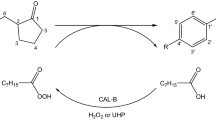
Abstract
The lipase catalyzed kinetic resolution of three trans-1-(2-hydroxycyclohexyl)-indoles in both batch and continuousflow systems is reported. Ring opening of cyclohexene oxide by the corresponding indole followed by enzymatic acylation with vinyl acetate resulted in novel, highly enantioenriched indole-substituted cyclohexanols and cyclohexyl acetates. The effect of the temperature on enantiomeric ratio (E) and productivity (specific reaction rate, rflow) in the continuous-flow mode acylation was studied at analytical scale in the 0–70 °C range. Preparative scale kinetic resolution of the three indole derivatives was performed in mixed continuous- and recirculation-flow mode resulting in almost complete conversion and good to excellent enantiomeric purity of the products.
Similar content being viewed by others
References
Barden, T. C. Indoles: Industrial, Agricultural and Over-the-Counter Uses. In Heterocyclic Scaffolds II (Topics in Heterocyclic Chemistry, Vol 26); Gribble, G. W., Ed.; Springer-Verlag: Berlin Heidelberg, 2010, pp. 31–46.
Kaushik, N. K.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, C. H.; Verma, A. K.; Choi, E. H. Chem. Commun. 2011, 47, 3625–3627.
Berger, M; Gray, J. A.; Roth, B. L. Annu. Rev. Med. 2009, 60, 355–366.
Phillipson, O. T. Neurobiol. Aging 2014, 35, 847–857.
Borroto-Escuela, D. O.; Romero-Fernandez, W.; Narvaez, M.; Oflijan, J.; Agnati, L. F.; Fuxe, K. Biochem. Biophys. Res. Commun. 2014, 443, 278–284.
Chilton, W. S.; Bigwood, J.; Gensen, R. E. J. Psychedelic Drugs 1979, 11, 61–69.
Jaishree, B.; Manjulatha, K.; Girish, M.; Adil, S.; Purohit, M. G. ARKI-VOC 2009, 12, 217–231.
Murphy, J. A.; Scott, K. A.; Sinclair, R. S.; Lewis, N. Tetrahedron Lett. 1997, 38, 7295–7298.
Bonollo, S.; Lanari, D.; Vaccaro, L. Eur.J. Org. Chem. 2011, 14, 2587–2598.
Smith, J. G. Synthesis 1984, 8, 629–656.
Cooper, G.; Irwin, W. J. J. Chem. Soc., Perkin Trans. 1 1976, 5, 545–549.
Kotsuki, H.; Hayashida, K.; Shimanouchi, T.; Nishizawa, H. J. Org. Chem. 1996, 61, 984–990.
Glas, H.; Thiel, W. R. Tetrahedron Lett. 1998, 39, 5509–5510.
Desai, H.; D’Souza, B. R.; Foether, D.; Johnson, B. F.; Lindsay, H. A. Synthesis 2007, 6, 902–910.
Porcar, R.; Sans, V.; Ríos-Lombardía, N.; Gotor-Fernández, V.; Gotor, V.; Burguete, M. I.; García-Verdugo, E.; Luis, S. V. ACS Catal. 2012, 2, 1976–1983.
Poppe, L.; Novák, L. Selective Biocatalysis: A Synthetic Approach; Wiley-VCH: Weinheim, 1992.
Industrial Biotransformations, 2nd ed.; Liese, A.; Seelbach, K.; Wandrey, C., Eds.; Wiley-VCH: Weinheim, 2006.
Faber, K. Biotransformations in Organic Chemistry, 6th ed.; Springer: Berlin-Heilderberg, 2011.
Boros, Z.; Hornyánszky, G.; Nagy, J.; Poppe, L.: Stereoselective Hydrolase-Catalyzed Processes in Continuous-Flow Mode. In Cascade Biocatalysis: Stereoselective and Environmentally Friendly Reactions; Riva, S.; Fessner, W., Eds.; Wiley-VCH: Weinheim, 2014 (in press).
Bornscheuer, U. T.; Kazlauskas, R. J. Hydrolases in Organic Synthesis: Regio-and Stereoselective Biotransformations; Wiley-VCH: Weinheim, New York, 2006.
Schmid, A.; Dordick, J. S.; Hauer, B.; Kiener, A.; Wubbolts, M.; Witholt, B. Nature 2001, 409, 258–268.
Practical Methods for Biocatalysis and Biotransformations; Whittal, J.; Sutton, P., Eds.; John Wiley & Sons, Ltd.: Chichester, 2010.
Green Chemistry in the Pharmaceutical Industry; Dunn, P. J.; Wells, A. S.; Williams M. T., Eds.; Wiley-VCH: Weinheim, 2010.
