Abstract
The last 3 years have seen the emergence of promising targeted therapies for the treatment of hepatocellular carcinoma (HCC). Sorafenib has been the mainstay of treatment for a decade and newer modalities were ineffective and did not confer any increased therapeutic benefit until the introduction of lenvatinib which was approved based on its non-inferiority to sorafenib. The subsequent success of regorafenib in HCC patients who progress on sorafenib treatment heralded a new era of second-line treatment and was quickly followed by ramucirumab, cabozantinib, and the most influential, immune checkpoint inhibitors (ICIs). Over the same period combination therapies, including anti-angiogenesis agents with ICIs, dual ICIs and targeted agents in conjunction with surgery or other loco-regional therapies, have been extensively investigated and have shown promise and provided the basis for exciting clinical trials. Work continues to develop additional novel therapeutic agents which could potentially augment the presently available options and understand the underlying mechanisms responsible for drug resistance, with the goal of improving the survival of patients with HCC.
Similar content being viewed by others
Introduction
Primary liver cancer remains a major problem for all health care systems worldwide and is associated with a significant clinical, economic, and psychological burden. Hepatocellular carcinoma (HCC) accounts for ~90% of cases of non-metastatic tumors of the liver.1 During the past decades, research has shed light on the epidemiology, risk factors, and molecular and genetic profiles of HCC‚ contributing to the evolution of strategies for prevention, surveillance, early diagnosis, and treatment.2,3 Liver resection, ablation, and liver transplantation are potentially curative but require diagnosis at a sufficiently early stage. Unfortunately, a significant proportion of HCC patients present with intermediate and advanced stage disease often, despite diligent surveillance, and curative treatments are frequently not possible.4 In these patients, systemic therapy remains essential and its pivotal role and potential have stimulated considerable research over the past decade. In this review, we examine recent advances in targeted therapy and discuss the impact this has had on the management of HCC. We also provide an overview of the most important areas of HCC research including novel clinical trials and technical platforms which promise to facilitate substantial progress within the next decade.
Approved first-line agents for HCC
Sorafenib
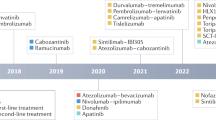
The success of SHARP and Asia-Pacific trial promoted the approval of sorafenib as first-line targeted therapy for advanced HCC,5,6,7,8,9 ushering in the era of systemic treatment. Subsequently, virtually all trials were centered around sorafenib and it was used as a control with which novel first-line agents were compared and evaluated in an attempt to improve the prognosis of patients with HCC. Unfortunately, despite a number of trials which compared these novel agents including sunitinib,10 brivanib,11 cediranib,12 linifanib,13 dovotinib,14 and immune-checkpoint inhibitors (ICIs) to sorafenib, none achieved the predefined primary end points (Fig. 1). In addition, during the decade when these agents were investigated, the median overall survival (OS) of sorafenib monotherapy as first-line treatment for advanced HCC increased from 10.7 months (SHARP) to 14.7 months (CheckMate-459), further consolidating its position. Meanwhile, the anti-tumor activity and safety of sorafenib have been validated in real-world setting. Subanalyses of the SHARP and Asia-Pacific trials found sorafenib was effective and safe irrespective of disease etiology, disease burden, ECOG (Eastern Cooperative Oncology Group performance status) status, etc.15,16,17 The safety of sorafenib was consistent across Child-Pugh A and B patients in clinical practice18 and the occurrence of side effects such as hand-foot syndrome and diarrhea were associated with an improved OS.19 Baseline hepatic function, clinicopathological factors, and etiology also affect the prognosis in HCC patients treated with sorafenib.20 In addition, sorafenib exerts anti-tumor effects with recurrent tumors following liver transplantation, conferring a survival advantage when compared with best supportive care (BSC).21,22,23 Noticeably, the application of sorafenib in clinical practice displays significant regional variations and incompliance with guidelines besides its usage as first-line therapy. It is common that initially unresectable HCCs got downstaged after sorafenib treatment and underwent curative-intent surgery24,25,26,27,28 and locoregional therapies before sorafenib were commonly encountered in real-world settings.29,30
The clinical benefit of sorafenib however remains modest and the complex molecular pathogenesis of HCC stimulated the investigation of combinations of sorafenib with other molecular targeting drugs. Sorafenib has been combined with anti-angiogenic agents, MEK/ERK pathway inhibitors, mTOR pathway inhibitors, histone deacetylase inhibitors, EGF/EGFR pathway inhibitors, and HGF/c-Met pathway inhibitors.31 Other agents such as interferon,32 selumetinib,33 capecitabine,34 tegafur-uracil,35 gemcitabine and oxaliplatin (GEMOX),36,37 and gemcitabine alone38 have also been evaluated but to date no treatments involving combinations containing sorafenib have succeeded in phase III trials.
Since sorafenib and TACE are both recommended therapies for advanced HCC, it is reasonable to expect that their combined use would confer benefits when compared with monotherapy. Results however highlighted regional differences and the heterogeneity of the trial protocols. In TACE 2, the multi-center, randomized, placebo-controlled, phase 3 European trial, when compared with TACE alone the addition of concurrent sorafenib, unlike the SPACE trial,39 did not improve progression free survival (PFS).40 It was also shown that the addition of sorafenib did not confer any survival benefit in patients with unresectable HCCs, who had already responded to TACE.41 Contrasting with these findings, retrospective studies from China have shown that combination therapy with sorafenib and TACE increased OS by more than 50% compared with TACE alone,42,43,44,45,46,47,48,49,50,51,52,53 which was supported by the findings from a number of other groups.54,55 Recently, the TACTICS trial, a randomized, multi-center prospective trial from Japan reported an improved PFS for TACE plus sorafenib compared with TACE alone (25.2 vs 13.5 months; p = 0.006),56 although this trial used a redefined PFS (not conventional PFS but time until “unTACEable” progression). The TACTICS trial also used time to any cause of death plus OS as primary endpoints (results not reported) and compared with sorafenib monotherapy, TACE plus sorafenib was only superior in controlling tumor progression and did not prolong OS.57,58
The acceptance of sorafenib as the standard to which other newer agents and non-surgical interventions are compared has resulted in studies comparing its use as monotherapy with TACE plus external beam radiotherapy59 and TACE plus intensity-modulated radiotherapy combined with sorafenib.60 In the SARAH study, selective internal radiotherapy with yttrium-90 resin microspheres did not produce any survival benefit compared with sorafenib in locally advanced and inoperable HCC (median OS, 8.0 vs 9.9, p = 0.18), and did not meet the primary endpoint of OS.61 Similarly, the addition of selective internal radiation therapy to sorafenib did not result in a significant improvement in OS compared with sorafenib alone.62 Bettinger et al.63 however did demonstrate that stereotactic body (external beam) radiotherapy employed as monotherapy (SBRT) was able to improve OS compared with sorafenib and SBRT with TACE also provided improved OS and PFS when compared with sorafenib and TACE in combination.64 In a recent trial of hepatic arterial infusion chemotherapy (HAIC; NCT02774187), He et al.65 reported that sorafenib plus HAIC with FOLFOX improved OS compared with sorafenib alone in advanced HCC when portal vein invasion was present, which was supported by other studies.66,67 Although the SCOOP-2 trial found sequential HAIC with cisplatin and sorafenib did not improve the survival benefit compared with sorafenib alone this is likely to have resulted from the study being underpowered for the primary and secondary endpoints.68
Due to the high recurrence rates following hepatectomy for HCC, approaches to adjuvant therapy has been extensively investigated although previous attempts, including the use of anti-viral agents, have been largely unsuccessful. Based on the palliative use and success of sorafenib its potential in the adjuvant setting was investigated and improved survivals following surgery anticipated. Unfortunately, this has not been demonstrated and it failed to reduce postoperative tumor recurrence in the STORM trial69 and other western studies.70 Explanations for the negative outcome in the STORM trial include high-dose modification rates, short treatment durations, and the enrollment of patients who were not at high risk of tumor recurrence (91% with no evidence of tumor satellites, 91% with one lesion, and 68% with no microscopic vascular invasion).71 Consistent with this viewpoint, Wang et al.72 reported no case of recurrence during the sorafenib dosing period whereas 4/14 patients suffered recurrence of their tumor within 7 months of discontinuation of sorafenib72 and persistent sorafenib intake following postoperative recurrence improved OS.73 Considering that patients who respond to sorafenib may belong to limited clinical or biological subsets, the effectiveness of sorafenib in an unselected population cohort supports its use in the adjuvant setting. A number of studies from the Far East including China, Japan, and Korea include patients with HCCs who are treated with hepatectomy despite their tumors being outside Barcelona Clinic Liver Cancer Classification (BCLC) guidelines, and although the results are difficult to compare due to heterogeneity of the protocols the results are positive. Sorafenib significantly reduces tumor recurrence in BCLC stage C patients74,75 and increases disease-free survival (DFS),76 and Zhuang et al.77 demonstrated that adjuvant therapy increased disease free survival (DFS) and OS. Sorafenib treatment following hepatectomy significantly prolonged the OS of advanced HCC rather than intermediate HCC.78 In addition to BCLC stage, patients who underwent hepatectomy and were pathologically diagnosed with microvascular invasion (MVI) also benefited from adjuvant sorafenib treatment.79 In line with these results, a large recent study with propensity score matching analysis also demonstrated that sorafenib significantly improved overall and recurrence-free survival following resection.80 The results from these studies which include all eligible patients suggest that more precise stratification would enable the identification of those patients who will benefit most from the use of adjuvant sorafenib and those in where additional treatment is not appropriate. Ongoing trials are attempting to evaluate the role of sorafenib in patients with MVI following radical resection (NCT02867280 and NCT02537158).
