Are fungicides a driver of European foulbrood disease in honey bee colonies pollinating blueberries?
- 1Department of Veterinary Pathology, Western College of Veterinary Medicine, Saskatoon, SK, Canada
- 2Department of Veterinary Microbiology, Western College of Veterinary Medicine, Saskatoon, SK, Canada
- 3Ministry of Agriculture, Government of Saskatchewan, Prince Albert, SK, Canada
- 4Agriculture and Agri-Food Canada, Beaverlodge, AB, Canada
- 5British Columbia Blueberry Council, Abbotsford, BC, Canada
- 6Department of Biochemistry, Microbiology and Immunology, College of Medicine, University of Saskatchewan, Saskatoon, SK, Canada
Introduction: Blueberry producers in Canada depend heavily on pollination services provided by honey bees (Apis mellifera L.). Anecdotal reports indicate an increased incidence of European foulbrood (EFB), a bacterial disease caused by Melissococcus plutonius, is compromising pollination services and colony health. Fungicidal products are commonly used in blueberry production to prevent fungal diseases such as anthracnose and botrytis fruit rot. Pesticide exposure has been implicated in honey bee immunosuppression; however, the effects of commercial fungicidal products, commonly used during blueberry pollination, on honey bee larval susceptibility to EFB have not been investigated.
Methods: Using an in vitro infection model of EFB, we infected first instar honey bee larvae with M. plutonius 2019 BC1, a strain isolated from an EFB outbreak in British Columbia, Canada, and chronically exposed larvae to environmentally relevant concentrations of fungicide products over 6 days. Survival was monitored until pupation or eclosion.
Results: We found that larvae chronically exposed to one, two, or three fungicidal products [Supra® Captan 80WDG (Captan), low concentration of Kenja™ 400SC (Kenja), Luna® Tranquility (Luna), and/or Switch® 62.5 WG (Switch)], did not significantly reduce survival from EFB relative to infected controls. When larvae were exposed to four fungicide products concurrently, we observed a significant 24.2% decrease in survival from M. plutonius infection (p = 0.0038). Similarly, higher concentrations of Kenja significantly reduced larval survival by 24.7–33.0% from EFB (p < 0.0001).
Discussion: These in vitro results suggest that fungicides may contribute to larval susceptibility and response to M. plutonius infections. Further testing of other pesticide combinations is warranted as well as continued surveillance of pesticide residues in blueberry-pollinating colonies.
1. Introduction
Honey bee pollination is crucial to blueberry production in North America, contributing 90% of the value of Canada’s blueberry crops each year (Government of Canada, 2018). Unfortunately, blueberry growers face a shortage of pollination services, in part due to a reported increased incidence of European foulbrood (EFB) disease in blueberry pollinating honey bee colonies (Wardell, 1982; Guarna et al., 2019; Olmstead et al., 2019; Thebeau et al., 2022). The negative economic consequences of EFB outbreaks include lost honey, reduced pollination service revenue, and increased treatment and colony replacement costs (Laate et al., 2020), thereby threatening the continued profitability of the blueberry and beekeeping industries and calling for scientific investigation of the predisposing factors for this disease.
European foulbrood occurs when the Gram-positive bacterium Melissococcus plutonius colonizes the midgut of honey bee larvae and outcompetes the larvae for nutrition (Forsgren, 2010; Laate et al., 2020). Clinical signs of EFB include yellow to brown, twisted and/or deflated larvae; dead larvae that dry to form a rubbery scale on the back of the brood cell; and a sour odor from affected brood due to secondary bacterial infection (Cheshire and Cheyne, 1885). EFB often emerges in honey bee colonies when under stress. For example, in the early spring when nursing bee populations are low and pollen and nectar resources are scarce in the environment, the colony’s brood may suffer from inadequate care and feeding, predisposing them to EFB (Forsgren, 2010; Kane and Faux, 2021).
Previously, colonies with EFB were observed to spontaneously recover when stressors such as inadequate food and water resources were alleviated (Forsgren, 2010); however, recent outbreaks of EFB associated with blueberry pollination have been described as refractory to traditional management practices (Olmstead et al., 2019; Laate et al., 2020; Thebeau et al., 2022). Proposed causes of the increased clinical severity of EFB include highly virulent (Djukic et al., 2018; Grossar et al., 2020; Thebeau et al., 2022) or antimicrobial-resistant strains of M. plutonius (Masood et al., 2022), and environmental factors such as the poor nutritional quality of blueberry pollen (Wardell, 1982; Olmstead et al., 2019); however, the role of pesticide exposure as a predisposing factor for EFB during blueberry pollination has been incompletely explored. For example, several studies have investigated the risk of pesticides on survival of honey bee brood through larval exposure to unrealistically high concentration of active ingredients (Mussen et al., 2004; Wade et al., 2019). Moreover, researchers studying synergistic effects of pesticides on larval survival have focused on combinations that also include insecticides with known negative effects (Prado et al., 2019; Wade et al., 2019; Wood et al., 2020). Nonetheless, the investigation of field-relevant concentrations of fungicide products containing proprietary ingredients has never been explored as a potential predisposing factor for EFB.
Fungicides are widely used in Canadian highbush blueberry production to prevent anthracnose and botrytis fruit rot (Everich et al., 2009; Province of British Columbia, 2022; Mussen et al., 2004). Frequently used fungicidal products include Supra® Captan 80WDG (Captan; active ingredient captan [N-Trichloromethylthio-4-cyclohexane-1,2-dicarboximide]), Kenja™ 400SC (Kenja; active ingredient isofetamid), Luna® Tranquility (Luna; active ingredients fluopyram and pyrimethanil) and Switch® 62.5 WG (Switch; active ingredients cyprodinil and fludioxonil) (Province of British Columbia, 2022). Captan is a broad-spectrum, dicarboximide fungicide (Mussen et al., 2004) that is commonly combined with Kenja, Luna, and Switch to prevent the development of resistance (Province of British Columbia, 2022). Modes of action of the active ingredients in these commonly used fungicidal products include succinate dehydrogenase inhibitors (isofetamid and fluopyram) which inhibit the mitochondrial electron transport chain (Umetsu and Shirai, 2020); anilinopyrimidines (pyrimethanil and cyprodinil) which inhibit methionine and protein biosynthesis (Fritz et al., 2003); and phenylpyrroles (fludioxonil) which disrupt cellular signal transduction (Bersching and Jacob, 2021).
Chronic exposure of honey bees to multiple fungicides during blueberry pollination is common (Graham et al., 2021; Guarna, 2021; Rondeau and Raine, 2022). A review of fungicide risk to bees identified a total of 90 different fungicides within North American and European honey bee colony derivatives, with the greatest number of fungicides present in pollen samples (Rondeau and Raine, 2022). Moreover, residue analysis of pooled honey and pollen samples from 3 to 5 colonies after blueberry pollination has confirmed concurrent detection of 4–5 fungicide residues within these colonies (Guarna, 2021), with fluopyram, pyrimethanil, cyprodinil, and fludioxonil detected in bee bread at concentrations up to 572 ng/g for fludioxonil. Fluopyram, pyrimethanil, cyprodinil, and fludioxonil have also been identified throughout Europe, North America, and Africa, with concentrations up to 16,400 ng/g fludioxonil reported in pollen (Rondeau and Raine, 2022). These four fungicides were also detected in blueberry pollinating colonies in the United States (Graham et al., 2021). Furthermore, residues of Captan have been found in concentrations as high as 18,970 ng/g in pollen collected from honey bee colonies pollinating crops, including blueberries, in the United States (Johnson et al., 2010; Mullin et al., 2010; Rondeau and Raine, 2022).
Chronic exposure to multiple fungicides may increase the susceptibility of honey bee colonies to EFB, considering that the exposure to combinations of agrochemicals has been found to elicit synergistic negative effects on honey bee adults and larvae (Johnson et al., 2013; Wade et al., 2019). For example, Wood et al. (2020) demonstrated chronic exposure to a fungicide and an insecticide decreased larval survival from EFB in vitro. Similarly, Bartling et al. (2021) showed that individual fungicide exposure decreased survival of adult bees infected with Pseudomonas. However, to our knowledge, there has been no investigation of potential synergistic effects of exposure to multiple fungicides on honey bee immunity and susceptibility to infectious disease.
