Abstract
The prevention of bacterial infections in the health care environment is paramount to providing better treatment. Covering a susceptible environment with an antimicrobial coating is a successful way to avoid bacterial growth. Research on the preparation of durable antimicrobial coatings is promising for both fundamental surface care and clinical care applications. Herein, we report a facile, efficient, and scalable preparation of MoO3 paint using a cost-effective ball-milling approach. The MoO3 nanoplates (synthesized by thermal decomposition of ammonium heptamolybdate) are used as a pigment and antibacterial activity moiety in alkyd resin binders and other suitable eco-friendly additives in the preparation of paint. Surface morphology, chemical states, bonding nature, and intermolecular interaction between the MoO3 and the alkyd resin were studied using Raman and x-ray photoelectron spectroscopic analysis. The antibacterial properties of a prepared MoO3 nanoplate against various bacterial strains (Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Klebsiella pneumoniae) was determined using the microdilution method. Bacterial strains exposed to an MoO3 paint coated surface exhibit a significant loss of viability in a time-dependent manner. Fundamental modes of antibacterial activities ascribed from a biocompatible and durable MoO3 nanostructure incorporated into an alkyd resin complex are discussed. The obtained experimental findings suggest the potential utility of prepared MoO3-based paint coating for the prevention of health care associated infections.
Export citation and abstract BibTeX RIS
Corrections were made to this article on 19 December 2014. The funding information was corrected.
1. Introduction
Health care associated infections (HAIs) are a type of infection that the patient and/or the public acquire during the course of health care treatment in hospitals [1]. In the United States, nearly 88 000 deaths per year due to HAIs have been reported [1]. The United States' Center for Disease Control and Prevention reported that the annual cost for the prevention of HAIs in US hospitals has ranged from $35.7 to $45 billion dollars [1, 2]. Rising death rates and increasing annual costs have become serious issues for the prevention of HAIs in the present decade. The ubiquitous nature of bacteria, and their ability to grow and transfer from any source to healthy persons through a variety of cross-contaminations in an environment, leads to several types of HAIs [3, 4]. Even when necessary prevention methods have been used to inhibit bacterial growth, the death rate due to HAIs is not significantly reduced. The development of health care related products, such as textiles, medical devices (catheters and implantable coatings), and paints, with antibacterial properties can significantly reduce several types of HAIs [5–8]. This fact motivated the researchers in the design and development of novel health care related products toward practical applications.
Inorganic nanomaterials have attracted significant attraction in the biomedical sector, especially in the development of biodiagnostics and nanomedicine products [9]. This is due to the efficacy of their distinct physicochemical properties compared to their bulk and their ability to control and manipulate biosystems [9, 10]. The ongoing global nanobiotechnology revolution is concerned not only with the preliminary research level, by elucidating the primary mechanism of engineering nanoparticles and their interaction with cellular systems, but is also aimed at developing practical products for commercial applications [6–8]. For instance, many nanoparticles, such as metals (Ag, Au, Cu), metal oxides (ZnO, CuO, TiO2, MgO), and carbon materials (graphene, graphene oxide, CNTs), have provided numerous opportunities in the biomedical field [10–18]. Recent studies have demonstrated the bactericidal effects of various nanomaterials and their fundamental modes of toxicity and cell death. However, in the view of the practical applications toward the design of commercial products, the nanoparticles must be formulated in the appropriate matrix. It is noteworthy to mention that the applications of nanomaterials with antibacterial properties are not limited to therapeutic applications, but can also useful for the development of antibacterial products [15–18]. A recent study suggested that nearly 35% of nano silver and 25% of TiO2 production in Europe is used by the painting industry [19].
Nanoparticles can be effectively functionalized with suitable polymer chemistry, allowing them to offer a class of advanced materials for various commercial products for the prevention of HAIs [15]. Out of the several nanomaterials studied for their antibacterial properties, silver nanoparticles possess exceptional broad-spectrum antibiotic capabilities [16]. Researchers have developed silver nanoparticle based ointments for topical use, catheters for implantable medical devices, and antibacterial textile fabrics that are available as a commercial product [17, 18, 20]. Even though silver nanoparticles are a promising antibacterial, the high cost of silver and the release of toxic silver ion limit their commercial applications [18, 20]. Compared with the other antibacterial products, research in the development of antibacterial coatings using silver nanoparticles is limited.