Lenvatinib
Following the approval of sorafenib for use in the treatment of HCC it takes more than a decade before the second first-line targeted agent for HCC emerged. Lenvatinib was approved for the first-line therapy in advanced HCC following the results of the REFLECT trial, a randomized phase III non-inferiority trial published by Kudo et al.81 Although not approved for long, further multi-center data from “real-world conditions” confirmed the efficacy of lenvatinib, regardless of previous tyrosine kinase inhibitor (TKI) therapies82,83 and lenvatinib monotherapy demonstrated anti-tumor activity for more than 4 years in unresectable HCC when portal vein invasion was present.84 In intermediate-stage HCC patients with tumors exceeding the up-to-seven criteria, for whom TACE is not helpful, lenvatinib could provide significant longer OS (37.9 vs. 21.3 months) and PFS (16.0 vs. 3.0 months).85 Lenvatinib pharmacokinetics in HCC is affected by body weight86,87 and a sufficient dose (relative dose intensity, RDI) is required to achieve a good therapeutic effect and consequently improved outcomes and prognosis are associated with the preservation of liver function which reduces the number of patients who need to discontinue their treatment.88,89,90,91 With lenvatinib, unlike other TKIs, there are issues with thyroid toxicity and surveillance for thyroid abnormalities during treatment is important.92 Hypothyroidism is not unusual and there are also fewer common reports of thyrotoxicosis and destructive thyroiditis.93 From a health economics standpoint however, lenvatinib is more cost effective than sorafenib.94,95
Second-line targeted agents for HCC
Still, sorafenib displays limited anti-tumor activity and some initially sorafenib-sensitive would eventually succumb to the disease, indicating the acquired resistance to sorafenib reduces its beneficial effects and an urgent need for second-line therapy.
Regorafenib
Initial attempts to discover effective second-line agents were unsuccessful and mirrored attempts to develop first-line agents which were superior to sorafenib.96 The RESORCE trial was a randomized, double blind, placebo-controlled, and phase III trial demonstrating the effectiveness of regorafenib in patients who had progressed on sorafenib treatment. This study finally confirmed the potential of second-line agents and ushered in the era of second-line and sequential therapy.97 Regorafenib provided survival benefit regardless of the rate of disease progression during prior sorafenib treatment or since the last sorafenib dose.98 This was confirmed by Yoo et al.99 in a retrospective study of safety and efficacy in Korean patients where data were consistent with those from the RESORCE trial. Regorafenib was even shown to be effective in patients with HCC recurrence following liver transplantation with a median OS of 12.9 months following regorafenib initiation and 38.4 months following sorafenib initiation (95% CI, 18.5–58.4) for the sorafenib followed by regorafenib sequential therapy.100
However, not all patients who progress on sorafenib are candidates for second-line therapy.101 In clinical practice only ~30% of patients are eligible for second-line regorafenib treatment.102 Good liver functional reserve and ECOG performance status during sorafenib treatment contributed to the efficacy and better outcomes of subsequent treatment,103,104 including lenvatinib.105 This may in part be due to the RDI required to achieve a clinically significant improvement in prognosis.106 This is supported by the demonstration that the new liver reserve function biomarker, albumin-bilirubin grade (ALBI),107 successfully identified regorafenib candidates and that in the selected cohort a median OS of 15.6 months was achieved compared with 6.8 months for non-candidates.108 Even in patients not eligible for regorafenib, the ones with an ECOG-PS score of 0, the absence of MVI, and TTP (time to progression) ≥4 months could still have acceptable postprogression survival.109 Long-term treatment with regorafenib has also been shown to reduce angiogenesis and improve portal hypertension (PHT) and acute administration ameliorates portal haemodynamics, suggesting that it may be especially suitable for patients with PHT and preserved liver function.110
Cabozantinib
Cabozantinib is another small molecule inhibitor of the tyrosine kinases which are implicated in the progression of HCC and the acquired resistance to sorafenib. Cabozantinib blocks the receptors involved in oncogenesis and angiogenesis including VEGFR 1, 2, 3, hepatocyte growth factor receptor (MET), AXL and the angiopoietin receptors TIE-2, RET, c-Kit and FLT-3 in vitro and in vivo. In CELESTIAL trial, cabozantinib achieved the primary endpoint with median OS of 10.2 months compared with 8.0 months for the placebo group111 and was consequently approved in the EU and USA. There remains a paucity of data however from real-world clinical practice examining the sequential treatment utilizing cabozantinib as the second-line agent, it is a costly option associated with frequent high-grade adverse events. Consequently, several studies have addressed the cost-effectiveness of cabozantinib using the cost and utility data extracted from the CELESTIAL trial. The conclusion from these studies is consistent and confirms that at its current cost point, the gain of quality-adjusted life-years for cabozantinib (QALYs, 0.067–0.16) and the incremental cost-effectiveness ratio (ICER, $156 437–$1,040,675) mean that it is not a cost-effective treatment option for patients with sorafenib-refractory HCC,112,113,114 compared with regorafenib (QALY, 0.18–0.25 and ICER, $201,797–$224,362).115,116
Ramucirumab
Ramucirumab is a fully human recombinant IgG1 monoclonal antibody targeting the VEGF2 receptor. Although ramucirumab failed to meet its primary endpoint as second-line treatment in the REACH trial,117 subgroup analysis found survival benefit in patients with AFP of 400 ng/ml or higher.118,119,120,121 This was later confirmed in the REACH-2 trial,122 which led to the approval of ramucirumab as second-line treatment for advanced HCC. REACH-2 is the first positive phase 3 trial in patients with HCC performed in a biomarker-selected patient cohort and more recent findings demonstrated that AFP-enriched HCCs displayed significant activation of VEGF which suggests the underlying mechanism of action and confirms the potential value of biomarker-driven clinical trials.123
Immune checkpoint therapy and TKI inhibitors
ICIs stand as the mainstream of immunotherapy. The CheckMate-040124 and KEYNOTE-224125 studies evaluated the safety and efficacy of nivolumab and pembrolizumab in patients with advanced HCC refractory to previous sorafenib treatment, which established the basis for accelerated approval by the FDA as second-line treatment. Subanalysis of CheckMate-040 data validated the safety and efficacy of nivolumab in Asian cohort.126 In an international real-world cohort study, ICIs have showed promising efficacy and safety in advanced HCCs as systemic first-/second-/third-/fourth-line treatment, with median OS and PFS of 11.0 and 4.6 months respectively127 and an excellent response to anti-PD-1 therapy has also been described in case report.128 Although the subsequent phase III KEYNOTE-240 trial did not meet its pre-specified statistical significance in respect of improved PFS and OS, the results were consistent with previous KEYNOTE-224.129 The KEYNOTE-394 presently underway in Asian patients may clarify the role of pembrolizumab in cases of advanced HCC with a viral background (NCT03062358). Recently, CheckMate-459, the multi-center phase III randomized sorafenib controlled trial evaluating nivolumab as first-line treatment for advanced HCC, failed to achieve its endpoints (ESMO 2019) but nivolumab did prolong OS (16.4 vs. 14.7 months) and achieve long-time disease control, less adverse events (AEs) and survival benefit regardless of the level of PD-L1 expression. Furthermore, nivolumab improved the survival of HCC patients whose etiology was HBV/HCV and did not reactivate hepatitis. Camrelizumab (SHR-1210, Hengrui Pharmaceutical), is an anti-PD-1 inhibitor from China investigated for the treatment of Hodgkin lymphoma and HCC. It has been shown to have antitumor activity in previously treated Chinese patients with advanced HCC in a multi-center, open-label, parallel-group, randomized, phase II trial (NCT02989922),130 providing evidence for the effectiveness of PD-1 therapy for HBV related HCC in Chinese patients. The results from other trials investigating novel ICIs including durvalumab, avelumab, tislelizumab, sintilimab, tremelimumab, ipilimumab, spartalizumab, and toripalimab will hopefully yield positive results and provide further options for the treatment of patients with HCC, particularly those who have relapsed on first-line treatments.
Further efforts to enhance the treatment effect of ICIs include dual ICIs treatment and combination therapy of ICIs with other kinds of targeted agents. For dual ICIs treatment, the initial results from CheckMate 9DW were astonishing: the objective response rate was 32%, higher than monotherapy of any ICIs alone. FDA has approved nivolumab in combination with ipilimumab for patients with HCC previously treated with sorafenib. Treatment modalities such as radiotherapy and anti-angiogenesis agents which affect antigen release or modulate the tumor microenvironments have the potential to increase the efficacy of immunotherapy and the combination of targeted agents with ICIs are attracting the attention of a number of research groups and in vitro studies and early-phase clinical trials assessing combination treatments have shown promising anti-tumor effects in patients with advanced HCC. In vitro evidence by Qui et al.131 demonstrated that lenvatinib and regorafenib could affect the expression of PD-L1 and real-time PCR results suggested that the mRNA expression of PD-L1 in the lenvatinib group was significantly higher than that in the control group, while its expression in the regorafenib group was significantly lower. When combined with anti-PD-1, lenvatinib can modulate cancer immunity in the tumor microenvironment and enhance antitumor activity.132,133 In July 2019, the FDA announced its approval of the first combination therapy employing the TKI lenvatinib with the ICI pembrolizumab based on the results from the KEYNOTE-524/Study 116 (NCT03006926) for the treatment of HCC. Recently, results from Study 117 (Phase Ib, NCT03418922) showed marginally better results for lenvatinib with nivolumab than lenvatinib with pembrolizumab. MET-mediated phosphorylation leads to a decreased expression of PD-L1 using the combination of MET inhibitors tivantinib and capmatinib, anti-PD1 and anti-PDL1 produced an additive effect which slows the growth of HCCs in mice.134 Clinically, based on the results from the experimental arm A of the GO 30140 study (NCT02715531), the FDA approved atezolizumab plus bevacizumab as breakthrough therapy for untreated advanced or metastatic HCC.135 Individual case studies also reported promising results for the use of combined TKI and anti-PD1/PD-L1 agents for advanced HCC.136,137,138 Such results were confirmed in the phase III trial IMbrave 150 study (NCT03434379) which reported that atezolizumab combined with bevacizumab resulted in better OS and PFS than sorafenib in patients with unresectable HCC.139 Other combination therapies include Galunisertib with nivolumab (NCT02423343), spartalizumab with and without capmatinib (NCT02795429), FGF401 with spartalizumab (NCT02325739), regorafenib with pembrolizumab (NCT03347292), cabozantinib with nivolumab (NCT03299946), avelumab with axitinib (NCT03289533), ramucirumab with durvalumab (NCT02572687), and XL888 with pembrolizumab (NCT03095781; Table 1).