Considering the chronic fungicide exposure of colonies pollinating blueberries and the previously reported negative effects of fungicides and adjuvants on honey bees, we urgently need to determine whether formulated fungicide exposure can explain EFB outbreaks during blueberry pollination in North America. Therefore, in this study, we used an in vitro larval infection model of EFB to investigate the effects of chronic exposure to four formulated fungicidal products commonly used in blueberry production on honey bee survival from EFB. Specifically, we sought to (1) determine the effects of field-relevant concentrations of individual fungicidal products on honey bee larval survival, (2) determine if individual fungicidal products increases mortality from EFB infection, and (3) determine if larvae co-exposed to combinations of two, three, or four fungicide products are more susceptible to EFB.
2. Materials and methods
2.1. Fungicide preparation
Four water soluble formulated fungicidal products were tested in this study: Supra® Captan 80WDG (Captan; Product 33,641, Lot BO9044965060, Terralink Horticulture, Abbotsford, BC, Canada), Kenja™ 400SC (Kenja; Product 31,758, Lot V31758-170324, Terralink Horticulture), Luna® Tranquility (Luna; Product 30,510, Lot NK43HX1965, Terralink Horticulture), and Switch® 62.5 WG (Switch; Product 28,189, Lot YGM9C28004, Terralink Horticulture). Products were stored in their concentrated form (wettable granules or liquid concentrate) in opaque containers at room temperature until use. Stock solutions of diluted fungicidal products were prepared in sterile water and stored at 4°C for up to 1 week until incorporation in the larval diet.
2.2. Fungicidal product concentration range determination
To determine a field-relevant concentration range for the four fungicidal products used in the experiment, we used BeeRex (United States Environmental Protection Agency, 2015), a United States Environmental Protection Agency tool for terrestrial pesticide risk assessment for honey bees (Table 1). Using BeeRex (United States Environmental Protection Agency, 2015), the total exposure to the active ingredients of the four fungicidal products for 6-day-old worker honey bee larvae was determined in two ways; (1) by input of the foliar application rate (kg/hectare) for the preventative control against botrytis fruit rot in highbush blueberries (Province of British Columbia, 2022), and (2) through input of the maximum residue concentrations in pollen/bee bread and honey of the active fungicide ingredients (Mullin et al., 2010; Guarna, 2021). For products with two active ingredients (Luna [125 g/l fluopyram and 375 g/l pyrimethanil] and Switch [37.5% cyprodinil and 25.0% fludioxonil]), we used residue information for the active ingredients in lowest concentration in the fungicidal product (i.e., fluopyram and fludioxonil) to calculate the exposure. The residue concentration for Switch (fludioxonil) was not available for honey and was replaced with the maximum bee bread residue concentration in the BeeRex model. The residue concentrations for isofetamid in pollen/bee bread and honey were not available.
To determine the fungicide product concentrations to be tested in vitro (Table 1), we considered the field application rate of the fungicide product, as well as residue data where available (Table 1). For Captan and Kenja, the calculated fungicide product exposure based on the application rate (19,000 and 6,000 ng/bee, respectively) caused a significant reduction in larval survival (Supplementary Figure S1); therefore, 10-, 100-, and 1,000-fold dilutions were performed to obtain high, medium, and low concentrations, respectively, for in vitro testing (Table 1). For Luna (i.e., fluopyram) and Switch (i.e., fludioxonil), the ‘high’ concentration tested in vitro corresponded to the BeeRex-calculated concentration based on the application rates (1,800 ng/bee and 3,000 ng/bee, respectively), while the ‘low’ concentration tested in vitro corresponded to the BeeRex-calculated concentration based on maximum reported residues quantified in pollen/bee bread and honey sampled from blueberry-pollinating colonies (Table 1). Medium concentrations were not calculated for Luna and Switch as both the high and low concentrations did not significantly decrease larval survival from control. The final concentrations in the larval diet were calculated based on the concentration of the active substance consumed over the 6-day larval period (160 μl, Schmehl et al., 2016).
2.3. Preparation of Melissococcus plutonius for in vitro larval infection
Melissococcus plutonius isolate 2019BC1 (Wood et al., 2020; Masood et al., 2022) was used to infect honey bee larvae in vitro. To prepare this isolate for larval infection, 100 μl of thawed liquid culture of 2019BC1, previously stored at −80°C in 20% glycerol, was inoculated into 100 ml of KSBHI liquid media (brain heart infusion, supplemented with 0.15 M KH2PO4, and 1% soluble starch) and incubated at 37°C under microaerophilic conditions (Arai et al., 2012) for 48 h, shaking at 200 rpm. The liquid culture was then stored in 1 ml aliquots with 20% glycerol at −80°C. The CFU/mL of the culture was determined by using a thawed culture aliquot and plating serial dilutions on KSBHI agar with 3 μg/ml nalidixic acid (Arai et al., 2012). On the day of larval infection, an aliquot of liquid culture was warmed to 37°C and diluted in PBS to a concentration of 1.0 × 105 CFU/ml based on the previously determined CFU/ml. Post larval infection, the CFU/ml of the thawed aliquot was intermittently re-determined for accuracy. While the re-evaluation of CFUs post larval infections would have ideally been performed for each M. plutonius infection day, we found minimal variability among aliquots when we confirmed M. plutonius CFUs post-infection.
2.4. In vitro larval rearing, fungicide exposure, and Melissococcus plutonius infection
Larval infection with M. plutonius and concurrent dietary exposure to fungicide products was adapted from the protocols of Schmehl et al. (2016) and Wood et al. (2020). Briefly, using recipes outlined by Schmehl et al. (2016), we prepared three diets, labeled ‘A,’ ‘B,’ and ‘C,’ using sterile royal jelly (Stakich Inc., Troy, MI, United States), glucose (Fisher Chemicals, Fair Lawn, NJ, United States), fructose (Fisher Chemicals), yeast extract (Becton, Dickson and Company), and sterile distilled water. Diets increased in sugar and protein content from ‘A’ to ‘C.’ For larval diets containing fungicidal products, diluted formulated fungicidal products replaced the distilled water fraction in all three diets. For all fungicidal products, the concentration remained constant within diets ‘A’ – ‘C’ (Table 1). Diets were stored at −20°C until use.
From mid-May until mid-August in 2020 and 2021, we produced age-synchronized frames of honey bee worker brood, by inserting an empty wax-drawn brood frame into a cage containing the queen in one or more of 15 honey bee colonies. After 24 h, frames with eggs were removed from the queen cage and incubated in the adjacent brood chamber for 3 days until hatching. Frames of first instar larvae were transported back to the laboratory for grafting using a portable incubator at 35°C.
In the laboratory, within a biological safety cabinet, first instar larvae were individually transferred (grafted) from the brood frame into 48-well sterile tissue culture plates (STCP; Figure 1). The day of grafting was considered day 0 (D0). Each well of the STCP contained a sterile, 1 cm in diameter plastic cup, each with 10 μl of control diet ‘A’ pre-warmed to 35°C. STCPs remained on an electric heating pad at 35°C during grafting.