Antibacterial paints are essential in hospitals, bathrooms, kitchens, and other locations with a high bacterial population. Referencing previous research on nano-based painting applications, Kumar et al demonstrated the use of silver nanoparticles, embedded in an alkyd resin, for their antibacterial properties [15]. Nanostructured silver vanadate has been used as an antibacterial additive in water-based paints [21] and metal oxide nanoparticles, such as ZnO and TiO2, have been used in paints for their pigment nature and as antibacterial additives [22, 23]. More recently, Natalio et al demonstrated the antibacterial and antifouling properties of vanadium pentoxide (V2O5) nanorods incorporated into paints [24]. These studies are examined at the research level and much work is still required for practical applications of nanomaterial-based paints. This data motivated us continue research in this area by developing a large-scale production of antibacterial nanopaint to be used as a cost-effective method for the prevention of HAIs.
The choice of materials used in the development of any product is important. Metal oxide nanoparticles can be an ideal choice for the preparation of paint due to their pigment nature, chemical resistance, and high thermal stability; they also have the advantage of being low cost [19, 25]. Among the metal oxides, nanostructured MoO3 has attracted attention due to its multifunctional properties, such as photocatalysis, gas sensors, supercapacitors, oxidative catalyst, photochromic coatings, and as additives in paint [26–28]. Recent studies have suggested the antibacterial properties of MoO3 nanomaterials [29, 30]. In this study, we prepared MoO3 paint using a ball-milling approach by embedding the MoO3 nanoplates in an alkyd resin matrix with nontoxic additives. The MoO3 paint coated surfaces were examined for their antibacterial properties against different pathogenic bacteria.
2. Experimental section
2.1. Materials and methods
Nanoparticles used in this study were prepared using the method discussed in our previous reports [10, 31]. Ammonium heptamolybdate tetra hydrate [(NH4)6Mo7O24·4H2O] was purchased from Dae Jung Chemicals and Metals Co., Ltd South Korea. All the chemicals used in this study were of research grade.
2.2. Preparation of MoO3 nanoplates
The MoO3 nanoplates were prepared using a one-step thermal decomposition approach with ammonium heptamolybdate as starting precursor. Briefly, 13 g of (NH4)6Mo7O24·4H2O was placed in a crucible and thermally treated with about 500 °C for 3 h. Following completion of the thermal reaction, MoO3 nanoplates were obtained and were used as pigment in the paint.
2.3. Preparation of MoO3 embedded alkyd paint
The MoO3 embedded alkyd paints were prepared using a high-energy ball-milling approach. MoO3 nanoplates (pigment), alkyd resin (binder), nanosized ZrO2 (inner coat drier), cobalt naphthenate (outer coat drier), nanosized ZnO (stabilizer), PVA (thickener), aluminum stearate (antisettling agent), and soya lecithin (wetting agent) were taken in an appropriate ratio (given in table 1) and allowed to mill for a period of 6 h using a tungsten carbide bowl with tungsten carbide balls (ball to powder ratio, 10:1) at a speed of 300 rpm. Subsequently, the toluene (solvent) was added to the milled product, and the milling process proceeded for an additional 30 min. Finally, homogenous MoO3 paint was obtained, which can be applied to any surface.
Table 1. Composition of MoO3 nanopaint and the role of its additives.
