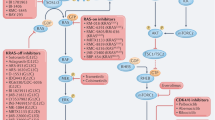
Key Points
-
The small GTPase KRAS is among the most frequently activated proteins in cancer. Despite this, extensive efforts to target KRAS over the past decades have yet to yield a clinical drug.
-
Advances in drug discovery approaches and technology have renewed hope for targeting this challenging oncogene, and several recent reports have demonstrated substantial progress to this end.
-
Analysis of RAS structure and dynamics suggests that targeting the GDP-bound off state of RAS may be a more viable approach than targeting the GTP-bound on state. Recent data indicating continued flux through the GTPase cycle for some oncogenic mutants make the GDP state even more promising. Several reported molecules now target nucleotide exchange and others allosterically target GTP binding.
-
Efforts to inhibit RAS-effector interactions and to impair RAS localization and trafficking through RAS-binding small molecules have continued to struggle. However, promising molecules have been reported to target these functions indirectly, without binding to RAS.
-
Stabilizing non-productive protein–protein complexes remains an attractive approach to inhibiting RAS; however, little progress has been demonstrated in this area.
Abstract
KRAS is the most frequently mutated oncogene in human cancer. In addition to holding this distinction, unsuccessful attempts to target this protein have led to the characterization of RAS as 'undruggable'. However, recent advances in technology and novel approaches to drug discovery have renewed hope that a direct KRAS inhibitor may be on the horizon. In this Review, we provide an in-depth analysis of the structure, dynamics, mutational activation and inactivation, and signalling mechanisms of RAS. From this perspective, we then consider potential mechanisms of action for effective RAS inhibitors. Finally, we examine each of the many recent reports of direct RAS inhibitors and discuss promising avenues for further development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bonfini, L., Karlovich, C., Dasgupta, C. & Banerjee, U. The Son of sevenless gene product: a putative activator of Ras. Science 255, 603–606 (1992).
Buday, L. & Downward, J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell 73, 611–620 (1993).
Ebinu, J. O. et al. RasGRP, a Ras guanyl nucleotide-releasing protein with calcium- and diacylglycerol-binding motifs. Science 280, 1082–1086 (1998).
Trahey, M. & McCormick, F. A cytoplasmic protein stimulates normal N-ras p21 GTPase, but does not affect oncogenic mutants. Science 238, 542–545 (1987).
Xu, G. et al. The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell 62, 599–608 (1990).
Wood, K. W., Sarnecki, C. & Roberts, T. M. & Blenis, J. Ras mediates nerve growth factor receptor modulation of three signal-transducing protein kinases: MAP kinase, Raf-1, and RSK. Cell 68, 1041–1050 (1992).
Howe, L. R. et al. Activation of the MAP kinase pathway by the protein kinase raf. Cell 71, 335–342 (1992).
Vojtek, A. B., Hollenberg, S. M. & Cooper, J. A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell 74, 205–214 (1993).
Moodie, S. A., Willumsen, B. M., Weber, M. J. & Wolfman, A. Complexes of Ras. GTP with Raf-1 and mitogen-activated protein kinase kinase. Science 260, 1658–1661 (1993).
Warne, P. H., Vician, P. R. & Downward, J. Direct interaction of Ras and the amino-terminal region of Raf-1 in vitro. Nature 364, 352–355 (1993).
Zhang, X. F. et al. Normal and oncogenic p21ras proteins bind to the amino-terminal regulatory domain of c-Raf-1. Nature 364, 308–313 (1993).
Sjölander, A., Yamamoto, K., Huber, B. E. & Lapetina, E. G. Association of p21ras with phosphatidylinositol 3-kinase. Proc. Natl Acad. Sci. USA 88, 7908–7912 (1991).
Rodriguez-Viciana, P. et al. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature 370, 527–532 (1994).
Kodaki, T., Woscholski, R., Hallberg, B., Rodriguez-Viciana Julian Downward, P. & Parker, P. J. The activation of phosphatidylinositol 3-kinase by Ras. Curr. Biol. 4, 798–806 (1994).
Brunn, G. J. et al. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J. 15, 5256–5267 (1996).
Hofer, F., Fields, S., Schneider, C. & Martin, G. S. Activated Ras interacts with the Ral guanine nucleotide dissociation stimulator. Proc. Natl Acad. Sci. USA 91, 11089–11093 (1994).