Immune-related adverse events (IRAEs) occur frequently during treatment with ICIs and the clinical consequences can be significant.140 Activation of the immune system leads to damage of normal healthy tissues and IRAEs can have myriad effects and involve a number of different organs and have been reported to produce colitis, hepatitis, pneumonitis, dermatitis, myocarditis, endocrine glands inflammation, and rheumatic and musculoskeletal phenotypes including inflammatory arthritis, arthralgia, myositis, and sicca syndrome.141 Although the precise pathophysiology underlying the IRAEs side effects during treatment with ICIs remains unknown, discontinuing administration and the use of steroids is generally effective. In severe cases, however, additional immunosuppressants may be required but based on current available evidence, immunosuppression for IRAEs does not appear to compromise the antitumor response to the ICI treatment.142,143
Promising agents and treatment regimens
Despite abovementioned targeted drugs, novel agents have been continuously under development (Table 2). Of note, apatinib, a novel inhibitor of VEGFR2 tyrosine kinase, has attracted considerable attention and there is now a significant body of work describing clinical experience with its use. Although less effective than sorafenib as a first-line treatment in a retrospective study,144 apatinib still displayed promising anti-tumor effects in sorafenib-resistant HCC,145,146,147 where portal vein invasion was present,148 when metastases have occured,149,150 and for unresectable and relapsed HCCs.151,152 Combination therapy in studies utilising apatinib with TACE have achieved better clinical effectiveness than TACE alone, with tolerable AEs.153,154,155,156,157,158,159,160,161 Recently, the combination of apatinib with the anti-PD-1 monoclonal antibody camrelizumab achieved partial response rates of 50%.153 The results of other ongoing trials including the phase III trial comparing TACE and apatinib with sorafenib as first-line treatment for locally advanced or metastatic and unresectable HCC (NCT 03764293) and the adjuvant apatinib after hepatectomy for the prevention of tumor recurrence (NCT03722875 and NCT03261791) will hopefully prove effective and add to the presently available therapeutic options.
These promising results have stimulated the investigation of other new agents, the combinations of agents and regimens, which have been thoroughly discussed in a recent review from Zhu and Sun.154 The combination of bevacizumab and erlotinib has been extensively evaluated as first-155 or second-line in advanced HCCs,156,157,158,159,160,161,162 but unfortunately the heterogeneous nature of the results precludes firm conclusions and recommendations. Recently, a single-arm meta-analysis of prospective studies found that combination therapy with bevacizumab and erlotinib used as second-line treatment was associated with a favorable PFS (16 weeks, P = 0.012) and OS (12 months, P = 0.048), suggesting that future well-designed and sufficiently powered large-scale RCTs should be able to identify the potential contribution of these agents.163
Preclinical evidence for cyclin-dependent kinase (CDK) targeting therapies in HCC has showed promise and supports their investigation,164,165,166 especially with the potential ability to abrogate the emergence of sorafenib resistance167 and sensitize HCC to regorafenib treatment.168 A number of CKD inhibitors are presently undergoing evaluation including palbociclib (NCT01356628), milciclib (NCT03109886), and ribociclib (NCT02524119). The anti-MET monoclonal antibody emibetuzumab exhibited the greatest antitumor activity in HCC when combined with ramucirumab and had an excellent safety profile169 and for HCC with high MET expression there was an almost 3-fold increase in PFS (8.1 vs. 2.8 months) relative to those with low MET expression, suggesting the potential for further biomarker-driven clinical trials. Rigosertib is a synthetic benzyl styryl sulfone small molecule inhibitor which has been used in the treatment of monomyelocytic leukemia and due to its activity as a RAS- and PLK1-signaling inhibitor, it was investigated in HCC patients who demonstrate upregulation of PLK1 during tumor development and HRAS expression in advanced HCC. High expression levels of PLK1 are also significantly correlated with poor patient survival and the multiple effects of rigosertib could be beneficially employed to produce a therapeutic “dual-hit” approach in selected patients.170 Donafenib is a novel multi-kinase inhibitor which is similar to sorafenib, displaying comparable or better safety and efficacy when treating advanced HCC in phase 1b trial and phase 3 studies using sorafenib as the control (NCT02645981).171 There are ongoing trials evaluating novel agents such as anlotinib, another multi-kinase inhibitor which is orally administered and targets VEGFR, fibroblast growth factor receptor (FGFR), platelet-derived growth factor receptors (PDGFR), and c-kit (NCT02809534). Tivozanib is another oral inhibitor of VEGFR-1/2/3 with promising activity against HCC in vivo (NCT01835223) and TRC105, which despite demonstrating clinical activity and being well tolerated in HCC patients following sorafenib, has not to date met prespecified criteria and its development in HCC continues as combination therapy with sorafenib (NCT02560779).
Biomarker-driven targeted therapy
Despite extensive research investigating potential biomarkers to aid the development of protocols for the treatment of HCC, none have so far been identified to be able to predict the effect of, or response to treatment with sorafenib.172,173,174,175,176,177,178,179,180,181,182 Although the molecular classification of HCC has been widely reported (Table 3) to date it remains unclear whether this basic genomic and proteomic data will prove valuable in guiding targeted therapies.183,184,185,186,187,188,189,190
The continued belief that the future lies with personalized treatment which will be made possible through the rapid developments in next generation sequencing and the precision medicine that it underpins, have encouraged the development of novel trial designs.191 These novel trials designs offer new hope that biomarker-driven targeted therapies can be modulated and tailored on an individual basis.192,193 Using the prospectively archived tumor tissue and baseline plasma samples from HCC patients receiving regorafenib in the RESORCE trial, it was found the plasma miRNA panel and genetic mutational signatures in tumors were able to predict the response to regorafenib.194 In BIOSTORM, the biomarker companion study of STORM, multigene signatures associated with improved RFS with adjuvant sorafenib treatment after hepatectomy were identified and in the future could be used to guide treatment protocols.195 This approach is supported by case series where patients with CDKN2A-inactivating, CTNNB1-activating, PTEN-inactivating, and MET-activating mutations received palbociclib (CDK4/6 inhibitor), celecoxib (COX-2/Wnt inhibitor), sirolimus (mTOR inhibitor), and cabozantinib, respectively, with a reduction of des-gamma-carboxy prothrombin and AFP following treatment.196
Approaches to developing biomarker-driven targeted therapy strategies have also been examined in vitro and inactivating mutations in TSC1 and TSC2 have been shown to confer sensitivity to the mTOR protein. Aurora kinases are known to be oncogenic and overexpressed in a variety of tumors including colon, breast and prostate cancer and HCC tumor and other genetic mutations are known to affect the response to tyrosine kinase inhibitors and amplification of the MET gene is associated with hypersensitivity to cabozantinib which inhibits the tyrosine kinases c-Met and VEGFR2.197,198 The Wnt/β-catenin and Akt/mTOR pathways have been investigated and are reported to be co-activated in 14.4% of HCCs with the result that inhibition of the Jak/Stat pathway has therapeutic potential.199 Considering the wide range and type of genetic alterations which may act as potential targets,185 it is reasonable to believe that with an appropriate and well-designed trial protocol it should be possible to identify and validate specific biomarkers which will predict the response to specific targeted agents. Thus, we can prevent the use of treatments which can have no therapeutic effect, and may be associated with significant, avoidable toxicity.
Drug resistance of targeted therapy for HCC
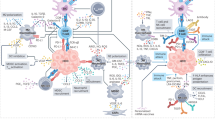
Drug resistance remains the principle cause of treatment failure during the use of targeted therapies200 and tumor heterogeneity and clonal evolution are the underlying mechanisms (Fig. 2), with the former mainly involved in primary resistance and the later acquired resistance.201 HCC is a remarkably heterogeneous disease exhibiting inter-patient heterogeneity, intertumoral heterogeneity among multifocal tumors and intratumoral heterogeneity (ITH) within tumors. This heterogeneity explains the attraction of targeted therapies and the search for biomarkers which can reliably predict the response to different agents. Nevertheless, it is also clear that the potential for this degree of heterogeneity renders the task of identifying single useful biomarkers or combinations of biomarkers extremely complex. On the other hand, the heterogeneity that has been identified does explain the, often quite dramatic, differences in the response to different therapeutic agents or combinations of agents.