| Material | Role | Weight Percentage % |
|---|---|---|
| MoO3 nanoplates (pigment) | Providing the color of the paint | 20.0 |
| Alkyd resin (binder) | Film-forming component of paint | 60.0 |
| Nanosized ZnO (stabilizer) | Reducing the color fading effect of the paint | 0.6 |
| Aluminum stearate (antisettling agent) | Preventing pigment and binder settling | 0.5 |
| Polyvinyl alcohol (thickener) | Improving the viscosity and preventing coagulation | 0.1 |
| Soya lecithin (wetting agent) | Wetting the pigment in the binder for uniform dispersion | 0.6 |
| Nanosized zirconia | Chemical cross-linking agent of unsaturated fatty acids | 0.6 |
| Cobalt napthenate (upper coat drier) | Active catalyst for the lipid autoxidation process | 0.6 |
| Toluene (thinner/solvent) | Dispersing agent | 17.0 |
2.4. Instrumentation
The ball-milling process used in the preparation of the paint was carried out on a Pulverisette 6 planetary monomill, Fritsch instruments. The crystal structure and orientation were determined using a Rigaku x-ray diffractometer (XRD), operated at 40 KeV and 40 mA with Cu Kα radiation, in the range of 10–60° with a step of 0.02°. The Fourier transform infrared spectroscopy (FT-IR) spectrum was measured using a Thermo Scientific FT-IR spectrometer with pure KBr as the background. The sample was mixed with KBr. The mixture was then dried and compressed into a transparent tablet for measurement. The surface morphology of the samples was studied using field emission scanning electron microscope (FE-SEM, JEOL JSM-7500F). The chemical composition and state of the elements present in the outermost part of the samples were studied using x-ray photoelectron spectroscopy (XPS) techniques using ESCA-2000, VG Microtech Ltd. Here, a monochromatic x-ray beam source at 1486.6 (aluminum anode) and 14 kV was used to scan the sample surface. A high-flux x-ray source with an aluminum anode was used for x-ray generation and a quartz crystal monochromator was used to focus and scan the x-ray beam on the sample. Raman spectra of MoO3 and MoO3 paint were examined using a LabRam HR800 micro-Raman spectroscope (Horiba Jobin-Yvon, France). The Raman system was operated at 10 mV laser power and an excitation wavelength of 514 nm with an Ar+ ion laser.
2.5. Test of antibacterial properties
2.5.1. Bacterial strains
We used bacterial strains Escherichia coli (ATCC 25922), Staphylococcus aureus (ATCC 25923), Pseudomonas aeruginosa (ATCC 27853), and Klebsiella pneumoniae (ATCC 10031) to evaluate the antibacterial activity.
2.5.2. Antibacterial activity of MoO3 nanoplates
The antibacterial activity of the MoO3 nanoplates was examined using the microdilution method and the minimum inhibitory concentration (MIC) was evaluated [30, 32]. The MIC is defined as the lowest concentration of an antimicrobial agent that allows no growth of a microorganism after overnight incubation when compared with that of the control. Luria–Bertani (LB) broth was used as the diluent for the bacterial strains. Inoculates were prepared by suspending growth from overnight cultures in sterile LB broth. A two-fold dilution of the sample in 96-well plates was prepared. Approximately 107 CFU mL−1 cells were inoculated; the final volume in each microwell plate was 0.2 mL and was incubated at 37 °C for 24 h. The microwell plates were read at 590 nm using the ELISA reader prior to and following incubation to determine their minimum inhibitory concentration (MIC values). The experiments were performed in triplicate and were repeated twice.
2.5.3. Effect of MoO3 paint surface on bacterial growth
The antibacterial activity of MoO3 nanopaint was studied using the procedure given in literature [33, 34]: The MoO3 paint coated glass substrates, alkyd resin coated substrates, and uncoated substrates were sterilized. Coated and uncoated (negative control) surfaces were exposed to 200 μl of each microbe at an initial concentration of 107 CFU mL−1 in nutrient broth, allowed to air dry, and kept at a controlled temperature. Following an appropriate time period, the material surfaces were washed three times with 2 mL of nutrient broth. A ten-fold serial dilution with nutrient broth was prepared, spread onto a nutrient agar plate, and incubated at 37 °C for 24 h. The number of colonies in each sample was reported and the percentage of bacterial reduction was calculated as follows:
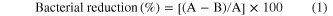
where A is the number of viable bacteria on the uncoated surface and B is the number of viable bacteria on the the paint-coated surface. All experiments were performed in triplicate and were repeated three times.
2.5.4. Statistical analysis
Data were analyzed using Biostat software (AnalystSoft Inc., Vancouver, British Columbia, Canada) for one-way analysis of variance for the statistical significance of the model (P < 0.05).