Spaargaren, M. & Bischoff, J. R. Identification of the guanine nucleotide dissociation stimulator for Ral as a putative effector molecule of R-ras, H-ras, K-ras, and Rap. Proc. Natl Acad. Sci. USA 91, 12609–12613 (1994).
Kikuchi, A., Demo, S. D., Ye, Z. H., Chen, Y. W. & Williams, L. T. ralGDS family members interact with the effector loop of ras p21. Mol. Cell. Biol. 14, 7483–7491 (1994).
White, M. A., Vale, T., Camonis, J. H., Schaefer, E. & Wigler, M. H. A role for the Ral guanine nucleotide dissociation stimulator in mediating Ras-induced transformation. J. Biol. Chem. 271, 16439–16442 (1996).
Casey, P. J., Solski, P. A., Der, C. J. & Buss, J. E. p21ras is modified by a farnesyl isoprenoid. Proc. Natl Acad. Sci. USA 86, 8323–8327 (1989).
Gutierrez, L., Magee, A. I., Marshall, C. J. & Hancock, J. F. Post-translational processing of p21ras is two-step and involves carboxyl-methylation and carboxy-terminal proteolysis. EMBO J. 8, 1093–1098 (1989).
Whyte, D. B. et al. K- and N-Ras are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. J. Biol. Chem. 272, 14459–14464 (1997).
Hancock, J. F., Paterson, H. & Marshall, C. J. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell 63, 133–139 (1990).
Milburn, M. V. et al. Molecular switch for signal transduction: structural differences between active and inactive forms of protooncogenic ras proteins. Science 247, 939–945 (1990). This study described the structural differences between the GDP-bound and GTP-bound states of RAS.
Rajakulendran, T., Sahmi, M., Lefrançois, M., Sicheri, F. & Therrien, M. A dimerization-dependent mechanism drives RAF catalytic activation. Nature 461, 542–545 (2009).
Lin, W.-C. et al. H-Ras forms dimers on membrane surfaces via a protein–protein interface. Proc. Natl Acad. Sci. USA 111, 2996–3001 (2014).
Muratcioglu, S. et al. GTP-dependent K-Ras dimerization. Structure 23, 1325–1335 (2015).
Nan, X. et al. Ras-GTP dimers activate the mitogen-activated protein kinase (MAPK) pathway. Proc. Natl Acad. Sci. USA 112, 7996–8001 (2015).
Parada, L. F., Tabin, C. J., Shih, C. & Weinberg, R. A. Human EJ bladder carcinoma oncogene is homologue of Harvey sarcoma virus ras gene. Nature 297, 474–478 (1982).
Santos, E., Tronick, S. R., Aaronson, S. A., Pulciani, S. & Barbacid, M. T24 human bladder carcinoma oncogene is an activated form of the normal human homologue of BALB- and Harvey-MSV transforming genes. Nature 298, 343–347 (1982).
Der, C. J., Krontiris, T. G. & Cooper, G. M. Transforming genes of human bladder and lung carcinoma cell lines are homologous to the ras genes of Harvey and Kirsten sarcoma viruses. Proc. Natl Acad. Sci. USA 79, 3637–3640 (1982).
Taparowsky, E. et al. Activation of the T24 bladder carcinoma transforming gene is linked to a single amino acid change. Nature 300, 762–765 (1982).
Forbes, S. A. et al. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 39, D945–D950 (2011).
Prior, I. A., Lewis, P. D. & Mattos, C. A. Comprehensive survey of Ras mutations in cancer. Cancer Res. 72, 2457–2467 (2012).
Tsai, F. D. et al. K-Ras4A splice variant is widely expressed in cancer and uses a hybrid membrane-targeting motif. Proc. Natl Acad. Sci. USA 112, 779–784 (2015).
Fasano, O. et al. Analysis of the transforming potential of the human H-ras gene by random mutagenesis. Proc. Natl Acad. Sci. USA 81, 4008–4012 (1984).
Gideon, P. et al. Mutational and kinetic analyses of the GTPase-activating protein (GAP)-p21 interaction: the C-terminal domain of GAP is not sufficient for full activity. Mol. Cell. Biol. 12, 2050–2056 (1992).
Buhrman, G., Holzapfel, G., Fetics, S. & Mattos, C. Allosteric modulation of Ras positions Q61 for a direct role in catalysis. Proc. Natl Acad. Sci. USA 107, 4931–4936 (2010).