The primary drug resistance mainly derives from interpatient and intratumoral heterogeneity while tumor evolution during treatment leads to spatial- and temporal-heterogeneity, which cause acquired resistance. Tumor heterogeneity and clonal evolution stands as the main reasons for targeted drug resistance
Unlike other primary cancers, multifocal lesions in liver is not uncommon whether derived from synchronous carcinogenesis or intrahepatic metastases. When multiple tumors occur, despite the fact that they originate from genetically similar cells and the potential etiology is the same, the lesions differ significantly (often very significantly) from each other demonstrating genomic alterations, varied biological behavior and loco-regional tumor microenvironment diversity which produces the well described differences in response to therapeutic agents.202,203 Within the tumor, intratumoral heterogeneity (ITH) has been found at genomic,204 epigenetic,205 transcriptional206, and protein level.207,208 Considering the level and extent of the intratumoral heterogeneity it is not surprising that the response to single treatment is equally heterogenous and irrespective of the efficacy of a single agent the most favorable outcome that can be reasonably expected is the eradication of a small portion of the total tumor cell burden leaving resistant clones surviving and responsible for progression.209 Although trunk mutational events in HCC are less heterogeneous210 and it is reported that single region sample could effectively recapitulate the genomic or proteomic features of HCC,211,212,213 which seems to shed light on overcoming drug resistance by ITH, tumor evolution due to selective pressure from targeted therapy brings new challenges.214 Thus, we have to find new ways to circumvent tumor heterogeneity and tumor evolution.
Fortunately, several novel techniques including single-cell sequencing, liquid biopsy, circulating tumor DNA (ctDNA), patient-derived cell-lines (PDC), patient-derived organoid (PDO), and patient-derived xenografts (PDX) should enable us to track cancer evolution in HCC. Compared with bulk tumor tissue sequencing, single-cell sequencing provides higher sensitivity and specificity, delineating tumor biology and characterization of cancer stem cell heterogeneity to understand the cellular diversity of HCC.215 Single-cell whole-genome sequencing has been used to examine the diverse modes of clonal evolution in HCC and relate this to tumor morphology. Variations are seen to occur early in tumor development but subsequently demonstrate stability during tumor progression. These findings suggest that treatment strategies could be developed based on the knowledge of tumor morphology.216
ctDNA represents a non-invasive, dynamic method to profile the tumor genome, predict treatment response, monitor disease progression, and help elucidate the mechanisms of drug resistance.217 In gastrointestinal cancers, ctDNA has been shown to outperform single-lesion tumor biopsies in discovering alterations which produce clinically relevant resistance and the mechanisms responsible for resistance to multiple agents.218 Novel alterations and parallel evolution which result in treatment-associated resistance are common in ctDNA, although the occurrence of multiple treatment related alterations in the circulation confound attempts to integrate the results into clinical protocols.219 When tumor biopsies are not available genetic profiling is possible using ctDNA which can provide a similar level of accuracy in respect of identifying somatic mutations and has the added advantage of being able to also detect de novo mutations.220 To date, few studies had examined the value of ctDNA to guide targeted therapy in HCC patients although preliminary results have demonstrated the feasibility of ctDNA in circumventing ITH,212 delineating tumor evolution,221,222 efficiently capturing mutations indicative of targeted therapy,196,223 and dynamically revealing genomic change during pharmacological treatment.224
Other approaches to understand the evolution of HCC and the mechanisms which underly the resistance to targeted therapy include next generation sequencing, the use of cancer organoids (PDOs) and PDX. PDO and PDX closely mimic hepatocarcinogenesis and preserve the tumor microenvironment making them excellent preclinical models for drug screening, biomarker development and research into the alterations and mechanism responsible for drug resistance.225,226,227 An example of this approach is our recent study using a patient-derived xenograft where we established 103 stable and transplantable xenograft lines that could be serially passaged, cryopreserved and revived. These lines maintained the diversity of HCC and the essential features of the original specimens at the histological, transcriptome, proteomic, and genomic levels. Using this model, we explored the predictive markers for sorafenib response and found that mitogen-activated protein kinase kinase kinase 1 (MAP3K1) might play an important role in sorafenib resistance and sorafenib response is impaired in patients with MAP3K1 down-expression.228 The combination of ctDNA sampling, next generation sequencing and PDX modeling has been applied to the clinical management of melanoma and facilitated personalized treatment.229 This approach using combinations of investigative techniques may also be applicable in the management of HCC and allow us to improve our understanding of the problems and opportunities for targeted therapy, tumor clonal evolution and interactions and facilitate the implementation of precision, personalized treatment (Fig. 3).
Unsolved issues in targeted therapy for HCC
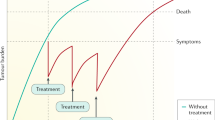
Several issues remain to be determined in the near future (Fig. 4). The best treatment strategy is still not clear, especially which targeted agent is the most appropriate for a specific HCC patient cohort. In the past, patients were faced with the harsh reality of knowing that other than sorafenib no therapeutic candidates were available. Today, a number of first and second-line options are available to clinicians. But in real-world medicine and most health care systems around the world, the majority of oncologist have to take into account the economic consequences before prescribing a particular treatment. Medical reimbursement or economic issues unfortunately remains an unavoidable consideration which influences decisions about treatment and the lack of our ability to provide clear guidance in respect of targeted treatment due to the myriad issues producing resistance to most therapies (vide supra) further complicates these decisions. We propose that the discovery and validation of novel biomarkers to reliably predict the response to treatment and define suitable candidates for a specific targeted agent, improving response rates and limiting avoidable toxicity in those who are unlikely to benefit, should be the focus of future HCC research.
In the clinical setting, we should ensure that we adhere to established treatment protocols particularly in respect of sequential targeted therapies or when a change of therapeutic agent is indicated. It is not uncommon for patients to be switched from sorafenib to lenvatinib, from regorafenib to lenvatinib, or from sorafenib to lenvatinib and to regorafenib when drug resistance develops, and these changes may be instigated by an oncologist or the patient themselves. While many patients do clearly benefit from these treatment changes, there is no evidence base underpinning many of these changes and this practice is undesirable as it confounds data collection at best and potentially exposes the responsible clinician to criticism especially where serious adverse events occur.
Conclusion
An iterative approach to targeted therapy has provided a wealth of data and encouraging results particularly in those patients where resistance to sorafenib develops. Although the understanding and management of HCC has changed dramatically due to the extensive basic and clinical research which has occurred over the last decade, HCC sadly remains a devastating disease which has a ubiquitous, enormous impact on health care systems across the world. The advances over this period however mean that patients can be comforted by the knowledge that there is a huge international effort underway from oncologists, hepatologists, and basic scientists to fully understand the mechanisms which are providing more rapid progress and ensure that the prognosis continues to improve.
References
Villanueva, A. Hepatocellular carcinoma. New Engl. J. Med. 380, 1450–1462 (2019).
Petrick, J. L. et al. International trends in hepatocellular carcinoma incidence, 1978–2012. Int. J. Cancer 147, 317–330 (2019).
Bertuccio, P. et al. Global trends and predictions in hepatocellular carcinoma mortality. J. Hepatol. 67, 302–309 (2017).
Yang, J. D. et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 16, 589–604 (2019).
Llovet, J. M. et al. Sorafenib in advanced hepatocellular carcinoma. New Engl. J. Med. 359, 378–390 (2008).
Cheng, A. L. et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 10, 25–34 (2009).
Liver, E. A. F. T. S. O. T. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J. Hepatol. 69, 182–236 (2018).
Zhou, J. et al. Guidelines for diagnosis and treatment of primary liver cancer in China (2017 Edition). Liver Cancer 7, 235–260 (2018).
2018 Korean Liver Cancer Association-National Cancer Center. Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Gut Liver 13, 227–299 (2019).
Cheng, A. L. et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J. Clin. Oncol. 31, 4067–4075 (2013).
Johnson, P. J. et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J. Clin. Oncol. 31, 3517–3524 (2013).
Zhu, A. X. et al. Efficacy, safety, pharmacokinetics, and biomarkers of cediranib monotherapy in advanced hepatocellular carcinoma: a phase II study. Clin. Cancer Res. 19, 1557–1566 (2013).
Cainap, C. et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J. Clin. Oncol. 33, 172–179 (2015).
Cheng, A. L. et al. Randomized, open-label phase 2 study comparing frontline dovitinib versus sorafenib in patients with advanced hepatocellular carcinoma. Hepatology 64, 774–784 (2016).
Bruix, J. et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J. Hepatol. 57, 821–829 (2012).
Cheng, A. L. et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma according to baseline status: subset analyses of the phase III Sorafenib Asia-Pacific trial. Eur. J. Cancer 48, 1452–1465 (2012).
Raoul, J. L. et al. Relationship between baseline hepatic status and outcome, and effect of sorafenib on liver function: SHARP trial subanalyses. J. Hepatol. 56, 1080–1088 (2012).
Marrero, J. A. et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: The GIDEON study. J. Hepatol. 65, 1140–1147 (2016).
Cho, J. Y. et al. Clinical parameters predictive of outcomes in sorafenib-treated patients with advanced hepatocellular carcinoma. Liver Int. 33, 950–957 (2013).
Rovesti, G. et al. Impact of baseline characteristics on the overall survival of HCC patients treated with sorafenib: ten years of experience. Gastrointest. Tumors 6, 92–107 (2019).
Kang, S. H. et al. Efficacy of sorafenib for the treatment of post-transplant hepatocellular carcinoma recurrence. J. Korean Med. Sci. 33, e283 (2018).
Alsina, A. E. et al. Can sorafenib increase survival for recurrent hepatocellular carcinoma after liver transplantation? A pilot study. Am. Surg. 80, 680–684 (2014).
Pfeiffenberger, J. et al. Sorafenib treatment is save and may affect survival of recurrent hepatocellular carcinoma after liver transplantation. Langenbeck’s Arch. Surg. 398, 1123–1128 (2013).