3. Results and discussion
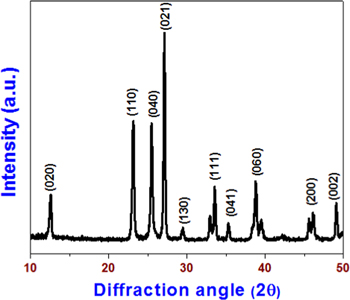
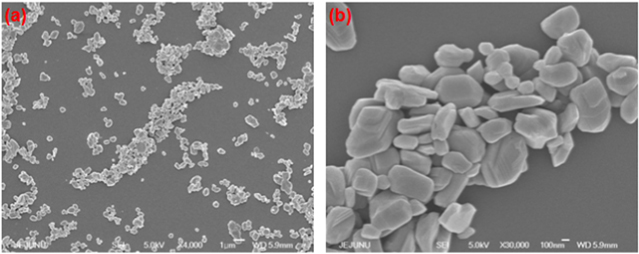
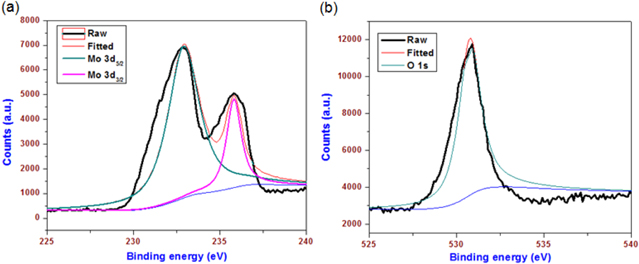
This study used a one-step thermal decomposition method for the preparation of MoO3 nanoplates with ammonium heptamolybdate as the starting precursor. Ammonium heptamolybdate is an analytical reagent used for the quantitative determination of phosphates, silicates, and arsenics [35]. Upon direct thermal treatment, the decomposition of ammonium heptamolybdate into molybdic acid through the evaporation of ammonia and water molecules occurred. Prolonged thermal treatment of molybdic acid resulted in the formation of MoO3 nanoplates. Nearly 10 g of MoO3 nanoplates were obtained using this method. Compared to the previous methods used for the synthesis of nano MoO3, such as chemical synthesis, the sonochemical method, and the hydrothermal method, the thermal decomposition approach used herein provided a unique method for gram-scale synthesis of MoO3 nanostructures. Figure 1 shows the x-ray diffraction pattern of the prepared MoO3 nanoplates, indicating the presence of sharp diffraction peaks, which are closely matched with the reflection lines of α-MoO3 (JCPDS, File No. 05-0508) [36]. No peaks related to any such impurities are found in the XRD pattern, confirming that a high-purity MoO3 was obtained using this thermal decomposition method. The surface morphology of the prepared nano MoO3 is examined using FE-SEM (as shown in figure 2). The low-magnification image [figure 2(a)] shows the presence of plate-like MoO3 nanostructures. The dimension of the MoO3 nanoplates is in the range of 300 to 700 nm, as observed from the high-magnification image [figure 2(b)]. Further, the chemical states of the elements present in the MoO3 nanoplates are studied using x-ray photoelectron spectra. Figures 3(a) and (b) shows the XPS deconvoluted spectra of Mo 3d and O 1s states in the MoO3 nanoplates. The presence of Mo 3d3/2 and 3d5/2 states in the MoO3 nanoplates at binding energies 232 and 236 eV is evident, as shown in figure 3(a). The oxygen atoms bonded with the molybdenum in MoO3 are observed at a binding energy of 532 eV. The observed data are in close agreement with the previous report on the XPS of MoO3 [37]. These physicochemical characterizations confirm the formation of high-purity MoO3 nanoplates through the thermal decomposition of ammonium molybdate.
Figure 1. X-ray diffraction pattern of MoO3 nanoplates.
Download figure:
Standard image High-resolution imageFigure 2. FE-SEMs of MoO3 nanoplates with different magnifications. (a) low-magnification (scale: 1 μm) and (b) high-magnification (scale: 100 nm).
Download figure:
Standard image High-resolution imageFigure 3. X-ray photoelectron spectra of MoO3 nanoplates (a) Mo 3d deconvoluted spectra and (b) O 1s deconvoluted spectra.
