Ti | 0.81 ± 0.14 a | 1.48 ± 0.01 b | 0.67 ± 0.23 a | 24.16 ** |
| Tl | 0.65 ± 0.15 a | 1.20 ± 0.20 b | 1.71 ± 0.30 c | 16.44 ** |
| Zn | 5.77 ± 1.63 a | 24.13 ± 0.14 b | 6.56 ± 0.52 a | 330.92 *** |
| Total (g/kg) | 9.42 ± 0.32 b | 14.02 ± 1.24 c | 7.60 ± 0.75 a | 59.38 *** |
| EC50 (mg/mL) | F 1 | ||
|---|---|---|---|
| TeW | 8.70 ± 0.54 a, A | 9.15 ** | 31.32 * |
| TeE | 17.37 ± 2.12 d, B | ||
| CeW | 11.96 ± 1.70 ab, A | 0.04 | |
| CeE | 11.71 ± 0.49 ab, A | ||
| CSeW | 13.42 ± 2.14 bc, A | 2.36 | |
| CseE | 15.75 ± 0.17 cd, A | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cebrián-Tarancón, C.; Fernández-Roldán, F.; Sánchez-Gómez, R.; Salinas, R.; Llorens, S. Vine-Shoots as Enological Additives. A Study of Acute Toxicity and Cytotoxicity. Foods 2021, 10, 1267. https://doi.org/10.3390/foods10061267
Cebrián-Tarancón C, Fernández-Roldán F, Sánchez-Gómez R, Salinas R, Llorens S. Vine-Shoots as Enological Additives. A Study of Acute Toxicity and Cytotoxicity. Foods. 2021; 10(6):1267. https://doi.org/10.3390/foods10061267
Chicago/Turabian StyleCebrián-Tarancón, Cristina, Francisco Fernández-Roldán, Rosario Sánchez-Gómez, Rosario Salinas, and Silvia Llorens. 2021. "Vine-Shoots as Enological Additives. A Study of Acute Toxicity and Cytotoxicity" Foods 10, no. 6: 1267. https://doi.org/10.3390/foods10061267