Barbier, L. et al. Liver resection after downstaging hepatocellular carcinoma with sorafenib. Int. J. Hepatol. 2011, 791013 (2011).
Barbier, L. et al. Safety of liver resection for hepatocellular carcinoma after sorafenib therapy: a multicenter case-matched study. Ann. Surg. Oncol. 20, 3603–3609 (2013).
Kitajima, T. et al. Complete pathological response induced by sorafenib for advanced hepatocellular carcinoma with multiple lung metastases and venous tumor thrombosis allowing for curative resection. Clin. J. Gastroenterol. 8, 300–305 (2015).
Yoshimoto, T. et al. The outcome of sorafenib therapy on unresectable hepatocellular carcinoma: experience of conversion and salvage hepatectomy. Anticancer Res. 38, 501–507 (2018).
Takeyama, H. et al. Impact of surgical treatment after sorafenib therapy for advanced hepatocellular carcinoma. Surg. Today 48, 431–438 (2018).
Park, J. W. et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 35, 2155–2166 (2015).
Kudo, M. et al. Regional differences in sorafenib-treated patients with hepatocellular carcinoma: GIDEON observational study. Liver Int. 36, 1196–1205 (2016).
Gao, J. J., Shi, Z. Y., Xia, J. F., Inagaki, Y. & Tang, W. Sorafenib-based combined molecule targeting in treatment of hepatocellular carcinoma. World J. Gastroenterol. 21, 12059–12070 (2015).
Itokawa, N. et al. Effects of sorafenib combined with low-dose interferon therapy for advanced hepatocellular carcinoma: a pilot study. Int. J. Clin. Oncol. 21, 676–683 (2016).
Tai, W. M. et al. A phase Ib study of selumetinib (AZD6244, ARRY-142886) in combination with sorafenib in advanced hepatocellular carcinoma (HCC). Ann. Oncol. 27, 2210–2215 (2016).
Patt, Y., Rojas-Hernandez, C., Fekrazad, H. M., Bansal, P. & Lee, F. C. Phase II trial of sorafenib in combination with capecitabine in patients with hepatocellular carcinoma: INST 08-20. Oncologist 22, 1158–e1116 (2017).
Azim, H. A. et al. Sorafenib plus tegafur-uracil (UFT) versus sorafenib as first line systemic treatment for patients with advanced stage HCC: a Phase II trial (ESLC01 study). J. Hepatocell. Carcinoma 5, 109–119 (2018).
Assenat, E. et al. Sorafenib alone vs. sorafenib plus GEMOX as 1(st)-line treatment for advanced HCC: the phase II randomised PRODIGE 10 trial. Br. J. Cancer 120, 896–902 (2019).
Liu, Y. et al. First-line gemcitabine and oxaliplatin (GEMOX) plus sorafenib, followed by sorafenib as maintenance therapy, for patients with advanced hepatocellular carcinoma: a preliminary study. Int. J. Clin. Oncol. 20, 952–959 (2015).
Srimuninnimit, V., Sriuranpong, V. & Suwanvecho, S. Efficacy and safety of sorafenib in combination with gemcitabine in patients with advanced hepatocellular carcinoma: a multicenter, open-label, single-arm phase II study. Asia Pac. J. Clin. Oncol. 10, 255–260 (2014).
Lencioni, R. et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J. Hepatol. 64, 1090–1098 (2016).
Meyer, T. et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol. Hepatol. 2, 565–575 (2017).
Kudo, M. et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur. J. Cancer 47, 2117–2127 (2011).
Qu, X. D. et al. The efficacy of TACE combined sorafenib in advanced stages hepatocellullar carcinoma. BMC Cancer 12, 263 (2012).
Bai, W. et al. Sorafenib in combination with transarterial chemoembolization improves the survival of patients with unresectable hepatocellular carcinoma: a propensity score matching study. J. Dig. Dis. 14, 181–190 (2013).
Hu, H. et al. Sorafenib combined with transarterial chemoembolization versus transarterial chemoembolization alone for advanced-stage hepatocellular carcinoma: a propensity score matching study. PLoS ONE 9, e96620 (2014).
Zhu, K. et al. Hepatocellular carcinoma with portal vein tumor thrombus: treatment with transarterial chemoembolization combined with sorafenib–a retrospective controlled study. Radiology 272, 284–293 (2014).
Wan, X. et al. Retrospective analysis of transarterial chemoembolization and sorafenib in Chinese patients with unresectable and recurrent hepatocellular carcinoma. Oncotarget 7, 83806–83816 (2016).
Yao, X., Yan, D., Zeng, H., Liu, D. & Li, H. Concurrent sorafenib therapy extends the interval to subsequent TACE for patients with unresectable hepatocellular carcinoma. J. Surg. Oncol. 113, 672–677 (2016).
Yao, Q., Zhang, H., Xiong, B. & Zheng, C. Combination of sorafenib and TACE inhibits portal vein invasion for intermediate stage HCC: a single center retrospective controlled study. Oncotarget 8, 79012–79022 (2017).
Huang, Y. et al. Overall survival in response to sorafenib with transarterial chemoembolization for BCLC stage B hepatocellular carcinoma: propensity score analysis. Int. J. Clin. Pharmacol. Ther. 55, 498–508 (2017).
Lei, X. F. et al. Effect and safety of sorafenib in patients with intermediate hepatocellular carcinoma who received transarterial chemoembolization: a retrospective comparative study. World J. Clin. Cases 6, 74–83 (2018).
Ren, B. et al. Transarterial chemoembolization (TACE) combined with sorafenib versus TACE alone for unresectable hepatocellular carcinoma: A Propensity Score Matching Study. J. Cancer 10, 1189–1196 (2019).
Zhu, K. et al. Medium or large hepatocellular carcinoma: sorafenib combined with transarterial chemoembolization and radiofrequency ablation. Radiology 288, 300–307 (2018).
Yuan, J. et al. Transarterial chemoembolization (TACE) combined with sorafenib in treatment of HBV background hepatocellular carcinoma with portal vein tumor thrombus: A Propensity Score Matching Study. BioMed. Res. Int. 2019, 2141859 (2019).
Aktas, G. et al. Sorafenib with TACE improves the survival of hepatocellular carcinoma patients with more than 10 cm tumor: a single-center retrospective study. J. BUON 22, 150–156 (2017).
Varghese, J. et al. Combination of TACE and sorafenib improves outcomes in BCLC stages B/C of hepatocellular carcinoma: a single centre experience. Ann. Hepatol. 16, 247–254 (2017).
Kudo, M. et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 69, 1492–1501 (2019).
Choi, G. H. et al. Sorafenib alone versus sorafenib combined with transarterial chemoembolization for advanced-stage hepatocellular carcinoma: results of propensity score analyses. Radiology 269, 603–611 (2013).
Wu, F. X. et al. The safety and efficacy of transarterial chemoembolization combined with sorafenib and sorafenib mono-therapy in patients with BCLC stage B/C hepatocellular carcinoma. BMC Cancer 17, 645 (2017).
Yoon, S. M. et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: A Randomized Clinical Trial. JAMA Oncol. 4, 661–669 (2018).
Zhao, Y. et al. Safety and efficacy of transcatheter arterial chemoembolization plus radiotherapy combined with sorafenib in hepatocellular carcinoma showing macrovascular invasion. Front. Oncol. 9, 1065 (2019).
Vilgrain, V. et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 18, 1624–1636 (2017).
Ricke, J. et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J. Hepatol. 71, 1164–1174 (2019).
Bettinger, D. et al. Stereotactic body radiation therapy as an alternative treatment for patients with hepatocellular carcinoma compared to sorafenib: a propensity score analysis. Liver Cancer 8, 281–294 (2019).
Shen, L. et al. Combination therapy after TACE for hepatocellular carcinoma with macroscopic vascular invasion: stereotactic body radiotherapy versus sorafenib. Cancers 10, 516 (2018).
He, M. et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 5, 953–960 (2019).
Nomura, T. et al. Efficacy of combined modality therapy with sorafenib following hepatic arterial injection chemotherapy and three-dimensional conformal radiotherapy for advanced hepatocellular carcinoma with major vascular invasion. Mol. Clin. Oncol. 11, 447–454 (2019).
Kawaoka, T. et al. Comparison of hepatic arterial infusion chemotherapy versus sorafenib monotherapy in patients with advanced hepatocellular carcinoma. J. Dig. Dis. 16, 505–512 (2015).
Kondo, M. et al. Randomized, phase II trial of sequential hepatic arterial infusion chemotherapy and sorafenib versus sorafenib alone as initial therapy for advanced hepatocellular carcinoma: SCOOP-2 trial. BMC Cancer 19, 954 (2019).
Bruix, J. et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 16, 1344–1354 (2015).
Antoniou, E. A. et al. Sorafenib as an adjuvant therapy for resectable hepatocellular carcinoma: a single center experience. J. BUON 21, 1189–1194 (2016).
Kelley, R. K. Adjuvant sorafenib for liver cancer: wrong stage, wrong dose. Lancet Oncol. 16, 1279–1281 (2015).
Wang, S. N., Chuang, S. C. & Lee, K. T. Efficacy of sorafenib as adjuvant therapy to prevent early recurrence of hepatocellular carcinoma after curative surgery: a pilot study. Hepatol. Res. 44, 523–531 (2014).
Zhang, W. et al. Adjuvant sorafenib reduced mortality and prolonged overall survival and post-recurrence survival in hepatocellular carcinoma patients after curative resection: a single-center experience. Biosci. Trends 8, 333–338 (2014).