Download figure:
Standard image High-resolution imageThe MoO3-based alkyd paint is prepared using a ball-milling approach, as shown in the composition given in table 1. Herein, the MoO3 nanoplate prepared using the thermal decomposition approach mentioned previously is used as a pigment (color providing agent) material in the paint. All other additives used in the composition of the paint are nontoxic in nature. Following the balling process, which ran for 6 h, a homogeneous colloidal paint was formed that can be applied to any surface (as shown in figure 4). Drying of paint is a process in which the colloidal paint suspension will form a film on the surface onto which it was applied [15]. A binder is very important in the drying process of the paint because it is the film-forming component in the paint composition [38]. In our study, we used alkyd resin as the binder, which is a well-known binder used in the decorative paints. Alkyd resins are composed of polyunsaturated fatty acids and lipid chains, which will undergo a free radical mediated chain reaction in the presence of atmospheric oxygen [15]. This mechanism, known as a lipid autoxidation reaction, further results in the formation of uniform surfaces [39, 40]. The use of driers in the paint composition is to catalyze the drying time of the paint. In our study, we used two types of driers, cobalt napthenate (exterior drier) and nano zirconia (interior drier), in the MoO3 paint. Cobalt napthenate is an active catalyst for the lipid autoxidation process, which catalyzed the drying of outer surface of the paint [41], whereas nanozirconia is a chemical cross-linking agent of unsaturated fatty acids [42], which enhances the chemical cross-linking process in the inner region of the paint during the drying process. To improve the viscosity and prevent coagulation of the MoO3 paint, a combination of thickener (PVA) and antisettling agent (aluminum stearate) is used. We used nanosized ZnO as stabilizer in the alkyd paint, which is a type of additive used to inhibit or prevent the separation of pigment material from the binder and to reduce color fading [43].
Figure 4. Digital photographs of bare and MoO3 nanopaint coated glass substrates.
Download figure:
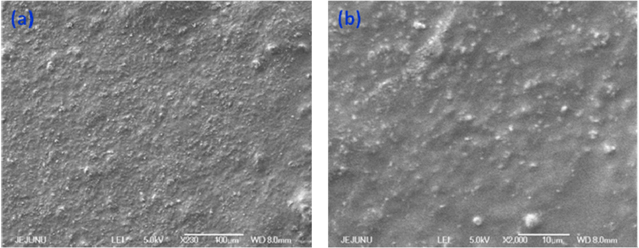
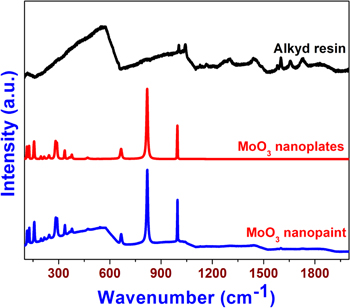
Standard image High-resolution imageThe surface morphology of the MoO3 paint coatings are analyzed using FE-SEM. Figures 5(a) and (b) shows the FE-SEM micrographs of the prepared paint coatings with low and high magnifications. It is evidenced from the FE-SEM micrographs that the MoO3 nanoplates are dispersed well in the alkyd resin matrix. The high dispersibility of the MoO3 nanoplates in the alkyd resin is due to the effectiveness of the ball-milling process. Raman spectroscopy is a nondestructive tool used to examine the crystallinity, defects states, and bonding interactions of nanoscale materials, such as graphene, metal oxides, several polymers, and paint coatings [44, 45] and was used to study the bonding interactions between MoO3 and alkyd resin in the prepared paint. Figure 6 shows the comparative Raman spectrum of the MoO3 nanoplates, alkyd resin, and the final paint. The Raman spectrum of MoO3 nanoplates shows the presence of well-defined Raman bands in the region 100 to 1000 cm−1, suggesting the