Scheffzek, K. et al. The Ras–RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science 277, 333–338 (1997).
Smith, M. J., Neel, B. G. & Ikura, M. NMR-based functional profiling of RASopathies and oncogenic RAS mutations. Proc. Natl Acad. Sci. USA 110, 4574–4579 (2013).
Seeburg, P. H., Colby, W. W., Capon, D. J., Goeddel, D. V. & Levinson, A. D. Biological properties of human c-Ha-ras1 genes mutated at codon 12. Nature 312, 71–75 (1984).
Hunter, J. C. et al. Biochemical and structural analysis of common cancer-associated KRAS mutations. Mol. Cancer Res. 13, 1325–1335 (2015).
Wang, M.-T. et al. K-Ras promotes tumorigenicity through suppression of non-canonical Wnt signaling. Cell 163, 1237–1251 (2015).
Johnson, L. et al. K-ras is an essential gene in the mouse with partial functional overlap with N-ras. Genes Dev. 11, 2468–2481 (1997).
Zhang, Z. et al. Wildtype Kras2 can inhibit lung carcinogenesis in mice. Nat. Genet. 29, 25–33 (2001).
Bentley, C. et al. A requirement for wild-type Ras isoforms in mutant KRas-driven signalling and transformation. Biochem. J. 452, 313–320 (2013).
Staffas, A., Karlsson, C., Persson, M., Palmqvist, L. & Bergo, M. O. Wild-type KRAS inhibits oncogenic KRAS-induced T-ALL in mice. Leukemia 29, 1032–1040 (2014).
Kong, G. et al. Loss of wild-type Kras promotes activation of all Ras isoforms in oncogenic Kras-induced leukemogenesis. Leukemia 30, 1542–1551 (2016).
Matallanas, D. et al. Mutant K-Ras activation of the proapoptotic MST2 pathway is antagonized by wild-type K-Ras. Mol. Cell 44, 893–906 (2011).
Knight, Z. A. & Shokat, K. M. Chemical genetics: where genetics and pharmacology meet. Cell 128, 425–430 (2007).
de Vos, A. M. et al. Three-dimensional structure of an oncogene protein: catalytic domain of human c-H-ras p21. Science 239, 888–893 (1988).
Tong, L., de Vos, A. M., Milburn, M. V. & Kim, S.-H. Crystal structures at 2.2 Å resolution of the catalytic domains of normal ras protein and an oncogenic mutant complexed with GDP. J. Mol. Biol. 217, 503–516 (1991).
Kraulis, P. J., Domaille, P. J., Campbell-Burk, S. L., Van Aken, T. & Laue, E. D. Solution structure and dynamics of ras p21.GDP determined by heteronuclear three- and four-dimensional NMR spectroscopy. Biochemistry 33, 3515–3531 (1994).
Ito, Y. et al. Regional polysterism in the GTP-bound form of the human c-Ha-Ras protein. Biochemistry 36, 9109–9119 (1997).
Ostrem, J. M., Peters, U., Sos, M. L., Wells, J. A. & Shokat, K. M. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature 503, 548–551 (2013).
Gorfe, A. A., Grant, B. J. & McCammon, J. A. Mapping the nucleotide and isoform-dependent structural and dynamical features of Ras proteins. Structure 16, 885–896 (2008). This paper details molecular dynamics simulations revealing increased flexibility in KRAS relative to NRAS and HRAS.
Spoerner, M., Herrmann, C., Vetter, I. R., Kalbitzer, H. R. & Wittinghofer, A. Dynamic properties of the Ras switch I region and its importance for binding to effectors. Proc. Natl Acad. Sci. USA 98, 4944–4949 (2001). This paper identifies both an active and an inactive state within GTP-bound RAS.
Ford, B., Skowronek, K., Boykevisch, S., Bar-Sagi, D. & Nassar, N. Structure of the G60A mutant of Ras: implications for the dominant negative effect. J. Biol. Chem. 280, 25697–25705 (2005).
Shima, F. et al. Structural basis for conformational dynamics of GTP-bound Ras protein. J. Biol. Chem. 285, 22696–22705 (2010).
Araki, M. et al. Solution structure of the state 1 conformer of GTP-bound H-Ras protein and distinct dynamic properties between the state 1 and state 2 conformers. J. Biol. Chem. 286, 39644–39653 (2011).