Li, J., Hou, Y., Cai, X. B. & Liu, B. Sorafenib after resection improves the outcome of BCLC stage C hepatocellular carcinoma. World J. Gastroenterol. 22, 4034–4040 (2016).
Xia, F. et al. Adjuvant sorafenib after heptectomy for Barcelona Clinic Liver Cancer-stage C hepatocellular carcinoma patients. World J. Gastroenterol. 22, 5384–5392 (2016).
Liao, Y. et al. Sorafenib therapy following resection prolongs disease-free survival in patients with advanced hepatocellular carcinoma at a high risk of recurrence. Oncol. Lett. 13, 984–992 (2017).
Zhuang, L. et al. Sorafenib combined with hepatectomy in patients with intermediate-stage and advanced hepatocellular carcinoma. Arch. Med. Sci. 13, 1383–1393 (2017).
Zhu, H., Ye, B., Qiao, Z., Zeng, L. & Li, Q. Hepatectomy combined with sorafenib in patients with intermediate-advanced hepatocellullar carcinoma. J. BUON 24, 1382–1389 (2019).
Huang, Y. et al. Should we apply sorafenib in hepatocellular carcinoma patients with microvascular invasion after curative hepatectomy? OncoTargets Ther. 12, 541–548 (2019).
Zhang, X. P. et al. Postoperative adjuvant sorafenib improves survival outcomes in hepatocellular carcinoma patients with microvascular invasion after R0 liver resection: a propensity score matching analysis. HPB 21, 1687–1696 (2019).
Kudo, M. et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391, 1163–1173 (2018).
Hiraoka, A. et al. Clinical features of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions: multicenter analysis. Cancer Med. 8, 137–146 (2019).
Hiraoka, A. et al. Therapeutic potential of lenvatinib for unresectable hepatocellular carcinoma in clinical practice: multicenter analysis. Hepatol. Res. 49, 111–117 (2019).
Takeda, H. et al. Long-term antitumor effect of lenvatinib on unresectable hepatocellular carcinoma with portal vein invasion. Hepatol. Res. 49, 594–599 (2019).
Kudo, M. et al. Lenvatinib as an initial treatment in patients with intermediate-stage hepatocellular carcinoma beyond up-to-seven criteria and child-pugh a liver function: a proof-of-concept study. Cancers 11, 1084 (2019).
Ikeda, M. et al. Safety and pharmacokinetics of lenvatinib in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 22, 1385–1394 (2016).
Ikeda, K. et al. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J. Gastroenterol. 52, 512–519 (2017).
Hiraoka, A. et al. Prognostic factor of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions-Multicenter analysis. Cancer Med. 8, 3719–3728 (2019).
Ueshima, K. et al. Impact of baseline ALBI grade on the outcomes of hepatocellular carcinoma patients treated with lenvatinib: A Multicenter Study. Cancers 11, 592 (2019).
Sasaki, R. et al. Response to lenvatinib is associated with optimal relativedose intensity in hepatocellular carcinoma: experience in clinical settings. Cancers 11, 1769 (2019).
Takahashi, A. et al. Impact of relative dose intensity of early-phase lenvatinib treatment on therapeutic response in hepatocellular carcinoma. Anticancer Res. 39, 5149–5156 (2019).
Koizumi, Y. et al. Lenvatinib-induced thyroid abnormalities in unresectable hepatocellular carcinoma. Endocr. J. 66, 787–792 (2019).
Hirooka, M. et al. Destructive thyroiditis induced by lenvatinib in three patients with hepatocellular carcinoma. Intern. Med. 58, 791–795 (2019).
Kobayashi, M. et al. Cost-effectiveness analysis of lenvatinib treatment for patients with unresectable hepatocellular carcinoma (uHCC) compared with sorafenib in Japan. J. Gastroenterol. 54, 558–570 (2019).
Kodama, K. et al. Correlation between early tumor marker response and imaging response in patients with advanced hepatocellular carcinoma treated with lenvatinib. Oncology 97, 75–81 (2019).
Zhu, A. X. et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA 312, 57–67 (2014).
Bruix, J. et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389, 56–66 (2017).
Finn, R. S. et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: Additional analyses from the phase III RESORCE trial. J. Hepatol. 69, 353–358 (2018).
Yoo, C. et al. Multicenter retrospective analysis of the safety and efficacy of regorafenib after progression on sorafenib in Korean patients with hepatocellular carcinoma. Invest. New Drugs 37, 567–572 (2019).
Iavarone, M. et al. Preliminary experience on safety of regorafenib after sorafenib failure in recurrent hepatocellular carcinoma after liver transplantation. Am. J. Transplant. 19, 3176–3184 (2019).
Kuzuya, T. et al. Clinical characteristics and outcomes of candidates for second-line therapy, including regorafenib and ramucirumab, for advanced hepatocellular carcinoma after sorafenib treatment. Hepatol. Res. 49, 1054–1065 (2019).
Uchikawa, S. et al. Clinical outcomes of sorafenib treatment failure for advanced hepatocellular carcinoma and candidates for regorafenib treatment in real-world practice. Hepatol. Res. 48, 814–820 (2018).
Ogasawara, S. et al. Characteristics of patients with sorafenib-treated advanced hepatocellular carcinoma eligible for second-line treatment. Invest. New Drugs 36, 332–339 (2018).
Terashima, T. et al. Analysis of the liver functional reserve of patients with advanced hepatocellular carcinoma undergoing sorafenib treatment: prospects for regorafenib therapy. Hepatol. Res. 48, 956–966 (2018).
Hiraoka, A. et al. Important clinical factors in sequential therapy including lenvatinib against unresectable hepatocellular carcinoma. Oncology 97, 277–285 (2019).
Wang, W. et al. Sorafenib-regorafenib sequential therapy in japanese patients with unresectable hepatocellular carcinoma-relative dose intensity and post-regorafenib therapies in real world practice. Cancers 11, 1517 (2019).
Johnson, P. J. et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J. Clin. Oncol. 33, 550–558 (2015).
Yukimoto, A. et al. Using ALBI score at the start of sorafenib treatment to predict regorafenib treatment candidates in patients with hepatocellular carcinoma. Jpn J. Clin. Oncol. 49, 42–47 (2019).
Kuzuya, T. et al. Prognostic factors associated with postprogression survival in advanced hepatocellular carcinoma patients treated with sorafenib not eligible for second-line regorafenib treatment. Oncology 95, 91–99 (2018).
Uschner, F. E. et al. The multikinase inhibitor regorafenib decreases angiogenesis and improves portal hypertension. Oncotarget 9, 36220–36237 (2018).
Abou-Alfa, G. K. et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. New Engl. J. Med. 379, 54–63 (2018).
Soto-Perez-de-Celis, E., Aguiar, P. N., Cordon, M. L., Chavarri-Guerra, Y. & Lopes, G. L. Cost-effectiveness of cabozantinib in the second-line treatment of advanced hepatocellular carcinoma. J. Natl Compr. Cancer Netw. 17, 669–675 (2019).
Liao, W. et al. Cost-effectiveness analysis of cabozantinib as second-line therapy in advanced hepatocellular carcinoma. Liver Int. https://doi.org/10.1111/liv.14257 (2019).
Shlomai, A., Leshno, M. & Goldstein, D. A. Cabozantinib for patients with advanced hepatocellular carcinoma: a cost-effectiveness analysis. Ther. Adv. Gastroenterol. 12, 1756284819878304 (2019).
Shlomai, A., Leshno, M. & Goldstein, D. A. Regorafenib treatment for patients with hepatocellular carcinoma who progressed on sorafenib-A cost-effectiveness analysis. PLoS ONE 13, e0207132 (2018).
Parikh, N. D., Singal, A. G. & Hutton, D. W. Cost effectiveness of regorafenib as second-line therapy for patients with advanced hepatocellular carcinoma. Cancer 123, 3725–3731 (2017).
Zhu, A. X. et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 16, 859–870 (2015).
Park, J. O. et al. Second-line ramucirumab therapy for advanced hepatocellular carcinoma (REACH): an East Asian and non-East Asian subgroup analysis. Oncotarget 7, 75482–75491 (2016).
Zhu, A. X. et al. Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma: analysis of REACH Trial Results by Child-Pugh Score. JAMA Oncol. 3, 235–243 (2017).
Kudo, M. et al. Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma: Japanese subgroup analysis of the REACH trial. J. Gastroenterol. 52, 494–503 (2017).
Chau, I. et al. Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib: Patient-focused outcome results from the randomised phase III REACH study. Eur. J. Cancer 81, 17–25 (2017).
Zhu, A. X. et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 20, 282–296 (2019).
Montal, R. et al. Molecular portrait of high alpha-fetoprotein in hepatocellular carcinoma: implications for biomarker-driven clinical trials. Br. J. Cancer 121, 340–343 (2019).
El-Khoueiry, A. B. et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389, 2492–2502 (2017).
Zhu, A. X. et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 19, 940–952 (2018).
Yau, T. et al. Nivolumab in advanced hepatocellular carcinoma: sorafenib-experienced Asian cohort analysis. J. Hepatol. 71, 543–552 (2019).
Scheiner, B. et al. Programmed cell death protein-1 (PD-1)-targeted immunotherapy in advanced hepatocellular carcinoma: efficacy and safety data from an international multicentre real-world cohort. Aliment. Pharmacol. Ther. 49, 1323–1333 (2019).
Roderburg, C. et al. Excellent response to anti-PD-1 therapy in a patient with hepatocellular carcinoma intolerant to sorafenib. Visc. Med. 35, 43–46 (2019).
Finn, R. S. et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase iii trial. J. Clin. Oncol. https://doi.org/10.1200/jco.19.01307 (2019).