presence of orthorhombic α-MoO3. The Raman band observed at 994 cm−1 represents the asymmetric Mo = O(1) stretching along the b-axis direction and the band observed at 816 cm−1 arises due to the symmetric Mo(3)-O-Mo(3) stretching vibrations along the a-axis direction [46]. The presence of well-defined Raman bands in the region below 200 cm−1 suggests the formation of highly crystalline MoO3 nanoplates [47]. The Raman spectra of bare alkyd resins show the presence of Raman bands at 565, 1046, 1305, 1435, 1604, 1659, and 1734 cm−1, which correspond to the polyunsaturated fatty acid and lipids present in the alkyd resin. This is in agreement with previous study on the Raman spectrum of alkyd resin [45]. The Raman spectra of the MoO3 paint show significant changes when compared with those of bare MoO3 and alkyd resin. The spectra of MoO3 paint show the bands corresponding to the pigment MoO3 (such as Mo = O(1) stretching band at 994 cm−1, the Mo(3)-O-Mo(3) stretching band at 816 cm−1, and the regions below 200 cm−1). However, the bands due to the fatty acid and lipid components (such as 1046, 1305, 1435, 1604, 1659, and 1734 cm−1) of the alkyd binder are not observed in the Raman spectra of the MoO3 paint. This is because of the fluorescence nature of inorganic pigment material (MoO3) in the paint, which may overwhelm the weaker scattering bands of the alkyd binders. This is in consistent with the previous findings on the Raman spectra of paints composed of TiO2, amorphous carbon, iron oxide, and graphene oxide pigments [45, 48].
Figure 5. FE-SEMs of MoO3 nanopaint surfaces with different magnifications. (a) Low-magnification and (b) high-magnification.
Download figure:
Standard image High-resolution imageFigure 6. Raman spectra of bare alkyd resin, MoO3 nanoplates, and MoO3 nanopaint.
Download figure:
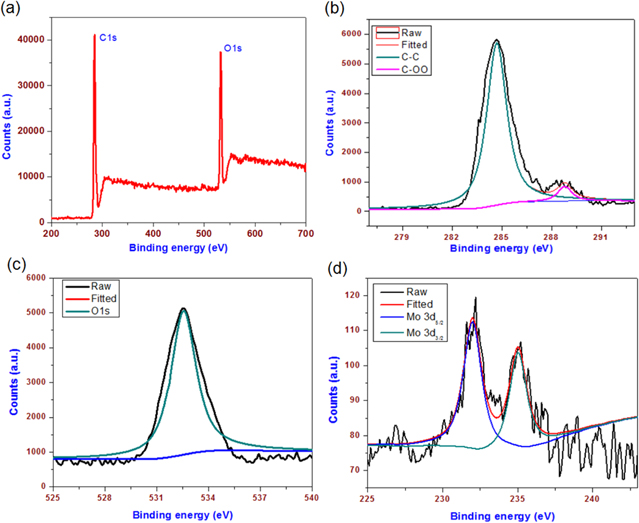
Standard image High-resolution imageThe chemical composition and state of elements present in the MoO3 paint are examined by XPS. The XPS survey spectrum of the alkyd resin (data not shown) shows the presence of carbon and oxygen related groups at 284.5 and 530 eV, respectively. The XPS survey spectrum of MoO3 paint is given in figure 7(a), which shows the presence of carbon- and oxygen-related moieties in the paint surface. The binding energies corresponding to the Mo-O transition of MoO3 in the paint are not clearly visible in the survey spectrum of the MoO3 paint. Figures 7(b) and (c) shows the XPS deconvoluted spectrum of C 1s and O 1s present in the paint surface. It shows the presence of carbon- and oxygen-related groups demonstrating the functional groups, such as C-C (284.6 eV), and C-OO (288.5 eV) groups from the alkyd resin component in the paint composition [48]. The deconvoluted spectra of Mo 3d, as given in figure 7(d), show the presence of Mo 3d3/2 and Mo 3d5/2 states at 233 and 235 eV, respectively [37]. In comparison with the spectra of bare MoO3 nanoplates (as shown in figure 3), the intensity of Mo 3d surface states is very low in the paint, which is due to the presence of alkyd resin bounded on the surface of nanoplates. Moreover, in contrast to the Raman spectra (where bands due to alkyd resin are diminished), the XPS spectra indicate the presence of both components of alkyd resin (binder) and the MoO3 (pigment) in the paint surface.