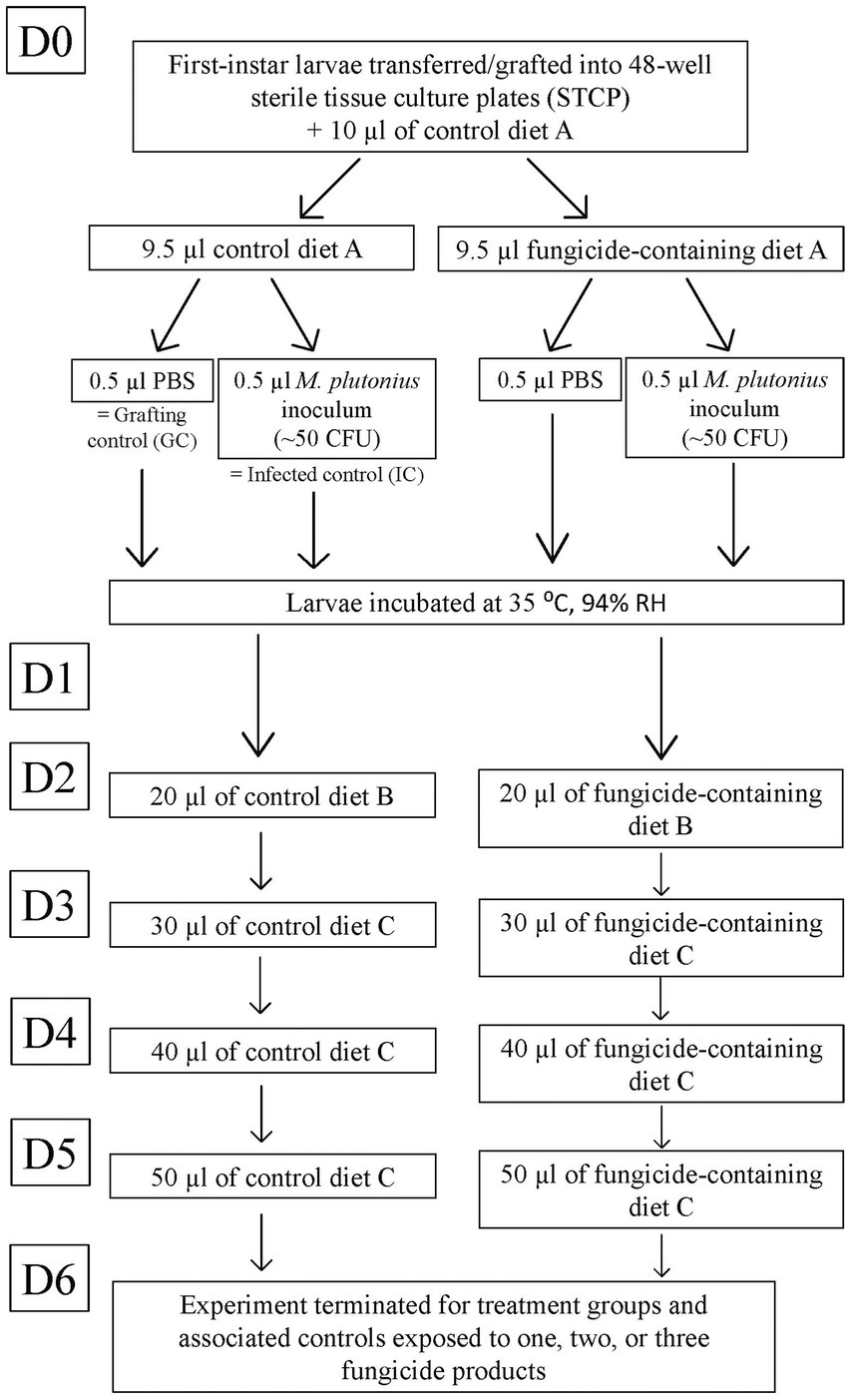
Figure 1. Diagram of experimental fungicide exposure and larval infection for larvae reared until day 6 (D6). Larvae exposed to one, two, or three fungicide products were reared for 6 days in vitro. The flow chart outlines timeline of infection and chronic fungicidal exposure. Larvae were monitored daily for survival.
Each STCP was divided into four groups of 12 larvae, including one negative control group (grafting control; GC) per plate to ensure adequate grafting and rearing techniques, and intermittent (once every 1–4 weeks) infection control groups (IC) to confirm successful M. plutonius infection. STCPs with <75% survival in the GC at D6 were removed from the study (Wood et al., 2020). Each fungicidal product was included in the larval diet at two to three different incremental concentrations (Table 1), with or without M. plutonius infection (Figure 1). Next, using the same experimental design, larvae were exposed to combinations of two, three, or four fungicide products (which corresponds to two to six active ingredients) alone or in combination with M. plutonius (Figure 2). For combination of two or three fungicide products, we selected the highest concentrations that did not significantly reduce survival (high concentration of Captan, Luna, and Switch, and the low concentration of Kenja); for larvae exposed to four fungicide products, we selected the low concentrations of Captan, Kenja, Luna, and Switch to approximate the exposure based on residues (Table 1). A minimum of three technical replicates (n = 36 larvae) and two to six biological replicates (different queens corresponding to different genetic lineages) were used for each treatment group (Supplementary Table S1).
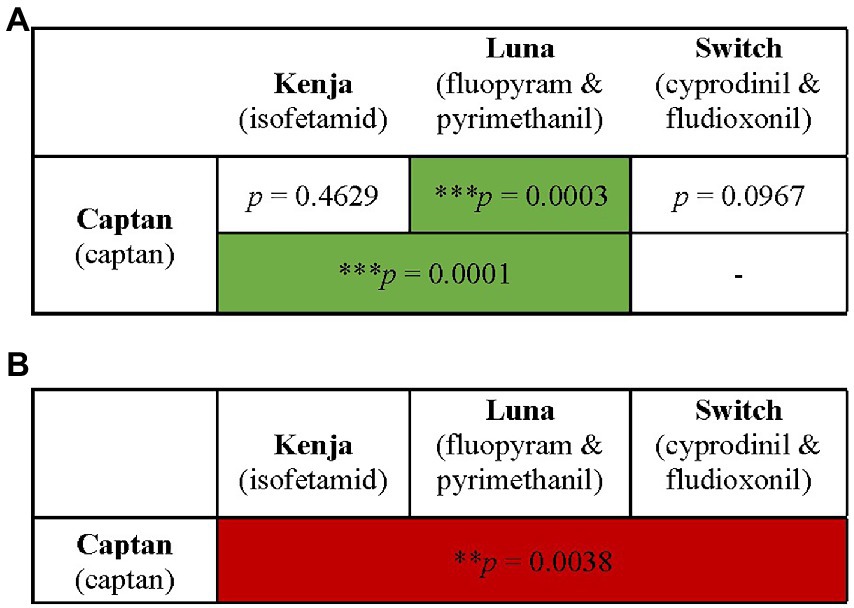
Figure 2. Summary of combination fungicide exposure groups and their effects on larval survival with M. plutonius infection. Honey bee larvae were exposed to combination of two, three (A), or four (B) fungicide products [i.e., two, three, four (A), or six (B) active ingredients]. Survival was monitored for 6 days (A) or 18 days (B). ** and *** indicate significant effects on survival with p < 0.01 and p < 0.001, respectively by a Mantel–Cox log rank test (green boxes indicate significant increases in survival and red boxes indicate significant decreases in survival). Combinations not tested are indicated by “-.”
After grafting, each larva received an additional 9.5 μl of control diet A (GC, IC), or fungicide product-containing diet A, combined with either 0.5 μl of M. plutonius inoculum [~50 CFU (mean = 61.8 CFU, SD = 24.0); Supplementary Table S1, or 0.5 μl of PBS (Figure 1)]. STCP with larvae were incubated at 35°C (mean = 34.69°C, SD = 0.24) within a desiccator containing approximately 400 ml of supersaturated potassium sulfate solution to maintain the relative humidity at approximately 94% (mean = 98.25%, SD = 5.25) (Schmehl et al., 2016). Temperature and relative humidity were recorded hourly in the desiccator using a thermometer hygrometer probe.
Larvae were fed according to the schedule described by Schmehl et al. (2016) and adapted by Wood et al. (2020). On day 1 (D1) of in vitro rearing, the larvae were not fed. From D2 to D5, larvae received 20, 30, 40, and 50 μl of either control (GC, IC) or fungicide product-containing diet B (D2) or diet C (D3-D5; Schmehl et al., 2016). Larval survival was monitored daily using a dissecting microscope. Dead larvae, characterized by darkened coloration, lack of mobility, and arrest of spiracle movement, were removed daily (Schmehl et al., 2016).
Survival data is presented until D6 for all treatment groups exposed to one, two, or three fungicide products. Experiments were limited to six days because larval survival from D0 to D6 was shown to be reflective of larval survival until adulthood (18 days; Supplementary Figure S2). Only the treatment groups exposed to four fungicide products, and corresponding controls, were reared to adulthood, as outlined below.