Shirouzu, M. et al. Mutations that abolish the ability of Ha-Ras to associate with Raf-1. Oncogene 9, 2153–2157 (1994).
Sung, Y.-J., Carter, M., Zhong, J.-M. & Hwang, Y.-W. Mutagenesis of the H-ras p21 at glycine-60 residue disrupts GTP-induced conformational change. Biochemistry 34, 3470–3477 (1995).
Moodie, S. A. et al. Different structural requirements within the switch II region of the Ras protein for interactions with specific downstream targets. Oncogene 11, 10308–10320 (1995).
Drugan, J. K. et al. Ras interaction with two distinct binding domains in Raf-1 may be required for Ras transformation. J. Biol. Chem. 271, 233–237 (1996).
Nur-E-Kamal, M. S., Sizeland, A., D'Abaco, G. & Maruta, H. Asparagine 26, glutamic acid 31, valine 45, and tyrosine 64 of Ras proteins are required for their oncogenicity. J. Biol. Chem. 267, 1415–1418 (1992).
Feig, L. A. & Cooper, G. M. Inhibition of NIH 3T3 cell proliferation by a mutant ras protein with preferential affinity for GDP. Mol. Cell. Biol. 8, 3235–3243 (1988).
Farnsworth, C. L. & Feig, L. A. Dominant inhibitory mutations in the Mg2+-binding site of RasH prevent its activation by GTP. Mol. Cell. Biol. 11, 4822–4829 (2009).
Cool, R. H. et al. The Ras mutant D119N is both dominant negative and activated. Mol. Cell. Biol. 19, 6297–6305 (1999).
Drosten, M. et al. Genetic analysis of Ras signalling pathways in cell proliferation, migration and survival. EMBO J. 29, 1091–1104 (2010).
Shieh, A., Ward, A. F., Donlan, K. L. & Harding-Theobald, E. R. Defective K-Ras oncoproteins overcome impaired effector activation to initiate leukemia in vivo. Blood 121, 4884–4893 (2013).
Castellano, E., Sheridan, C., Thin, M. Z. & Nye, E. Requirement for interaction of PI3-kinase p110α with RAS in lung tumor maintenance. Cancer Cell 24, 617–630 (2013).
Pacold, M. E. et al. Crystal structure and functional analysis of Ras binding to its effector phosphoinositide 3-kinase γ. Cell 103, 931–943 (2000).
John, J. et al. Kinetic and structural analysis of the Mg2+-binding site of the guanine nucleotide-binding protein p21H-ras. J. Biol. Chem. 268, 923–929 (1993).
Ford, B. et al. Characterization of a Ras Mutant with Identical GDP- and GTP-Bound Structures. Biochemistry 48, 11449–11457 (2009).
Traut, T. W. Physiological concentrations of purines and pyrimidines. Mol. Cell. Biochem. 140, 1–22 (1994).
John, J., Frech, M. & Wittinghofer, F. Biochemical properties of Ha-ras encoded p21 mutants and mechanism of the autophosphorylation reaction. J. Biol. Chem. 263, 11792–11799 (1989).
Huang, H. et al. Oncogenic K-Ras requires activation for enhanced activity. Oncogene 33, 532–535 (2014).
Ardito, C. M. et al. EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell 22, 304–317 (2012).
Navas, C. et al. EGF receptor signaling is essential for k-ras oncogene-driven pancreatic ductal adenocarcinoma. Cancer Cell 22, 318–330 (2012).
da Cunha Santos, G., Dhani, N., Tu, D. & Chin, K. Molecular predictors of outcome in a phase 3 study of gemcitabine and erlotinib therapy in patients with advanced pancreatic cancer: National Cancer Institute of Canada Clinical Trials Group Study PA.3. Cancer 116, 5599–5607 (2010).
Normanno, N., Bianco, C., De Luca, A. & Salomon, D. S. The role of EGF-related peptides in tumor growth. Front. Biosci. 6, D685–D707 (2001).
Pao, W. et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2, e17 (2005).
Karapetis, C. S. et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N. Engl. J. Med. 359, 1757–1765 (2008).
Amado, R. G. et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J. Clin. Oncol. 26, 1626–1634 (2008).
Takasaki, J. A novel G q/11-selective inhibitor. J. Biol. Chem. 279, 47438–47445 (2004).