Figure 7. X-ray photoelectron studies of MoO3 nanopaint coated surfaces. (a) Survey spectrum and (b) (c) (d) represent the deconvoluted spectra of C1s, O1s, and Mo 3d components, respectively.
Download figure:
Standard image High-resolution imageIn this study, at first, we examined the antibacterial properties of the MoO3 nanoplates against four different types of bacteria viz. E. coli, P. aeruginosa, S. aureus, and K. pneumoniae, by examining their MIC values. MIC value can be defined as the minimum amount of antibacterial agent required to inhibit the growth of bacteria and a study of MIC value of any antibacterial agent is very important before their application in the development of any products. The MIC values of MoO3 nanoplates and commercial antibiotic Ciprofloxacin against these bacterial strains, shown in table S1, were measured using the microdilution method [30, 32]. From our study, the MIC value of MoO3 nanoplates against E. coli, P. aeruginosa, S. aureus, and K. pneumoniae are found to be 8, 8, 16, and 32 μg ml−1, respectively. Ciprofloxacin exhibited an MIC of 16 (E. coli), 16 (P. aeruginosa), 128 (S. aureus), and 64 μg ml−1 (K. pneumoniae), respectively. Observed MIC values are in agreement with our previous report on the antibacterial activity of chemically synthesized MoO3 nanoplates [30]. However, there are differential parameters such as size, shape, and surface states of the nanomaterials that can strongly influence the antibacterial activity [49]. A study by Dellasega et al demonstrated that the bacterial toxicity of nanomaterials could differ for gram-positive and gram-negative bacteria due to their structural changes in their outer membrane [50]. Similarly, there is research showing that nanomaterial-mediated toxicity can vary depending on the source of bacteria [51].
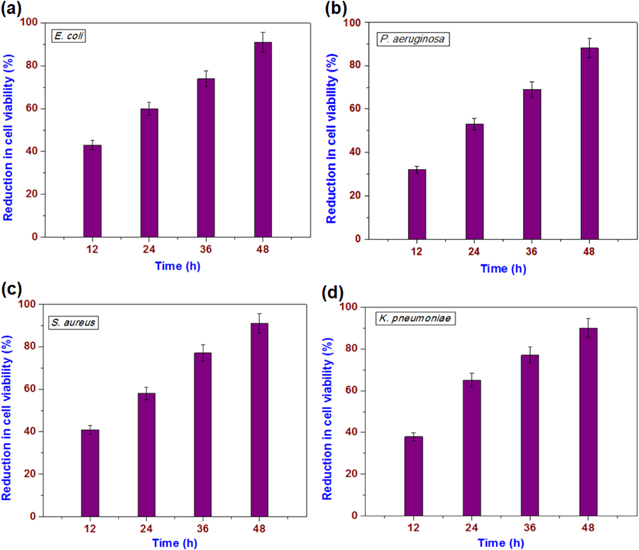
The antibacterial studies on the paint surface are more realistic than studies of the powder form because the paint can be used in places where the bacterial population is high, thereby controlling their growth. Studies have attempted to investigate the potential use of the prepared nanopaint to control microbial growth. The antibacterial properties of MoO3 nanopaint coated surfaces have been examined against four pathogenic bacteria viz. gram negative bacteria (E. coli, P. aeruginosa, and K. pneumoniae) and gram-positive bacteria (S. aureus) are examined using the method reported in the literature [33, 34]. Herein, the bacterial suspensions are exposed to the surface of the MoO3 paint coated substrates and after a certain time period (such as 12, 24, 36, and 48 h), the antibacterial activity was studied. The antibacterial activity of the MoO3 paint coated surface is shown in figure 8. It can be seen that the MoO3 paint surface is resistive to the growth of the bacteria. After 24 h of incubation, MoO3 nanopaint coated surface reduced 60, 65, 58, and 53% of viable cells against E. coli, K. pneumoniae, S. aureus, and P. aeruginosa, respectively (figure 8). With an increase in time, the cell viability was notably decreased, which clearly depicts the time-dependent antibacterial activity. As revealed from figure 8, after 48 h of incubation, the MoO3 nanopaint coated surface inhibited 91, 90, 91, and 88% of viable cells against E. coli, K. pneumoniae, S. aureus, and P. aeruginosa, respectively. Table S2 shows the time-dependent bacterial cell viability for the bare alkyd resin coatings, which clearly indicates that the bare resin coatings have only a negligible influence on the bacterial growth inhibition. This is in agreement with a previous study by Kumar et al [15]. Moreover, the effects of other additives, such as ZnO and ZrO2 (which are known to exhibit antibacterial properties), in the prepared paint on the antibacterial effect can be ruled out due to their lower weight fractions in the final product. Hence, the antibacterial properties of the MoO3 paint are due to the incorporation of MoO3 in the alkyd resin matrix.