On D6 of in vitro rearing, to prepare for pupation, honey bee larvae that consumed all larval diet were individually transferred to a new ‘pupal’ STCP containing a 1 cm in diameter circular Kimwipe™ tissue in each well and incubated at 35°C (mean = 34.50°C, SD = 0.31) within a desiccator containing approximately 400 ml of supersaturated sodium chloride to maintain the relative humidity at 75% (mean = 75.55%, SD = 6.16) (Schmehl et al., 2016). Temperature and relative humidity were recorded hourly. Larvae that did not consume all diet by D6 were kept in the larval desiccator at 94% humidity until death or until all diet was consumed, at which point surviving larvae were transferred to a pupal STCP. Pupal survival was monitored daily by visual inspection until honey bees emerged as adults at 15–18 days after grafting. Dead pupae, characterized by deflation or brown discoloration, were removed daily.
2.5. Statistical analysis
Stata 16 (StataCorp LLC, College Station, TX, United States) was used for analyses. Data are reported as the median and interquartile range. A Shapiro–Wilk test was used to assess normality. Pearson’s chi-squared test was used to compare percent survival among groups. Survival analysis was performed with a Mantel-Cox log-rank test. Level of significance was p < 0.05. A Bonferroni correction was used for multiple comparisons using the level of significance p < 0.016 with the Mantel–Cox log-rank test.
3. Results
Larval survival was not negatively affected by exposure to maximum field-relevant concentrations of Captan, Luna, and Switch compared to grafting control (GC) larvae (Figure 3A).
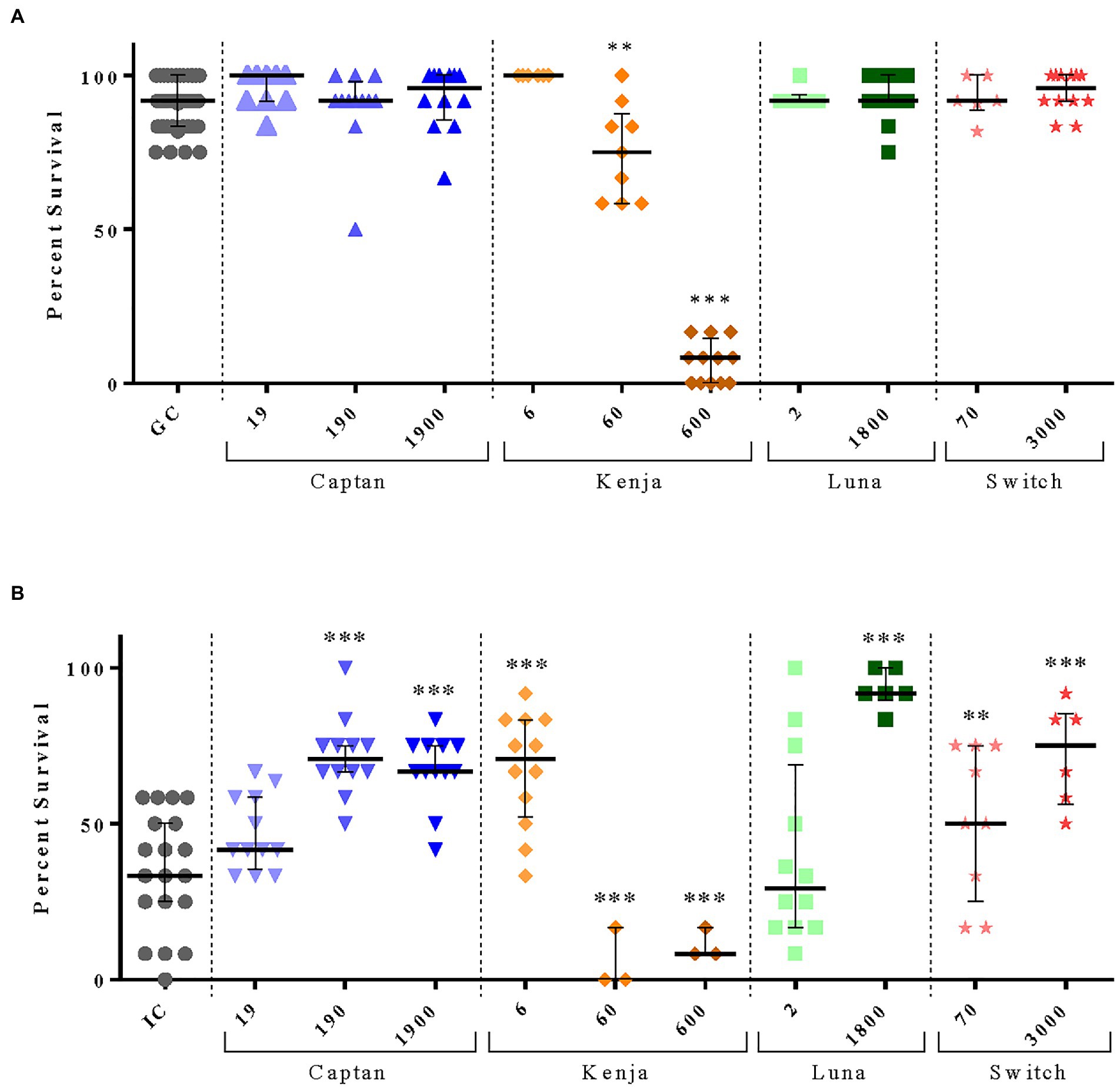
Figure 3. Effect of chronic fungicidal exposure on larval survival from European foulbrood disease in vitro. Honey bee larvae were reared in vitro for 6 days and chronically exposed to low, medium, and high concentrations of four different formulated fungicidal products. (A) Percent survival of 60–144 larvae chronically administered fungicidal product and compared to 501 grafting control (GC) larvae. (B) 36–144 larvae infected with 50 CFU of M. plutonius bacteria, chronically exposed to fungicides, and compared to 228 infected control (IC) larvae. Each dot represents one replicate (n = 12 larvae). Numbered categories on the x-axis represent the concentrations of the active fungicidal ingredient in ng/bee. Horizontal and vertical lines overlying the dots represent the median and interquartile range, respectively. **p < 0.01, ***p < 0.001, by a Pearson’s chi-squared test.
Surprisingly, larvae infected with M. plutonius and exposed to the medium and high concentrations of Captan, the high concentration of Luna, and the low and high concentrations of Switch, experienced significant 16.7–58.4% increases in survival compared to infected control (IC) larvae [Figure 3B, x2(1) = 31.70, p < 0.0001; x2(1) = 17.05, p < 0.0001; x2(1) = 55.46, p < 0.0001; x2(1) = 7.564, p = 0.006; x2(1) = 19.74, p < 0.0001; Supplementary Table S1].
Larval exposure to medium and high concentrations of Kenja, without M. plutonius infection, significantly decreased larval survival compared to GC by 16.7 and 83.4%, respectively [Figure 3A, x2(1) = 12.13, p = 0.0005; x2(1) = 169.4, p < 0.0001]. Similarly, when larvae were infected with M. plutonius and exposed to the medium or high concentrations of Kenja, larval survival significantly decreased by 33 and 24.7%, respectively, compared to IC larvae [Figure 3B, x2(1) = 46.96, p < 0.0001; x2(1) = 22.17, p < 0.0001]. Larval survival was not negatively affected by exposure to the low concentration of Kenja compared to GC (Figure 3A), whereas larvae infected with M. plutonius and exposed to the low concentration of Kenja experienced a significant 50.0% increase in survival compared to IC larvae [Figure 3B, x2(1) = 32.39, p < 0.0001].
Larval exposure to combinations of two or three fungicides did not negatively affect survival relative to GC (Figure 4), whereas larvae infected with M. plutonius and exposed to combinations of either Captan and Luna, or Captan, Kenja, and Luna, had significant 25.1 and 26.3% increases in larval survival compared to IC larvae, respectively [Figure 4, x2(1) = 13.15, p = 0.0003; x2(1) = 11.62, p = 0.0007].