Qin, S. et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. https://doi.org/10.1016/s1470-2045(20)30011-5 (2020).
Qiu, M. J. et al. Effects of liver-targeted drugs on expression of immune-related proteins in hepatocellular carcinoma cells. Clin. Chim. Acta 485, 103–105 (2018).
Kimura, T. et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 109, 3993–4002 (2018).
Kato, Y. et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS ONE 14, e0212513 (2019).
Li, H. et al. MET inhibitors promote liver tumor evasion of the immune response by stabilizing PDL1. Gastroenterology 156, 1849–1861.e1813 (2019).
Lee, M. S. et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 21, 808–820 (2020).
Chen, X., Zhang, Y., Zhang, N., Ge, Y. & Jia, W. Lenvatinib combined nivolumab injection followed by extended right hepatectomy is a feasible treatment for patients with massive hepatocellular carcinoma: a case report. OncoTargets Ther. 12, 7355–7359 (2019).
Liu, Z., Li, X., He, X., Xu, Y. & Wang, X. Complete response to the combination of Lenvatinib and Pembrolizumab in an advanced hepatocellular carcinoma patient: a case report. BMC Cancer 19, 1062 (2019).
Joerger, M., Guller, U., Bastian, S., Driessen, C. & von Moos, R. Prolonged tumor response associated with sequential immune checkpoint inhibitor combination treatment and regorafenib in a patient with advanced pretreated hepatocellular carcinoma. J. Gastrointest. Oncol. 10, 373–378 (2019).
Finn, R. S. et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. New Engl. J. Med. 382, 1894–1905 (2020).
Postow, M. A., Sidlow, R. & Hellmann, M. D. Immune-related adverse events associated with immune checkpoint blockade. New Engl. J. Med. 378, 158–168 (2018).
Cappelli, L. C., Gutierrez, A. K., Bingham, C. O. III & Shah, A. A. Rheumatic and musculoskeletal immune-related adverse events due to immune checkpoint inhibitors: a systematic review of the literature. Arthritis Care Res. 69, 1751–1763 (2017).
Horvat, T. Z. et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial sloan kettering cancer center. J. Clin. Oncol. 33, 3193–3198 (2015).
Weber, J. S. et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J. Clin. Oncol. 35, 785–792 (2017).
Wang, Y., Gou, Q., Xu, R., Chen, X. & Zhou, Z. Efficacy and safety of sorafenib versus apatinib in the treatment of intermediate and advanced hepatocellular carcinoma: a comparative retrospective study. OncoTargets Ther. 11, 3407–3413 (2018).
Han, Z., He, Z., Wang, C. & Wang, Q. The effect of apatinib in the treatment of sorafenib resistant metastatic hepatocellular carcinoma: a case report. Medicine 97, e13388 (2018).
Zhang, Y., Fan, W., Wang, Y., Huang, G. & Li, J. Apatinib for patients with sorafenib-refractory advanced hepatitis B virus related hepatocellular carcinoma: Results of a Pilot Study. Cancer Control. 26, 1073274819872216 (2019).
Zhu, H., Ma, X., Zhao, Y. & Duo, J. The excellent antitumor effect of apatinib alone as second-line therapy in a patient with sorafenib-refractory hepatocellular carcinoma: a case report. Medicine 97, e11214 (2018).
Yang, X., Wu, G. & Xu, G. Apatinib treatment of advanced hepatocellular carcinoma with portal vein and inferior vena cava tumor thrombus: a case report. Medicine 98, e14582 (2019).
Zhu, H., Zhao, Y. & Wang, X. The radiosensitive effect of apatinib for hepatocellular carcinoma patient with big paraspinal metastasis: a case report. Medicine 97, e9598 (2018).
Du, X. et al. Efficacy of apatinib in advanced hepatocellular carcinoma with lung metastasis: a retrospective, multicenter study. J. BUON 24, 1956–1963 (2019).
Zhen, L. et al. The efficacy and safety of apatinib treatment for patients with unresectable or relapsed liver cancer: a retrospective study. J. Cancer 9, 2773–2777 (2018).
Yu, W. C., Zhang, K. Z., Chen, S. G. & Liu, W. F. Efficacy and Safety of apatinib in patients with intermediate/advanced hepatocellular carcinoma: a prospective observation study. Medicine 97, e9704 (2018).
Xu, J. et al. Anti-PD-1 antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study. Clin. Cancer Res. 25, 515–523 (2019).
Zhu, X. D. & Sun, H. C. Emerging agents and regimens for hepatocellular carcinoma. J. Hematol. Oncol. 12, 110 (2019).
Hsu, C. H. et al. Bevacizumab with erlotinib as first-line therapy in Asian patients with advanced hepatocellular carcinoma: a multicenter phase II study. Oncology 85, 44–52 (2013).
Thomas, M. B. et al. Phase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinoma. J. Clin. Oncol. 27, 843–850 (2009).
Kaseb, A. O. et al. Efficacy of bevacizumab plus erlotinib for advanced hepatocellular carcinoma and predictors of outcome: final results of a phase II trial. Oncology 82, 67–74 (2012).
Philip, P. A. et al. Phase 2 study of bevacizumab plus erlotinib in patients with advanced hepatocellular cancer. Cancer 118, 2424–2430 (2012).
Yau, T. et al. Phase II study of bevacizumab and erlotinib in the treatment of advanced hepatocellular carcinoma patients with sorafenib-refractory disease. Invest. New Drugs 30, 2384–2390 (2012).
Govindarajan, R., Siegel, E., Makhoul, I. & Williamson, S. Bevacizumab and erlotinib in previously untreated inoperable and metastatic hepatocellular carcinoma. Am. J. Clin. Oncol. 36, 254–257 (2013).
Kaseb, A. O. et al. Phase II trial of bevacizumab and erlotinib as a second-line therapy for advanced hepatocellular carcinoma. OncoTargets Ther. 9, 773–780 (2016).
Thomas, M. B. et al. A randomized phase II open-label multi-institution study of the combination of bevacizumab and erlotinib compared to sorafenib in the first-line treatment of patients with advanced hepatocellular carcinoma. Oncology 94, 329–339 (2018).
He, L. et al. Efficacy of bevacizumab combined with erlotinib for advanced hepatocellular carcinoma: a single-arm meta-analysis based on prospective studies. BMC Cancer 19, 276 (2019).
Bollard, J. et al. Palbociclib (PD-0332991), a selective CDK4/6 inhibitor, restricts tumour growth in preclinical models of hepatocellular carcinoma. Gut. https://doi.org/10.1136/gutjnl-2016-312268 (2016).
Shen, S., Dean, D. C., Yu, Z. & Duan, Z. Role of cyclin-dependent kinases (CDKs) in hepatocellular carcinoma: therapeutic potential of targeting the CDK signaling pathway. Hepatol. Res. 49, 1097–1108 (2019).
Wang, C. CDK12 inhibition mediates DNA damage and is synergistic with sorafenib treatment in hepatocellular carcinoma. Gut. 69, 727–736 (2019).
Hsu, C. et al. Cyclin E1 inhibition can overcome sorafenib resistance in hepatocellular carcinoma cells through Mcl-1 suppression. Clin. Cancer Res. 22, 2555–2564 (2016).
Xu, J. et al. Inhibition of cyclin E1 sensitizes hepatocellular carcinoma cells to regorafenib by mcl-1 suppression. Cell Commun. Signal. 17, 85 (2019).
Harding, J. J. et al. A phase Ib/II study of ramucirumab in combination with emibetuzumab in patients with advanced cancer. Clin. Cancer Res. 25, 5202–5211 (2019).
Dietrich, P. et al. Combined effects of PLK1 and RAS in hepatocellular carcinoma reveal rigosertib as promising novel therapeutic “dual-hit” option. Oncotarget 9, 3605–3618 (2018).
Liu, J. et al. Safety, pharmacokinetics and efficacy of donafenib in treating advanced hepatocellular carcinoma: report from a phase 1b trial. Die Pharmazie 74, 688–693 (2019).
Chiang, D. Y. et al. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res. 68, 6779–6788 (2008).
Sawey, E. T. et al. Identification of a therapeutic strategy targeting amplified FGF19 in liver cancer by Oncogenomic screening. Cancer Cell 19, 347–358 (2011).
Arao, T. et al. FGF3/FGF4 amplification and multiple lung metastases in responders to sorafenib in hepatocellular carcinoma. Hepatology 57, 1407–1415 (2013).
Herraez, E. et al. Expression of SLC22A1 variants may affect the response of hepatocellular carcinoma and cholangiocarcinoma to sorafenib. Hepatology 58, 1065–1073 (2013).
Huang, X. Y. et al. alphaB-crystallin complexes with 14-3-3zeta to induce epithelial-mesenchymal transition and resistance to sorafenib in hepatocellular carcinoma. Hepatology 57, 2235–2247 (2013).
Horwitz, E. et al. Human and mouse VEGFA-amplified hepatocellular carcinomas are highly sensitive to sorafenib treatment. Cancer Discov. 4, 730–743 (2014).
Scartozzi, M. et al. VEGF and VEGFR genotyping in the prediction of clinical outcome for HCC patients receiving sorafenib: the ALICE-1 study. Int. J. Cancer 135, 1247–1256 (2014).
Lee, Y. S. et al. SLC15A2 genomic variation is associated with the extraordinary response of sorafenib treatment: whole-genome analysis in patients with hepatocellular carcinoma. Oncotarget 6, 16449–16460 (2015).
Lo, J. et al. Nuclear factor kappa B-mediated CD47 up-regulation promotes sorafenib resistance and its blockade synergizes the effect of sorafenib in hepatocellular carcinoma in mice. Hepatology 62, 534–545 (2015).