Figure 8. Loss of bacterial cell viability (%) with exposure to MoO3 nanopaint coated surfaces after different time periods. (a) E. coli, (b) P. aeruginosa, (c) S. aureus, and (d) K. Pneumoniae.
Download figure:
Standard image High-resolution imageThe mechanism of the antibacterial effect of nanocoatings and nanopaints is still under debate because many factors influence the bactericidal effect. In general, the mechanism of nanomaterials toxicity toward bacteria can be due to either membrane or oxidative stress [52]. The former represents the interaction of bacteria with nanoparticles through direct contact, whereas the latter represents the involvement of a reactive oxygen species [4, 52]. Studies on the antibacterial property of the nano MoO3 is very limited compared to the other inorganic nanoparticles. It is suggested that the toxicity of MoO3 is due to the formation of hydroxonium ions on surface of MoO3 [29]. Our recent study suggested the disruption of bacterial cell wall due to MoO3 exposure, which can result in cell death [30]. Previous studies also demonstrated the antibacterial properties of MoO3 based thin films [53]. These mechanisms generally arise due to the size and solution effect of the nanomaterials, which cannot be the primary mechanism for the bacterial inactivation on the paint surface [29, 30, 54]. This is because the physicochemical properties of nanomaterials and their corresponding solution structures cannot be available in their solid state, especially after their formulation in paints. Because the experiments are completed in a dry state, it is believed that direct contact between the bacterial species and the paint surface is required for the bactericidal effects. Previous work by Kumar et al on in situ preparation of silver nanoparticle based paint for bacterial growth inhibition showed the paint had promising bactericidal effects [15]. This study revealed that the bacterial toxicity of silver embedded nanoparticles is due to the synergic effect of silver nanoparticles and silver metal ions present in the paint. Similarly, silver vanadate nanomaterials were also investigated for their application in paint as an antibacterial additive [21]. The other significant issue in the toxicity is due to the release of metal ions from the nanoparticle [15]. However, in this study, the release of molybdenum ions from the paint surface can be ruled out because the examination is not in an aqueous environment, which can limit the dissolution of metal ions. There are numerous other factors influencing antibacterial effects, such as the pigment–volume concentration, surface energy, and interfacial reactions that occurred between the bacteria and the paint surface, which are also considered for bacterial growth inhibition [54]. Overall, this study shows the bacterial growth inhibition properties of MoO3 paint coated surfaces; hence, it can be used in many places where the bacterial population is high to reduce the HAIs. It is well known that hygiene is of paramount importance in surgical and major treatment rooms in hospitals and the use of proven antibacterial coating is critical within these places [55]. At the same time, the color of the paint should be aesthetic and provide psychological relief. As MoO3 paint is an aesthetically pleasing milky green color and possesses the necessary antimicrobial properties, it can be used to paint the walls of surgical rooms, Intensive Care Unit, central kitchens, and other parts of the hospitals.
4. Conclusion
The MoO3 nanoplate embedded alkyd paint with suitable nontoxic additives was prepared using the ball-milling approach. The bonding interactions between MoO3 and the alkyd resin and between the surface states of the prepared MoO3 paint have been examined by Raman and XPS analysis. Our experimental results demonstrated that the prepared MoO3 paint exhibited good antibacterial properties against pathogenic bacteria. With the advantage of being a low cost, large-scale production with antibacterial properties, the as-prepared MoO3 paint can be used for the prevention of HAIs.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (2013R1A2A2A01068926).