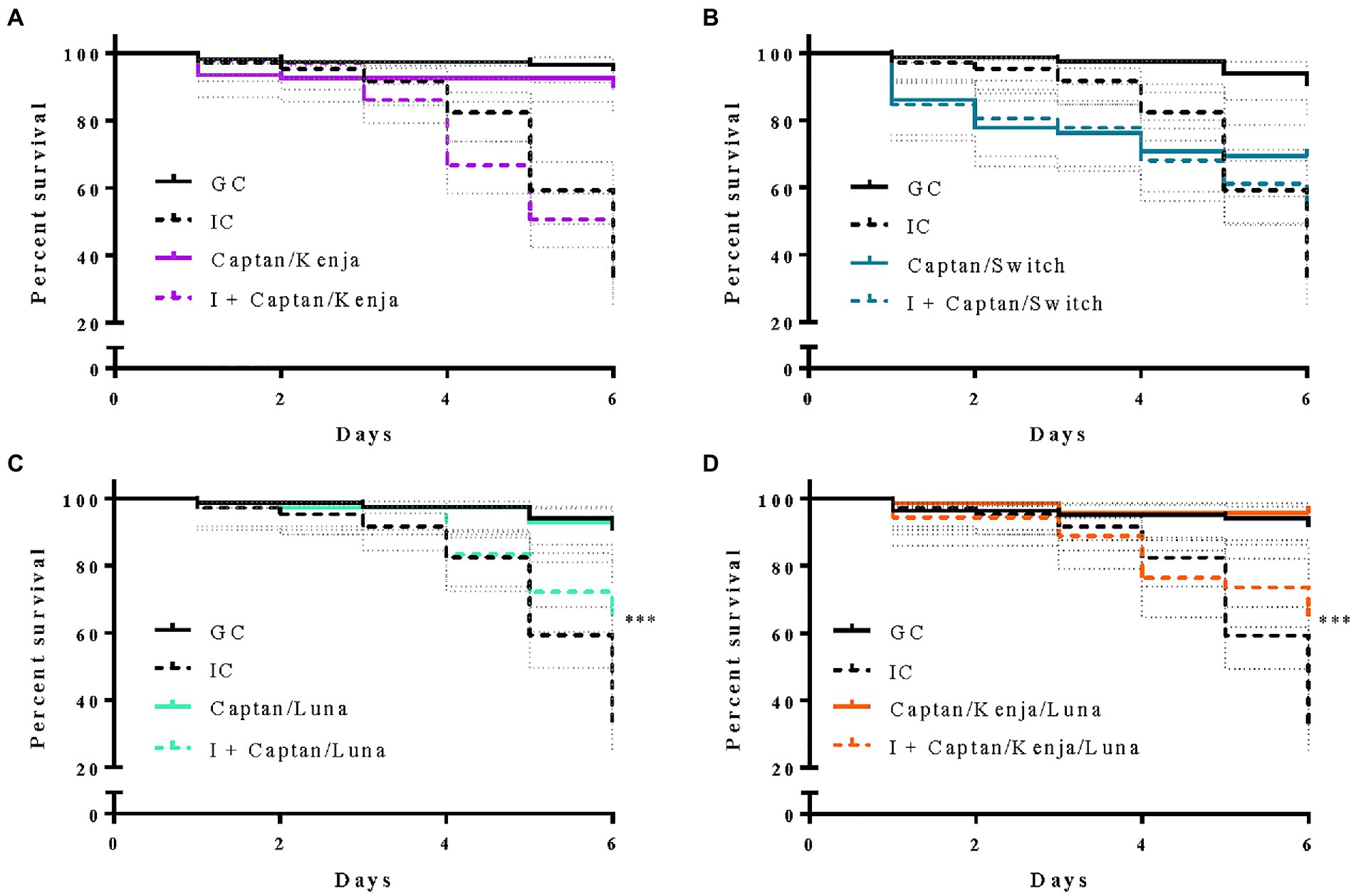
Figure 4. Effects of chronic exposure to combinations of fungicidal products on larval survival from European foulbrood disease in vitro. Solid and dashed lines represent percent survival ± confidence interval (dotted grey lines) over 6 days of in vitro rearing of 84–119 grafting control larvae (GC; solid black lines), 72–108 larvae chronically administered a combination of fungicide products (solid colored lines), 105 infected control larvae (IC; dashed black lines) inoculated with 50 colony forming units (CFU) of M. plutonius 2019 BC1, and 72–144 larvae that were infected (I) with 50 CFU of M. plutonius 2019 BC1 and subsequently administered fungicide product combinations in their diet (dashed colored lines). Fungicide-exposed larvae received (A) high concentration Captan and low concentration Kenja, (B) high concentrations of Captan and Switch, (C) high concentrations of Captan and Luna, and (D) high concentration Captan, low concentration Kenja, and high concentration Luna. ***p < 0.001, by a Mantel–Cox log rank test.
Larval survival was not significantly decreased by concurrent exposure to low concentrations of four fungicidal products (Figure 5); however, when combined with M. plutonius infection, exposure to four fungicidal products resulted in a significant 24.2% decrease in larval survival compared to IC larvae [Figure 5, x2(1) = 8.398, p = 0.0038].
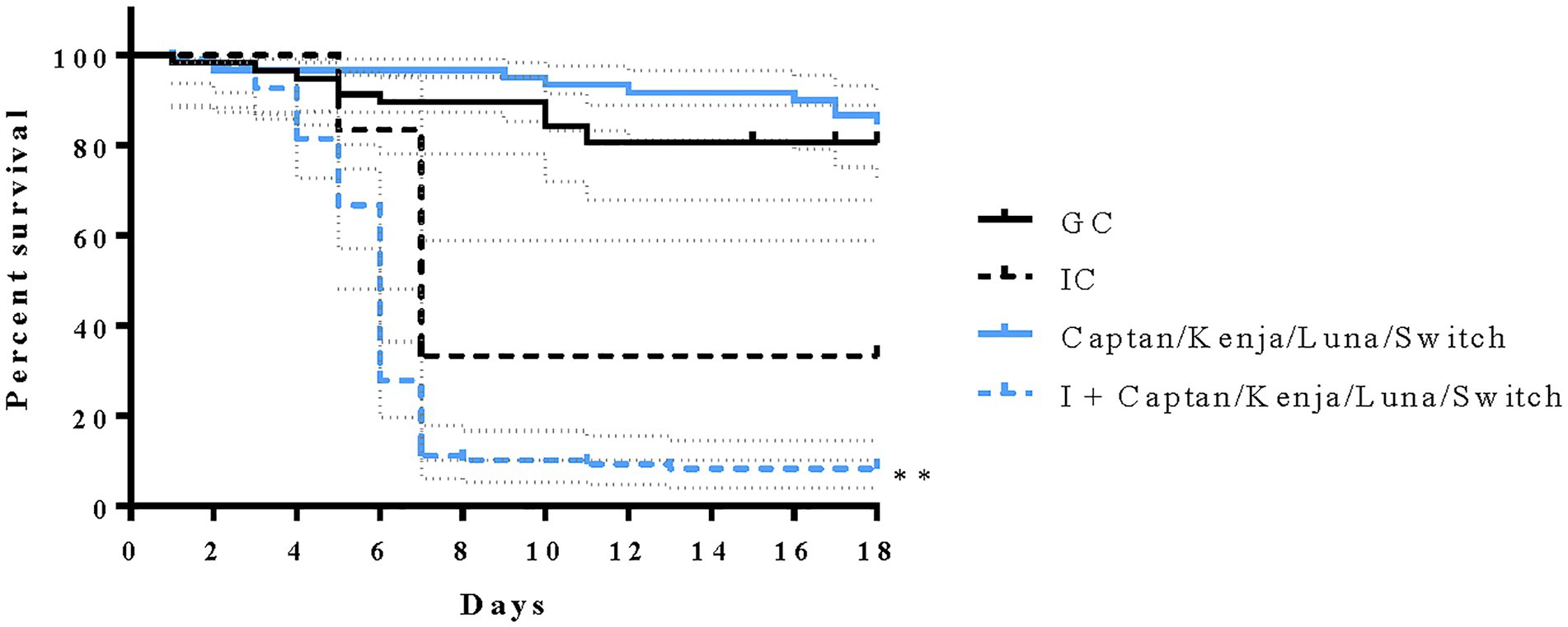
Figure 5. Effects of chronic exposure to four fungicidal products on larval survival from European foulbrood disease in vitro. Solid and dashed lines represent the percent survival ± confidence interval (dotted grey lines) of honey bees until adult emergence for 58 grafting control larvae (GC; solid black line), 60 larvae chronically exposed to four fungicides simultaneously (solid blue line), 12 infected control larvae (IC; dashed black line) inoculated with 50 colony forming units (CFU) of M. plutonius 2019 BC1, and 108 larvae that were infected (I) with 50 CFU of M. plutonius and subsequently administered fungicidal product in the diet (dashed blue line). Fungicide-exposed larvae received low concentrations of Captan, Kenja, Luna, and Switch. **p < 0.01 by a Mantel–Cox log rank test.