Kaibori, M. et al. Increased FGF19 copy number is frequently detected in hepatocellular carcinoma with a complete response after sorafenib treatment. Oncotarget 7, 49091–49098 (2016).
Tong, M. et al. Efficacy of annexin A3 blockade in sensitizing hepatocellular carcinoma to sorafenib and regorafenib. J. Hepatol. 69, 826–839 (2018).
Boyault, S. et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology 45, 42–52 (2007).
Hoshida, Y. et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 69, 7385–7392 (2009).
Schulze, K. et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat. Genet. 47, 505–511 (2015).
Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell 169, 1327–1341.e1323 (2017).
Sia, D. et al. Identification of an immune-specific class of hepatocellular carcinoma, based on molecular features. Gastroenterology 153, 812–826 (2017).
Kurebayashi, Y. et al. Landscape of immune microenvironment in hepatocellular carcinoma and its additional impact on histological and molecular classification. Hepatology 68, 1025–1041 (2018).
Jiang, Y. et al. Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma. Nature 567, 257–261 (2019).
Shimada, S. et al. Comprehensive molecular and immunological characterization of hepatocellular carcinoma. EBioMedicine 40, 457–470 (2019).
Collins, F. S. & Varmus, H. A new initiative on precision medicine. New Engl. J. Med. 372, 793–795 (2015).
McNeil, C. NCI-MATCH launch highlights new trial design in precision-medicine era. J. Natil Cancer Inst. https://doi.org/10.1093/jnci/djv193 (2015).
Mullard, A. NCI-MATCH trial pushes cancer umbrella trial paradigm. Nat. Rev. Drug Discov. 14, 513–515 (2015).
Teufel, M. et al. Biomarkers associated with response to regorafenib in patients with hepatocellular carcinoma. Gastroenterology 156, 1731–1741 (2019).
Pinyol, R. et al. Molecular predictors of prevention of recurrence in HCC with sorafenib as adjuvant treatment and prognostic factors in the phase 3 STORM trial. Gut 68, 1065–1075 (2019).
Ikeda, S. et al. Next-generation sequencing of circulating tumor dna reveals frequent alterations in advanced hepatocellular carcinoma. The Oncologist 23, 586–593 (2018).
Caruso, S. et al. Analysis of liver cancer cell lines identifies agents with likely efficacy against hepatocellular carcinoma and markers of response. Gastroenterology 157, 760–776 (2019).
Xiang, Q. et al. Cabozantinib suppresses tumor growth and metastasis in hepatocellular carcinoma by a dual blockade of VEGFR2 and MET. Clin. Cancer Res. 20, 2959–2970 (2014).
Toh, T. B., Lim, J. J., Hooi, L., Rashid, M. & Chow, E. K. Targeting Jak/Stat pathway as a therapeutic strategy against SP/CD44+ tumorigenic cells in Akt/beta-catenin-driven hepatocellular carcinoma. J. Hepatol. https://doi.org/10.1016/j.jhep.2019.08.035 (2019).
Fisher, R., Pusztai, L. & Swanton, C. Cancer heterogeneity: implications for targeted therapeutics. Br. J. Cancer 108, 479–485 (2013).
McGranahan, N. & Swanton, C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell 168, 613–628 (2017).
Xue, R. et al. Variable intra-tumor genomic heterogeneity of multiple lesions in patients with hepatocellular carcinoma. Gastroenterology 150, 998–1008 (2016).
Xu, L. X. et al. Genomic and transcriptional heterogeneity of multifocal hepatocellular carcinoma. Ann. Oncol. https://doi.org/10.1093/annonc/mdz103 (2019).
Zhai, W. et al. The spatial organization of intra-tumour heterogeneity and evolutionary trajectories of metastases in hepatocellular carcinoma. Nat. Commun. 8, 4565 (2017).
Lin, D. C. et al. Genomic and epigenomic heterogeneity of hepatocellular carcinoma. Cancer Res. 77, 2255–2265 (2017).
Ling, S. et al. Extremely high genetic diversity in a single tumor points to prevalence of non-Darwinian cell evolution. Proc. Natl Acad. Sci. USA 112, E6496–6505 (2015).
Friemel, J. et al. Intratumor heterogeneity in hepatocellular carcinoma. Clin. Cancer Res. 21, 1951–1961 (2015).
Buczak, K. et al. Spatial tissue proteomics quantifies inter- and intratumor Heterogeneity in Hepatocellular Carcinoma (HCC). Mol. Cell. Proteom. 17, 810–825 (2018).
Gao, Q. et al. Cell culture system for analysis of genetic heterogeneity within hepatocellular carcinomas and response to pharmacologic agents. Gastroenterology 152, 232–242 e234 (2017).
Torrecilla, S. et al. Trunk mutational events present minimal intra- and inter-tumoral heterogeneity in hepatocellular carcinoma. J. Hepatol. 67, 1222–1231 (2017).
Shen, Y. C. et al. Reliability of a single-region sample to evaluate tumor immune microenvironment in hepatocellular carcinoma. J. Hepatol. https://doi.org/10.1016/j.jhep.2019.09.032 (2019).
Huang, A. et al. Circumventing intratumoral heterogeneity to identify potential therapeutic targets in hepatocellular carcinoma. J. Hepatol. 67, 293–301 (2017).
Ding, X. et al. Genomic and epigenomic features of primary and recurrent hepatocellular carcinomas. Gastroenterology 157, 1630–1645.e1636 (2019).
Craig, A. J., von Felden, J., Garcia-Lezana, T., Sarcognato, S. & Villanueva, A. Tumour evolution in hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. https://doi.org/10.1038/s41575-019-0229-4 (2019).
Zheng, H. et al. Single-cell analysis reveals cancer stem cell heterogeneity in hepatocellular carcinoma. Hepatology 68, 127–140 (2018).
Duan, M. et al. Diverse modes of clonal evolution in HBV-related hepatocellular carcinoma revealed by single-cell genome sequencing. Cell Res. 28, 359–373 (2018).
Ossandon, M. R. et al. Circulating tumor DNA assays in clinical cancer research. J. Natl Cancer Inst. https://doi.org/10.1093/jnci/djy105 (2018).
Parikh, A. R. et al. Liquid versus tissue biopsy for detecting acquired resistance and tumor heterogeneity in gastrointestinal cancers. Nat. Med. 25, 1415–1421 (2019).
Zill, O. A. et al. The landscape of actionable genomic alterations in cell-free circulating tumor DNA from 21,807 advanced cancer patients. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.CCR-17-3837 (2018).
Ng, C. K. Y. et al. Genetic profiling using plasma-derived cell-free DNA in therapy-naive hepatocellular carcinoma patients: a pilot study. Ann. Oncol. 29, 1286–1291 (2018).
Cai, Z. X. et al. Circulating tumor DNA profiling reveals clonal evolution and real-time disease progression in advanced hepatocellular carcinoma. Int. J. Cancer 141, 977–985 (2017).
Chen, G. et al. Clonal evolution in long-term follow-up patients with hepatocellular carcinoma. Int. J. Cancer 143, 2862–2870 (2018).
Labgaa, I. et al. A pilot study of ultra-deep targeted sequencing of plasma DNA identifies driver mutations in hepatocellular carcinoma. Oncogene. https://doi.org/10.1038/s41388-018-0206-3 (2018).
Ikeda, S., Lim, J. S. & Kurzrock, R. Analysis of tissue and circulating tumor DNA by next-generation sequencing of hepatocellular carcinoma: implications for targeted therapeutics. Mol. Cancer Ther. 17, 1114–1122 (2018).
Sachs, N. et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell 172, 373–386.e310 (2018).
Vlachogiannis, G. et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 359, 920–926 (2018).
Hidalgo, M. et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 4, 998–1013 (2014).
Hu, B. et al. Establishment of a hepatocellular carcinoma patient-derived xenograft platform and its application in biomarker identification. Int. J. Cancer https://doi.org/10.1002/ijc.32564 (2019).
Girotti, M. R. et al. Application of sequencing, liquid biopsies, and patient-derived xenografts for personalized medicine in melanoma. Cancer Discov. 6, 286–299 (2016).
Acknowledgements
This study was jointly supported by the National Key R&D Program of China (2019YFC1315800, 2019YFC1315802), the National Key Research and Development Program (2016YFF0101405), National Natural Science Foundation of China (No.81830102, 81772578, 81802991, and 81830102), STCSM (18YF1403600), Shanghai Municipal Health Commission Collaborative Innovation Cluster Project (2019CXJQ02), Projects from the Shanghai Science and Technology Commission (19441905000), and Shanghai Municipal Key Clinical Specialty.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, A., Yang, XR., Chung, WY. et al. Targeted therapy for hepatocellular carcinoma. Sig Transduct Target Ther 5, 146 (2020). https://doi.org/10.1038/s41392-020-00264-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-020-00264-x
This article is cited by
-
RARRES1 inhibits hepatocellular carcinoma progression and increases its sensitivity to lenvatinib through interaction with SPINK2
Biology Direct (2024)
-
Inhibition of growth of hepatocellular carcinoma by co-delivery of anti-PD-1 antibody and sorafenib using biomimetic nano-platelets
BMC Cancer (2024)
-
Nano-modified viruses prime the tumor microenvironment and promote the photodynamic virotherapy in liver cancer
Journal of Biomedical Science (2024)
-
Machine learning-based disulfidptosis-related lncRNA signature predicts prognosis, immune infiltration and drug sensitivity in hepatocellular carcinoma
Scientific Reports (2024)
-
Ferroptosis: a new hunter of hepatocellular carcinoma
Cell Death Discovery (2024)