Nishimura, A. et al. Structural basis for the specific inhibition of heterotrimeric Gq protein by a small molecule. Proc. Natl Acad. Sci. USA 107, 13666–13671 (2010). Along with reference 85, this article details effective inhibition of a G protein by means of impaired nucleotide release.
Wells, J. A. & McClendon, C. L. Reaching for high-hanging fruit in drug discovery at protein–protein interfaces. Nature 450, 1001–1009 (2007).
Brennan, D. F. et al. A Raf-induced allosteric transition of KSR stimulates phosphorylation of MEK. Nature 472, 366–369 (2011).
Athuluri-Divakar, S. K. et al. A small molecule RAS-mimetic disrupts RAS association with effector proteins to block signaling. Cell 165, 643–655 (2016).
Liu, J. et al. Calcineurin is a common target of cyclophilin–cyclosporin A and FKBP–FK506 complexes. Cell 66, 807–815 (1991).
Chung, J., Kuo, C. J., Crabtree, G. R. & Blenis, J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell 69, 1227–1236 (1992).
Sabers, C. J. et al. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells. J. Biol. Chem. 270, 815–822 (1995).
Peyroche, A. et al. Brefeldin A acts to stabilize an abortive ARF–GDP–Sec7 domain protein complex. Mol. Cell 3, 275–285 (1999). This study demonstrates effective inhibition of a GTPase by stabilizing a non-productive protein–protein interaction.
Jackson, J. H. et al. Farnesol modification of Kirsten-ras exon 4B protein is essential for transformation. Proc. Natl Acad. Sci. USA 87, 3042–3046 (1990).
Reiss, Y., Goldstein, J. L., Seabra, M. C., Casey, P. J. & Brown, M. S. Inhibition of purified p21ras farnesyl:protein transferase by Cys-AAX tetrapeptides. Cell 62, 81–88 (1990).
Kohl, N. et al. Selective inhibition of ras-dependent transformation by a farnesyltransferase inhibitor. Science 260, 1934–1937 (1993).
James, G. et al. Benzodiazepine peptidomimetics: potent inhibitors of Ras farnesylation in animal cells. Science 260, 1937–1942 (1993).
Liu, M. et al. Targeting the protein prenyltransferases efficiently reduces tumor development in mice with K-RAS-induced lung cancer. Proc. Natl Acad. Sci. USA 107, 6471–6476 (2010).
Appels, N. M., Beijnen, J. H. & Schellens, J. H. Development of farnesyl transferase inhibitors: a review. Oncologist 10, 565–578 (2005).
Sousa, S., Fernandes, P. & Ramos, M. Farnesyltransferase inhibitors: a detailed chemical view on an elusive biological problem. Curr. Med. Chem. 15, 1478–1492 (2008).
Taveras, A. G. et al. Ras oncoprotein inhibitors: the discovery of potent, ras nucleotide exchange inhibitors and the structural determination of a drug-protein complex. Bioorg. Med. Chem. 5, 125–133 (1997).
Patgiri, A., Yadav, K. K., Arora, P. S. & Bar-Sagi, D. An orthosteric inhibitor of the Ras–Sos interaction. Nat. Chem. Biol. 7, 585–587 (2011).
Maurer, T. et al. Small-molecule ligands bind to a distinct pocket in Ras and inhibit SOS-mediated nucleotide exchange activity. Proc. Natl Acad. Sci. USA 109, 5299–5304 (2012).
Sun, Q. et al. Discovery of small molecules that bind to K-Ras and inhibit Sos-mediated activation. Angew. Chem. 124, 6244–6247 (2012).
Shima, F. et al. In silico discovery of small-molecule Ras inhibitors that display antitumor activity by blocking the Ras-effector interaction. Proc. Natl Acad. Sci. USA 110, 8182–8187 (2013).
Hocker, H. J. et al. Andrographolide derivatives inhibit guanine nucleotide exchange and abrogate oncogenic Ras function. Proc. Natl Acad. Sci. USA 110, 10201–10206 (2013).
Lim, S. M. et al. Therapeutic targeting of oncogenic K-Ras by a covalent catalytic site inhibitor. Angew. Chem. 53, 199–204 (2013).
Leshchiner, E. S. et al. Direct inhibition of oncogenic KRAS by hydrocarbon-stapled SOS1 helices. Proc. Natl Acad. Sci. USA 112, 1761–1766 (2015).