4. Discussion
In this investigation, we tested the effects of four formulated fungicide products used in highbush blueberry production on honey bee larval survival from EFB using an in vitro larval infection model (Schmehl et al., 2016; Wood et al., 2020). Previous investigators have found that at least four fungicide residues were detectable in pollen from blueberry pollinating honey bee colonies (Graham et al., 2021; Guarna, 2021; Rondeau and Raine, 2022), supporting the relevancy of our study.
Chronic oral larval exposure to the fungicide products Supra® Captan 80WDG (Captan), Luna® Tranquility (Luna), and Switch® 62.5 WG (Switch), when applied individually, did not negatively impact larval survival compared to grafting controls (GCs), nor did they have any significant negative effect on larval survival from EFB. The lack of significant negative effects following fungicide product exposure with and without M. plutonius infection are not surprising, as other researchers also have not found significant negative effects on honey bee larval survival from oral (or contact) exposure to field-relevant concentrations of the active fungicide ingredients in Captan, Luna, and Switch (Everich et al., 2009; Wood et al., 2020). While our highest tested concentrations of the active ingredients in Captan, Luna, and Switch reflected the highest reported residues of these active ingredients in pollen/bee bread and honey (Graham et al., 2021, 2022; Rondeau and Raine, 2022), the transfer rate of residues from pollen into royal jelly and worker jelly is believed to be low (Bohme et al., 2018, 2019; Milone et al., 2021), and thus, our concentrations are likely an over estimation of exposure. Although most fungicides detected in honey have systemic properties, fludioxonil is a non-systemic fungicide and its residues have never been reported in honey (Rondeau and Raine, 2022), therefore we used residue concentrations for pollen/bee bread in place of honey. As residue concentrations in pollen/bee bread are many folds higher than concentrations found in nectar/honey (Graham et al., 2021, 2022; Rondeau and Raine, 2022), this again illustrates our tested concentrations as a potential over exposure. On the other hand, water is also an important constituent in worker larval diet (McCune et al., 2021). As fungicides have been shown to accumulate in naturally occurring water sources (Zubrod et al., 2019), this may further contribute to increased fungicide exposure to honey bee larvae.
One limitation of this study is that we did not confirm fungicide exposure by measuring the concentration of active fungicide ingredients in the experimental diet. Accordingly, we cannot exclude possible fungicide degradation during diet preparation and freezing until time of use, or errors in manipulation that may have led to incorrect concentrations. Additionally, we only monitored larval survival until day 6 for treatment groups of larvae exposed to one, two, or three fungicidal products, thus limiting our ability to observe differences in pupal survival among treatment groups.
In the absence of M. plutonius infection, larval exposure to medium and high concentrations of Kenja™ 400SC (Kenja) were associated with a significant decline in larval survival compared to control larvae. Likewise, relative to infected controls (ICs), there was a significant decrease in survival when larvae were infected with M. plutonius and exposed to medium and high concentrations of Kenja. Isofetamid, the active ingredient in Kenja, is a newly developed fungicide and was only registered in Canada, United States, and Japan recently in 2014, 2015, and 2017, respectively (Umetsu and Shirai, 2020), and field-realistic residue concentrations of isofetamid in honey bee hive matrices have not been reported to date (Rondeau and Raine, 2022). Furthermore, Bellisai et al. (2021) found elevated residues of 3.65 mg/kg isofetamid in other fruit crops (whole raspberry plant) following foliar application of the product, emphasizing the need to further quantify field-realistic honey bee exposure to this fungicide during blueberry pollination.
Combined larval exposure to two or three fungicidal products did not significantly decrease larval survival from EFB compared to infected controls. Similar to our study, Prado et al. (2019) found that oral exposure to pyrimethanil had no negative effect on larval survival when combined with other fungicides. The exposure to low, non-toxic concentrations of fungicides (Lewis et al., 2016) that may not negatively impact immune function (O’Neal et al., 2018), are possible explanations for not observing negative effects on larval survival. Importantly, only selected combinations of fungicidal products commonly used during bloom in highbush blueberry production were tested in this study; we cannot exclude that other combinations of fungicidal products may impact larval survival from EFB.
However, when infected larvae were chronically exposed to a combination of four fungicide products with four different modes of action, there was a significant decrease in larval survival relative to infected controls. Given that multiple studies have reported the presence of ≥4 fungicidal residues in pollen samples (Rondeau and Raine, 2022), including those collected from blueberry-pollinating honey bee colonies (Graham et al., 2021; Guarna, 2021), these results provide a rationale for concern as we tested concentrations that were based on reported field-level concentrations or application rates. Other researchers have similarly found synergistic negative effects on larval survival after co-exposure to fungicides and insecticides (Wade et al., 2019; Wood et al., 2020), but to our knowledge, this is the first report of significant negative effects on larval survival following exposure to a combinations of fungicide products without insecticides. Decreased survival from pathogen infection in response to fungicide exposure may be explained by decreased immune function (O’Neal et al., 2018). Furthermore, proprietary ingredients present in these fungicide products may also contribute to the increased larval EFB mortality observed after exposure to multiple fungicides, as pesticide adjuvants have been previously implicated in enhancement of pesticide toxicity to honey bees (Mullin et al., 2015; Walker et al., 2022).
Surprisingly, larvae infected with M. plutonius and exposed the low concentration of Kenja or Switch, the medium concentration of Captan, and the high concentration of Captan, Luna, or Switch, had a significant increase in larval survival from EFB compared to infected control larvae. Likewise, an increase in survival was observed when larvae were infected with M. plutonius and exposed to combinations of high concentrations of Captan and Luna, and high concentrations of Captan, Luna, and the low concentration of Kenja. This observation may be explained by direct bacterial inhibition of the fungicide products on M. plutonius. While the antimicrobial properties of royal jelly have been previously reported to decrease M. plutonius viability in the diet (Takamatsu et al., 2017; Vezeteu et al., 2017; Floyd et al., 2020; Masood et al., 2022), the potential bactericidal effects of these fungicidal products on M. plutonius is unknown, and an area that warrants further investigation.
Our results demonstrate that chronic exposure of fungicide products used in highbush blueberry production only negatively impacts honey bee larval susceptibility to EFB in vitro when larvae are exposed to the four fungicidal products Captan, Kenja, Luna, and Switch combined, or when larvae are exposed to medium and high concentrations of Kenja. Accordingly, fungicidal exposure may be a driving force for the reported increase in incidence of EFB during blueberry pollination; however, comprehensive fungicide residue analysis is warranted, as well as the continued investigation of other host, pathogen, or environmental factors influencing the disease ecology of EFB.
5. Author’s note
Honey bee pollination contributes significantly to blueberry production in Canada and the United States each year; however, outbreaks of European foulbrood disease is an evolving problem that threatens the supply of honey bee pollination services to the blueberry industry. Investigating the risk factors which contribute to EFB disease during blueberry pollination is an important step in safeguarding honey bee colony health and maintaining profitability of both beekeepers and blueberry growers. Our in vitro study suggests that fungicide products commonly used in highbush blueberry production may predispose honey bee larvae to disease, as exposure to medium and high concentrations of Kenja, and exposure to four fungicide products concurrently increased larval susceptibility to EFB. While further evaluation of field-relevant fungicide exposure for colonies pollinating blueberries is required, our study facilitates the understanding of pesticide risk to honey bees pollinating crops and contributes to the ongoing efforts to enhance sustainability of blueberry pollination in North America.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
SW, ES, JT, GW, MG, EG, and AR contributed to the conception and design of the study. JT, AC, and DL acquired the data. JT, AC, DL, FM, IK, CK, MZ, and SB contributed to animal care. IM assisted in bacterial protocols. LS assisted with fungicide preparation. MG contributed to sections of the manuscript. JT analyzed the data, drafted and revised the work. All authors contributed to the manuscript revision, read, and approved the submitted version.