Busto, E.; Gotor-Fernández, V.; Gotor, V. Chem. Soc. Rev. 2010, 39, 4504–4523.
Humble, M. S.; Berglund, P. Eur. J. Org. Chem. 2011, 3391–3401.
Zhou, Z.; Hartmann, M. Top. Catal. 2012, 55, 1081–1100.
Ghanem, A.; Aboul-Enein, H. Y. Chirality 2005, 17, 1–15.
Ghisalba, O.; Meyer, H. P.; Wohlgemuth, R. Industrial Biotransformations. In Encyclopedia of Industrial Biotechnology: Bioprocess, Bioseparation, and Cell Technology; Flickinger, M. C., Ed.; John Wiley & Sons: Hoboken, 2010.
Hoyos, P.; Pace. V.; Alcántara, A. R. Adv. Synth. Catal. 2012, 354, 2585–2611.
Turner, N. J.; Curr. Opin. Chem. Biol. 2004, 8, 114–119.
Clouthier, M.; Pelletier, J. N. Chem. Soc. Rev. 2012, 41, 1585–1605.
van Rantwijk, F.; Sheldon, R. A.; Tetrahedron, 2004, 60, 501–519.
Garcia-Urdiales, E.; Alfonso, I.; Gotor, V. Chem. Rev. 2005, 105, 313–354.
Sun, J. H.; Dai, R. J.; Meng, W. W.; Deng, Y. L. Catal. Commun. 2010, 11, 987–991.
Poulhès, F.; Vanthuyne, N.; Bertrand, M. P.; Gastaldi, S.; Gil, G. J. Org. Chem. 2011, 76, 7281–7286.
Brem, J.; Bencze, L. C.; Liljeblad, A.; Turcu, M. C.; Paizs, C.; Irimie, F. D.; Kanerva, L. T. Eur. J. Org. Chem. 2012, 17, 3288–3294.
De Miranda, A. S.; Gomes, J. C.; Rodrigues Jr., M. T.; Costa, I. C. R.; Almeida, W. P.; de O. Lopes, R.; Miranda, L. S. M.; Coelho, F.; de Souza, R. O. M. A. J. Mol. Catal. B: Enzym. 2013, 91, 77–80.
Boros, Z.; Falus, P.; Márkus, M.; Weiser, D.; Oláh, M.; Hornyánszky, G.; Nagy, J.; Poppe, L. J. Mol. Catal. B: Enzym. 2013, 85–86, 119–125.
Rouhi A. M. Chem. Eng. News 2004, 82, 49–58.
Jas, G.; Kirschning, A. Chem. Eur. J. 2003, 9, 5708–5723.
Ceylan, S.; Kirschning, A. In Recoverable and Recyclable Catalysts; Benaglia, M., Ed.; John Wiley & Sons, Ltd.: New York, 2009, pp. 379–410.
Mak, X.Y.; Laurino, P.; Seeberger, P. H. Beilstein J. Org. Chem. 2009, 5, 19.
Rasheed, M.; Elmore, S. C.; Wirth, T. In Catalytic Methods in Asymmetric Synthesis - Advanced Materials, Techniques, and Applications; Gruttadauria, M.; Giacalone, F., Eds.; John Wiley & Sons, Inc.: Hoboken, 2011, pp. 345–372.
Yuryev, R.; Strompen, S.; Liese, A. Beilstein J. Org. Chem. 2011, 7, 1449–1467.
Microreactors in Organic Synthesis, 2nd ed.; Wirth, T., Ed.; Wiley-VCH: Weinheim, 2013.
Sheldon, R. A. Adv. Synth. Catal. 2007, 349, 1289–1307.
Mateo, C.; Palomo, J. M.; Fernandez-Lorente, G.; Guisan, J. M.; Fernandez-Lafuente, R. Enzyme Microb. Technol. 2007, 40, 1451–1463.
Hanefeld, U.; Gardossi, L.; Magner, E., Chem. Soc. Rev. 2009, 38, 453–468.
Garcia-Galan, C., Berenguer-Murcia, A.; Fernandez-Lafuente, R.; Rodrigues, R. C. Adv. Synth. Catal. 2011, 353, 2885–2904.
Sheldon, R. A.; van Pelt, S. Chem. Soc. Rev. 2013, 42, 6223–6235.
Rodrigues, R. C.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Fernández-Lafuente, R. Chem. Soc. Rev. 2013, 42, 6290–6307.
Miyazaki, M.; Maeda, H. Trends Biotechnol. 2006, 24, 463–470.
Kawakami, K.; Abe, D.; Urakawa, T.; Kawashima, A.; Oda, Y.; Takahashi, R.; Sakai, S. J. Sep. Sci. 2007, 30, 3077–3084.