Winter, J. J. G. et al. Small molecule binding sites on the Ras:SOS complex can be exploited for inhibition of Ras activation. J. Med. Chem. 58, 2265–2274 (2015).
Ganguly, A. K. et al. Detection and structural characterization of ras oncoprotein-inhibitors complexes by electrospray mass spectrometry. Bioorg. Med. Chem. 5, 817–820 (1997).
Ganguly, A. K. et al. Interaction of a novel GDP exchange inhibitor with the Ras protein. Biochemistry 37, 15631–15637 (1998).
Lacal, J. C. & Aaronson, S. A. Activation of ras p21 transforming properties associated with an increase in the release rate of bound guanine nucleotide. Mol. Cell. Biol. 6, 4214–4220 (1986).
Hattori, S. et al. Neutralizing monoclonal antibody against ras oncogene product p21 which impairs guanine nucleotide exchange. Mol. Cell. Biol. 7, 1999–2002 (1987).
Colombo, S., Peri, F., Tisi, R., Nicotra, F. & Martegani, E. Design and characterization of a new class of inhibitors of Ras activation. Ann. NY Acad. Sci. 1030, 52–61 (2004).
Palmioli, A. et al. Selective cytotoxicity of a bicyclic Ras inhibitor in cancer cells expressing K-RasG13D. Biochem. Biophys. Res. Commun. 386, 593–597 (2009).
Okamoto, T. et al. Stabilizing the pro-apoptotic BimBH3 helix (BimSAHB) does not necessarily enhance affinity or biological activity. ACS Chem. Biol. 8, 297–302 (2013).
Burns, M. C. et al. Approach for targeting Ras with small molecules that activate SOS-mediated nucleotide exchange. Proc. Natl Acad. Sci. USA 111, 3401–3406 (2014). This paper demonstrates paradoxical inhibition of RAS signalling by molecules that increase levels of RAS–GTP.
Roberts, A. E. et al. Germline gain-of-function mutations in SOS1 cause Noonan syndrome. Nat. Genet. 39, 70–74 (2006).
Tartaglia, M. et al. Gain-of-function SOS1 mutations cause a distinctive form of Noonan syndrome. Nat. Genet. 39, 75–79 (2006).
Margarit, S. M. et al. Structural evidence for feedback activation by Ras. GTP of the Ras-specific nucleotide exchange factor SOS. Cell 112, 685–695 (2003).
Boriack-Sjodin, P. A., Margarit, S. M., Bar-Sagi, D. & Kuriyan, J. The structural basis of the activation of Ras by Sos. Nature 394, 337–343 (1998).
Sondermann, H. et al. Structural analysis of autoinhibition in the Ras activator Son of sevenless. Cell 119, 393–405 (2004).
Koeppe, J. R., Seitova, A., Mather, T. & Komives, E. A. Thrombomodulin tightens the thrombin active site loops to promote protein C activation. Biochemistry 44, 14784–14791 (2005).
Patricelli, M. P. et al. Selective inhibition of oncogenic KRAS output with small molecules targeting the inactive state. Cancer Discov. 6, 316–329 (2016). Along with reference 136, this study demonstrates substantial flux through the RAS cycle even in the presence of the oncogenic G12C mutation.
Herrmann, C. et al. Sulindac sulfide inhibits Ras signaling. Oncogene 17, 1769–1776 (1998).
Müller, O. et al. Identification of potent Ras signaling inhibitors by pathway-selective phenotype-based screening. Angew. Chem. 43, 450–454 (2004).
Waldmann, H. et al. Sulindac-derived Ras pathway inhibitors target the Ras–Raf interaction and downstream effectors in the Ras pathway. Angew. Chem. 43, 454–458 (2004).
Pan, M.-R., Chang, H.-C. & Hung, W.-C. Non-steroidal anti-inflammatory drugs suppress the ERK signaling pathway via block of Ras/c-Raf interaction and activation of MAP kinase phosphatases. Cell. Signal. 20, 1134–1141 (2008).
Kato-Stankiewicz, J. et al. Inhibitors of Ras/Raf-1 interaction identified by two-hybrid screening revert Ras-dependent transformation phenotypes in human cancer cells. Proc. Natl Acad. Sci. USA 99, 14398–14403 (2002).
González-Pérez, V. et al. Genetic and functional characterization of putative Ras/Raf interaction inhibitors in C. elegans and mammalian cells. J. Mol. Signal 5, 2 (2010).