Funding
This research was funded by Project Apis m., Costco Canada, Mitacs, British Columbia Blueberry Council, the Saskatchewan Beekeepers Development Commission, Agriculture Development Fund, Results Driven Agriculture Research, Canadian Bee Research Fund, the Western College of Veterinary Medicine Interprovincial Undergraduate Student Summer Research Program, and the Interprovincial Graduate Student Fellowship.
Acknowledgments
The authors would like to thank summer students Melanie Roulin, Jessica E. DeBruyne, Brandele Brown, Mateo Castano Ospina, and Lara Reitsma, and postdoctoral fellows Igor Medici de Mattos and Mohsen Sharafi.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1073775/full#supplementary-material
SUPPLEMENTARY FIGURE 1 | HSurvival of larval honey bees reared in vitro for until eclosion (6 days and fed diet containing the fungicidal products Captan or Kenja at concentrations 19,000 and 6000 ng/bee, respectively.) following fungicide exposure. Percent survival ± confidence interval of (A) 108 honey bee larvae exposed to 19,000 ng/bee of Captan and (B) 144 honey bee larvae exposed to 6000 ng/bee Kenja until eclosion compared to 48-72 grafting control larvae (GC). The tested concentrations of Captan and Kenja represent the larval exposure based on the application rate used during blueberry pollination to prevent fungal diseases such as anthracnose and botrytis fruit rot (Everich et al., 2009; Province of British Columbia, 2022; Mussen et al., 2004). ****p < 0.0001, by a Mantel-Cox log rank test.
SUPPLEMENTARY FIGURE 2 | Effects of chronic exposure to fungicidal products on larval survival from European foulbrood disease (EFB) in vitro until eclosion or emergence. Percent survival ± confidence interval of honey bee larvae infected with M. plutonius (dashed lines) and exposed to low concentrations of Luna and Switch (colored lines). Infected (I), fungicide-exposed larvae were compared to infected control larvae (IC). Survival analysis of data from day 0-18 Larvae monitored until emergence (A, C, E) yielded had the same statistical relationship between infected experimental groups compared to survival analysis of data from day 0-as when the same data was truncated to 6 days (B, D, F). *p < 0.05, ***p < 0.001, by a Mantel-Cox log rank test.
References
Arai, R., Tominaga, K., Wu, M., Okura, M., Ito, K., Okamura, N., et al. (2012). Diversity of Melissococcus plutonius from honeybee larvae in Japan and experimental reproduction of European foulbrood with cultured atypical isolates. PLoS ONE 7:e33708. doi: 10.1371/journal.pone.0033708
Bartling, M. T., Thümecke, S., Russert, J. H., Vilcinskas, A., and Lee, K.-Z. (2021). Exposure to low doses of pesticides induces an immune response and the production of nitric oxide in honeybees. Sci. Rep. 11:6819. doi: 10.1038/s41598-021-86293-0
Bellisai, G., Bernasconi, G., Brancato, A., Carrasco Cabrera, L., Ferreira, L., Giner, G., et al. (2021). Modification of the existing maximum residue levels for isofetamid in raspberries, blackberries and dewberries. EFSA J. 19:6677. doi: 10.2903/j.efsa.2021.6677
Bersching, K., and Jacob, S. (2021). The molecular mechanism of fludioxonil action is different to osmotic stress sensing. J. Fungi 7:393. doi: 10.3390/jof7050393
Bohme, F., Bischoff, G., Zebitz, C. P. W., Rosenkranz, P., and Wallner, K. (2018). From field to food – will pesticide-contaminated pollen diet lead to a contamination of royal jelly? Apidologie 49, 112–119. doi: 10.1007/s13592-017-0533-3
Bohme, F., Bischoff, G., Zebitz, C. P. W., Rosenkranz, P., and Wallner, K. (2019). From field to food II – will pesticide-contaminated pollen diet lead to a contamination of worker jelly? J. Apic. Res. 58, 542–549. doi: 10.1080/00218839.2019.1614727
Cheshire, F. R., and Cheyne, W. W. (1885). The pathogenic history and the history under cultivation of a new Bacillus (B. alvei), the cause of a disease of the hive bee hitherto known as foul brood. J. R. Microsc. Soc. 5, 581–601. doi: 10.1111/j.1365-2818.1885.tb05794.x
Djukic, M., Erler, S., Leimbach, A., Grossar, D., Charriere, J.-D., Gauthier, L., et al. (2018). Comparative genomics and description of putative virulence factors of Melissococcus plutonius, the causative agent of European foulbrood disease in honey bees. Genes 9:419. doi: 10.3390/genes9080419
Everich, R., Schiller, C., Whitehead, J., Beavers, M., and Barrett, K. (2009). Effects of captan on Apis mellifera brood development under field conditions in California almond orchards. J. Econ. Entomol. 102, 20–29. doi: 10.1603/029.102.0104
Floyd, A. S., Mott, B. M., Maes, P., Copeland, D. C., McFrederick, Q. S., and Anderson, K. E. (2020). Microbial ecology of European foul brood disease in the honey bee (Apis mellifera): towards a microbiome understanding of disease susceptibility. Insects 11:555. doi: 10.3390/insects11090555
Forsgren, E. (2010). European foulbrood in honey bees. J. Invertebr. Pathol. 103, S5–S9. doi: 10.1016/j.jip.2009.06.016
Fritz, R., Lanen, C., Chapeland-Leclerc, F., and Leroux, P. (2003). Effect of the anilinopyrimidine fungicide pyrimethanil on the cystathionine β-lyase of Botrytis cinerea. Pestic. Biochem. Physiol. 77, 54–65. doi: 10.1016/S0048-3575(03)00094-4
Government of Canada. (2018). Statistical Overview of the Canadian Honey and Bee Industry and the Economic Contribution of Honey Bee Pollination. Available at: https://agriculture.canada.ca/en/agriculture-and-agri-food-canada/canadas-agriculture-sectors/horticulture/horticulture-sector-reports/statistical-overview-canadian-honey-and-bee-industry-and-economic-contribution-honey-bee-pollination#a5 (Accessed October 4, 2022).
Graham, K. K., Milbrath, M. O., Zhang, Y., Baert, N., McArt, S., and Isaacs, R. (2022). Pesticide risk to managed bees during blueberry pollination is primarily driven by off-farm exposures. Sci. Rep. 12:7189. doi: 10.1038/s41598-022-11156-1
Graham, K. K., Milbrath, M. O., Zhang, Y., Soehnlen, A., Baert, N., McArt, S., et al. (2021). Identities, concentrations, and sources of pesticide exposure in pollen collected by managed bees during blueberry pollination. Sci. Rep. 11:16857. doi: 10.1038/s41598-021-96249-z
Grossar, D., Kilchenmann, V., Forsgren, E., Charrière, J.-D., Gauthier, L., Chapuisat, M., et al. (2020). Putative determinants of virulence in Melissococcus plutonius, the bacterial agent causing European foulbrood in honey bees. Virulence 11, 554–567. doi: 10.1080/21505594.2020.1768338
Guarna, M. M., Higo, H., Foster, L., Pernal, S. F., and Wolf, V. P. (2019). Bee health and blueberry pollination. HiveLights 32:14.