Honda, T.; Miyazaki, M.; Yamaguchi, Y.; Nakamura, H.; Maeda, H. Lab Chip 2007, 7, 366–372.
Lam, L. K. P.; Hui, R. A.; Jones, J. B. J. Org. Chem. 1986, 51, 2047–2050.
Rantakylä, M.; Aaltonen, O. Biotechnol. Lett. 1994, 16, 825–830.
Molinari, F.; Mantegazza, L.; Villa, R.; Aragozzini, F. J. Ferment. Bioeng. 1998, 86, 62–64.
Ljubovič, E.; Majerič-Elenkov, M.; Avgadič, A.; Šunjič, V. Food Technol. Biotechnol. 1999, 37, 215–224.
López-Serrano, P.; Wegman, M. A.; van Rantwijk, F.; Sheldon, R. A. Tetrahedron: Asymmetry 2001, 12, 235–240.
Cainelli, G.; Galletti, P.; Giacomini, D.; Gualandi, A.; Quintavalla, A. Helv. Chim. Acta 2003, 86, 3548–3559.
Sakai, T. Tetrahedron: Asymmetry 2004, 15, 2749–2756.
Moon-Young, Y.; Lee, S. H.; Cheong, C. S.; Park, J. K. Enzyme Microb. Technol. 2004, 35, 574–580.
Chen, C. C.; Tsai, S. W. Enzyme Microb. Technol. 2005, 36, 127–132.
Lin, C.; Hiraga, Y.; Masaki, K.; Iefuji, H.; Ohkata, K. Biocatal. Biotransform. 2006, 24, 390–395.
Yang, G.; Wu, J.; Xu, G.; Yang, L. Appl. Microbiol. Biotechnol. 2009, 81, 847–853.
Cipiciani, A.; Bellezza, F.; Fringuelli, F.; Silvestrini, M. G. Tetrahedron: Asymmetry 2001, 12, 2277–2281.
Magnusson, A. O.; Takwa, M.; Hamberg, A.; Hult, K. Angew. Chem., Int. Ed. 2005, 44, 4582–4585.
Csajági, C.; Szatzker, G.; Tőke, E. R.; Ürge, L.; Darvas, F.; Poppe, L. Tetrahedron: Asymmetry 2008, 19, 237–246.
Barton, M. J.; Hamman, J. P.; Fichter, K. C.; Calton, G. J. Enzyme Microb. Technol. 1990, 12, 577–583.
Gharpure, S. J.; Sathiyanarayanan, A. M. Chem. Commun. 2011, 47, 3625–3627.
Chen, C. S.; Fujimoto, Y.; Girdaukas, G.; Sih, C. J. J. Am. Chem. Soc. 1982, 104, 7294–7299.
Boros, Z.; Abaháziová, E.; Oláh, M.; Sátorhelyi, P.; Erdélyi, B.; Poppe, L. Chim. Oggi 2012, 30, 26–29.
Choi, E.; Kim, Y.; Ahn, Y.; Park, J.; Kim, M. Tetrahedron: Asymmetry 2013, 24, 1449–1452.
Weiser, D.; Boros, Z.; Hornyánszky, G.; Tóth, A.; Poppe, L. Proc. Biochem. 2012, 47, 428–434.
Hellner, G.; Boros, Z.; Tomin, A.; Poppe, L. Adv. Synth. Catal. 2011, 353, 2481–2491.
Tomin, A.; Hornyánszky, G.; Kupai, K.; Dorkó, Z.; Ürge, L.; Darvas, F.; Poppe, L. Proc. Biochem. 2010, 45, 859–865.
Woodley, J. M.; Lilly, M. D. In AppliedBiocatalysis; Cabral, J. M. S.; Best, D.; Boros, L.; Tramper, J., Eds.; Harwood Academic: London, 1994, pp. 371–393.
Li, N.; Giorno, L.; Drioli, E. Ann. N.Y. Acad. Sci. 2003, 984, 436–452.
Huh, Y. S.; Jun, Y.; Hong, Y. K.; Hong, W. H.; Kim, D. H. J. Mol. Catal. B: Enzym. 2006, 43, 96–101.
Long, W. S.; Kamaruddin, A.; Bhatia, S. J. Membr. Sci. 2005, 247, 185–200.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Falus, P., Boros, Z., Kovács, P. et al. Lipase-Catalyzed Kinetic Resolution of 1-(2-Hydroxycyclohexyl)Indoles in Batch and Continuous-Flow Systems. J Flow Chem 4, 125–134 (2014). https://doi.org/10.1556/JFC-D-14-00011
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1556/JFC-D-14-00011