Chehade, K. A. H., Andres, D. A., Morimoto, H. & Spielmann, H. P. Design and synthesis of a transferable farnesyl pyrophosphate analogue to Ras by protein farnesyltransferase. J. Org. Chem. 65, 3027–3033 (2000).
Zimmermann, G. et al. Small molecule inhibition of the KRAS–PDEδ interaction impairs oncogenic KRAS signalling. Nature 497, 638–642 (2013).
Papke, B. et al. Identification of pyrazolopyridazinones as PDEδ inhibitors. Nat. Commun. 7, 11360 (2016).
Byrd, J. C. et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 369, 32–42 (2013).
Wang, M. L. et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 369, 507–516 (2013).
Stephen, A. G., Esposito, D., Bagni, R. K. & McCormick, F. Dragging ras back in the ring. Cancer Cell 25, 272–281 (2014).
Lito, P., Solomon, M., Li, L.-S., Hansen, R. & Rosen, N. Allele-specific inhibitors inactivate mutant KRAS G12C by a trapping mechanism. Science 351, 604–608 (2016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
J.M.L.O. and K.M.S. are joint inventors on a University of California Board of Regents-owned patent application covering inhibitors of KRAS, which has been licensed to Araxes Pharma LLC. They also hold stock in and are consultants to Araxes Pharma LLC.
Supplementary information
Supplementary information S1 (table)
Supplementary information (PDF 647 kb)
Glossary
- Effectors
-
Proteins that bind to the GTP-bound state of RAS in order to transmit downstream signals for proliferation, survival and differentiation.
- Intrinsic GTPase activity
-
GTPase activity (or nucleotide exchange) that is not catalysed by any other protein or chemical.
- Transformation
-
The process by which cells acquire the features of cancer. The degree of transformation refers to the extent to which cells have achieved a cancer-like phenotype.
- Tumorigenesis
-
The initial formation of a cancer, involving the transformation of normal cells into cancer cells.
- B factors
-
Measures of scattering in X-ray crystallography indicating the mobility of the atom. Higher B factors indicate more mobility.
- Electron density
-
A measure of the probability of electrons being present in a region. Because electrons are concentrated around atoms and bonds, in X-ray crystallography a model of the protein structure is built by fitting atoms within an electron density map calculated from the X-ray diffraction pattern.
- Nuclear magnetic resonance spectroscopy.
-
(NMR spectroscopy). A technique based on the absorption and re-emission of electromagnetic radiation by certain isotopes in a magnetic field. This technique may be carried out in solution and may be used to study protein structure, dynamics and interactions.
- Hydrogen–deuterium exchange
-
A technique in which solvent-accessible, labile hydrogen atoms (such as amide hydrogen atoms or side-chain hydrogen atoms) are exchanged for deuterium by incubating a protein in deuterated water (D2O). The exchange can then be evaluated using mass spectrometry or nuclear magnetic resonance spectroscopy. This technique can be used to determine whether regions of the protein are solvent accessible, and by proxy, whether they are mobile.
- Dynamics
-
Proteins are no longer thought to exist as a static shape, but rather as sets of shapes that interconvert on a range of timescales. Dynamics refers to the study of these movements within a protein structure, including the transitions themselves as well as the range of conformations a protein adopts.
- Electrophilic
-
An electron-poor chemical group that interacts with an electron-rich nucleophile to form a covalent bond. In this reaction, the nucleophile donates a pair of electrons to form a bond with the electrophile.
Rights and permissions
About this article
Cite this article
Ostrem, J., Shokat, K. Direct small-molecule inhibitors of KRAS: from structural insights to mechanism-based design. Nat Rev Drug Discov 15, 771–785 (2016). https://doi.org/10.1038/nrd.2016.139
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd.2016.139
This article is cited by
-
The energetic and allosteric landscape for KRAS inhibition
Nature (2024)
-
An overview of recent advancements in small molecules suppression of oncogenic signaling of K-RAS: an updated review
Molecular Diversity (2024)
-
Targeting KRAS-Mutated Gastrointestinal Malignancies with Small-Molecule Inhibitors: A New Generation of Breakthrough Therapies
Drugs (2024)
-
The current landscape of using direct inhibitors to target KRASG12C-mutated NSCLC
Experimental Hematology & Oncology (2023)
-
Recent advances in non-small cell lung cancer targeted therapy; an update review
Cancer Cell International (2023)