Johnson, R. M., Dahlgren, L., Siegfried, B. D., and Ellis, M. D. (2013). Acaricide, fungicide and drug interactions in honey bees (Apis mellifera). PLoS One 8:e54092. doi: 10.1371/journal.pone.0054092
Johnson, R. M., Ellis, M. D., Mullin, C. A., and Frazier, M. (2010). Pesticides and honey bee toxicity – USA. Apidologie 41, 312–331. doi: 10.1051/apido/2010018
Kane, T. R., and Faux, C. M. (2021). Honey Bee Medicine for the Veterinary Practitioner. doi: 10.1002/9781119583417
Laate, A. E., Emunu, J. P., Duering, A., and Ovinge, L. (2020). Potential Economic Impact of European and American Foulbrood on Alberta’s Beekeeping Industry. Available at: https://open.alberta.ca/dataset/029a345b-8621-4986-ad78-7fc6ddcd8b17/resource/25f7b78d-a359-428c-9648-9175c3634720/download/af-potential-economic-impact-european-american-foulbrood-on-albertas-beekeeping-industry.pdf (Accessed May 11, 2021).
Lewis, K. A., Tzilivakis, J., Warner, D. J., and Green, A. (2016). An international database for pesticide risk assessments and management. HERA 22, 1050–1064. doi: 10.1080/10807039.2015.1133242
Masood, F., Thebeau, J. M., Cloet, A., Kozii, I. V., Zabrodski, M. W., Biganski, S., et al. (2022). Evaluating approved and alternative treatments against an oxytetracycline-resistant bacterium responsible for European foulbrood disease in honey bees. Sci. Rep. 12:5906. doi: 10.1038/s41598-022-09796-4
McCune, F., Samson-Robert, O., Rondeau, S., Chagnon, M., and Fournier, V. (2021). Supplying honey bees with waterers: a precautionary measure to reduce exposure to pesticides. ESPR 28, 17573–17586. doi: 10.1007/s11356020-12147-3
Milone, J. P., Chakrabarti, P., Sagili, R. R., and Tarpy, D. R. (2021). Colony-level pesticide exposure affects honey bee (Apis mellifera L.) royal jelly production and nutritional composition. Chemosphere 263:128183. doi: 10.1016/j.chemosphere.2020.128183
Mullin, C. A., Chen, J., Fine, J. D., Frazier, M. T., and Frazier, J. L. (2015). The formulation makes the honey bee poison. Pestic. Biochem. Phys. 120, 27–35. doi: 10.1016/j.pestbp.2014.12.026
Mullin, C. A., Frazier, M., Frazier, J. L., Ashcraft, S., Simonds, R., vanEngelsdorp, D., et al. (2010). High levels of miticides and agrochemicals in north American apiaries: implications for honey bee health. PLoS One 5:e9754. doi: 10.1371/journal.pone.0009754
Mussen, E. C., Lopez, J. E., and Peng, C. Y. S. (2004). Effects of selected fungicides on growth and development of larval honey bees, Apis mellifera L. (Hymenoptera: Apidae). Environ. Entomol. 33, 1151–1154. doi: 10.1603/0046-225X-33.5.1151
O’Neal, S. T., Anderson, T. D., and Wu-Smart, J. Y. (2018). Interactions between pesticides and pathogen susceptibility in honey bees. Curr. Opin. Insect. Sci. 26, 57–62. doi: 10.1016/j.cois.2018.01.006
Olmstead, S., McCallum, R., and Shaw, J. (2019). Evaluating the Effect of Feeding Pollen Substitute to Honey Bee Colonies Destined for Wild Blueberry Pollination in Colchester County, Nova Scotia. Available at: https://www.perennia.ca/wp-content/uploads/2019/10/ATTTA-FactSheet-Oct-2019.pdf (Accessed May 24, 2021).
Prado, A., Pioz, M., Vidau, C., Requier, F., Jury, M., Crauser, D., et al. (2019). Exposure to pollen-bound pesticide mixtures induces longer-lived but less efficient honey bees. Sci. Total Environ. 650, 1250–1260. doi: 10.1016/j.scitotenv.2018.09.102
Province of British Columbia. (2022). Blueberries. Available at: https://www2.gov.bc.ca/gov/content/industry/agriservice-bc/production-guides/berries/blueberries (Accessed May 11, 2021).
Rondeau, S., and Raine, N. E. (2022). Fungicides and bees: a review of exposure and risk. Environ. Int. 165:107311. doi: 10.1016/j.envint.2022.107311
Schmehl, D. R., Tomé, H. V. V., Mortensen, A. N., Martins, G. F., and Ellis, J. D. (2016). Protocol for the in vitro rearing of honey bee (Apis mellifera L.) workers. J. Api. Res. 55, 113–129. doi: 10.1080/00218839.2016.1203530
Takamatsu, D., Osawa, A., Nakamura, K., Yoshiyama, M., and Okura, M. (2017). High-level resistance of Melissococcus plutonius clonal complex 3 strains to antimicrobial activity of royal jelly: royal jelly resistance of M. plutonius. Environ. Microbiol. Rep. 9, 562–570. doi: 10.1111/1758-2229.12590
Thebeau, J. M., Liebe, D., Masood, F., Kozii, I. V., Klein, C. D., Zabrodski, M. W., et al. (2022). Investigation of Melissococcus plutonius isolates from 3 outbreaks of European foulbrood disease in commercial beekeeping operations in western Canada. Can. Vet. J. 63, 935–942.
Umetsu, N., and Shirai, Y. (2020). Development of novel pesticides in the 21st century. Pestic. Sci. 45, 54–74. doi: 10.1584/jpestics.D20-201
United States Environmental Protection Agency. (2015). BeeREX, version 1.0. S EPA, O. Terrestrial Models: BeeREX. Environmental Protection Agency, Washington, DC. Available at: https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/models-pesticide-risk-assessment#terrestrial (Accessed October 4, 2022).
Vezeteu, T. V., Bobiş, O., Moritz, R. F. A., and Buttstedt, A. (2017). Food to some, poison to others — honeybee royal jelly and its growth inhibiting effect on European foulbrood bacteria. MicrobiologyOpen 6:e00397. doi: 10.1002/mbo3.397
Wade, A., Lin, C.-H., Kurkul, C., Regan, E. R., and Johnson, R. M. (2019). Combined toxicity of insecticides and fungicides applied to California almond orchards to honey bee larvae and adults. Insects 10:20. doi: 10.3390/insects10010020
Walker, E. K., Brock, G. N., Arvidson, R. S., and Johnson, R. M. (2022). Acute toxicity of fungicide-insecticide-adjuvant combinations applied to almonds during bloom on adult honey bees. Environ. Toxicol. Chem. 41, 1042–1053. doi: 10.1002/etc.5297
Wardell, G. (1982). European foulbrood: Association with Michigan blueberry pollination, and control. Ph.D dissertation. Michigan: Michigan State University.
Wood, S. C., Chalifour, J. C., Kozii, I. V., Medici de Mattos, I., Klein, C. D., Zabrodski, M. W., et al. (2020). In vitro effects of pesticides on European foulbrood in honeybee larvae. Insects 11:252. doi: 10.3390/insects11040252
Keywords: pesticides, fungicides, European foulbrood, honey bees (Apis mellifera), blueberries
Citation: Thebeau JM, Cloet A, Liebe D, Masood F, Kozii IV, Klein CD, Zabrodski MW, Biganski S, Moshynskyy I, Sobchishin L, Wilson G, Guarna MM, Gerbrandt EM, Ruzzini A, Simko E and Wood SC (2023) Are fungicides a driver of European foulbrood disease in honey bee colonies pollinating blueberries? Front. Ecol. Evol. 11:1073775. doi: 10.3389/fevo.2023.1073775
Edited by:
Pierre Lau, United States Department of Agriculture, United StatesReviewed by:
Adrian Fisher II, Arizona State University, United StatesSabrina Rondeau, University of Guelph, Canada
Copyright © 2023 Thebeau, Cloet, Liebe, Masood, Kozii, Klein, Zabrodski, Biganski, Moshynskyy, Sobchishin, Wilson, Guarna, Gerbrandt, Ruzzini, Simko and Wood. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jenna M. Thebeau, ✉ jenna.thebeau@usask.ca; Sarah C. Wood, ✉ sarah.wood@usask.ca
 Jenna M. Thebeau
Jenna M. Thebeau Allyssa Cloet1
Allyssa Cloet1  Elemir Simko
Elemir Simko Sarah C. Wood
Sarah C. Wood